Morpho-Molecular Characterization of Hypocrealean Fungi Isolated from Rice in Northern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation, and Examination
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Phylogenetic Analyses
3. Results
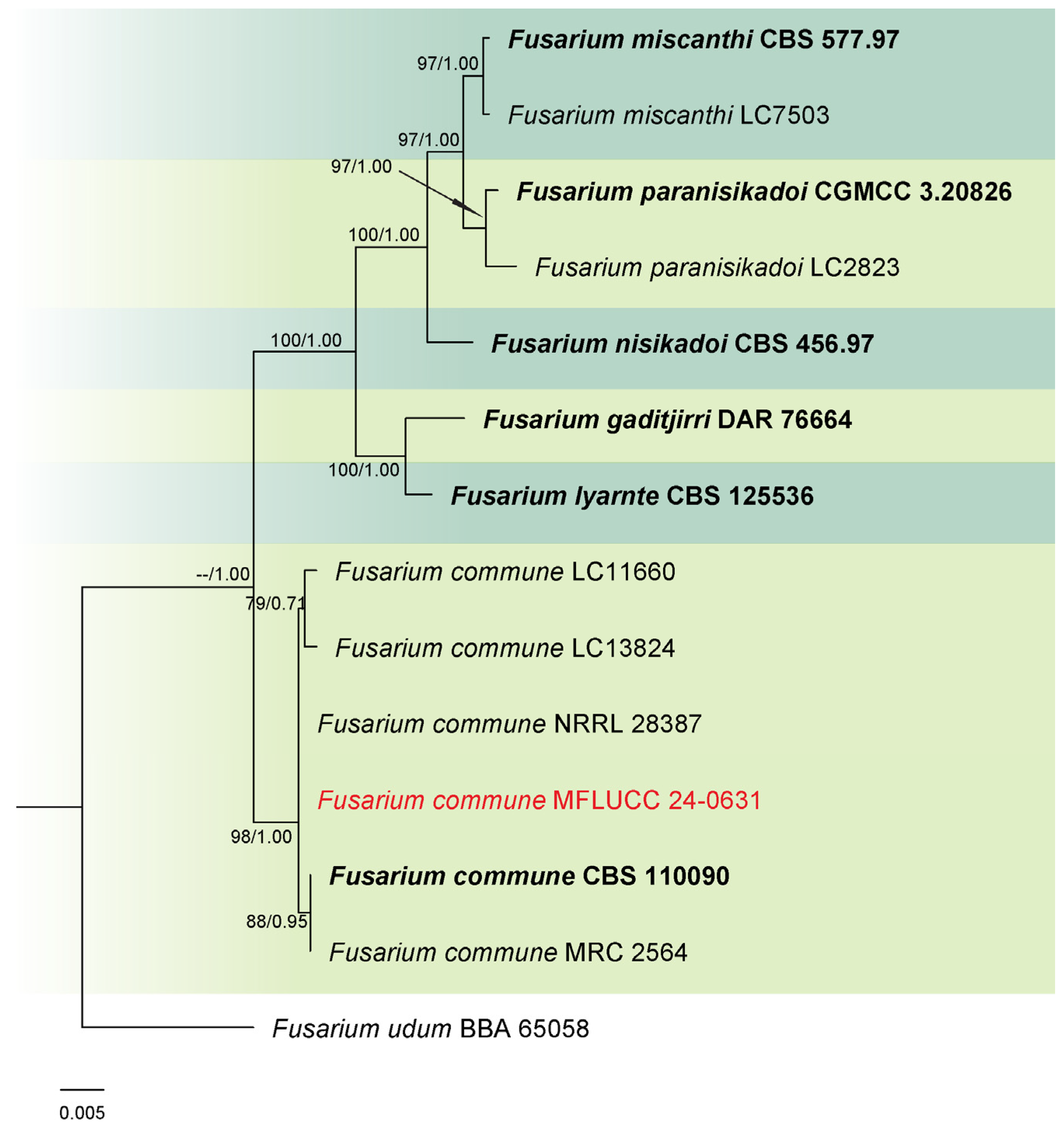
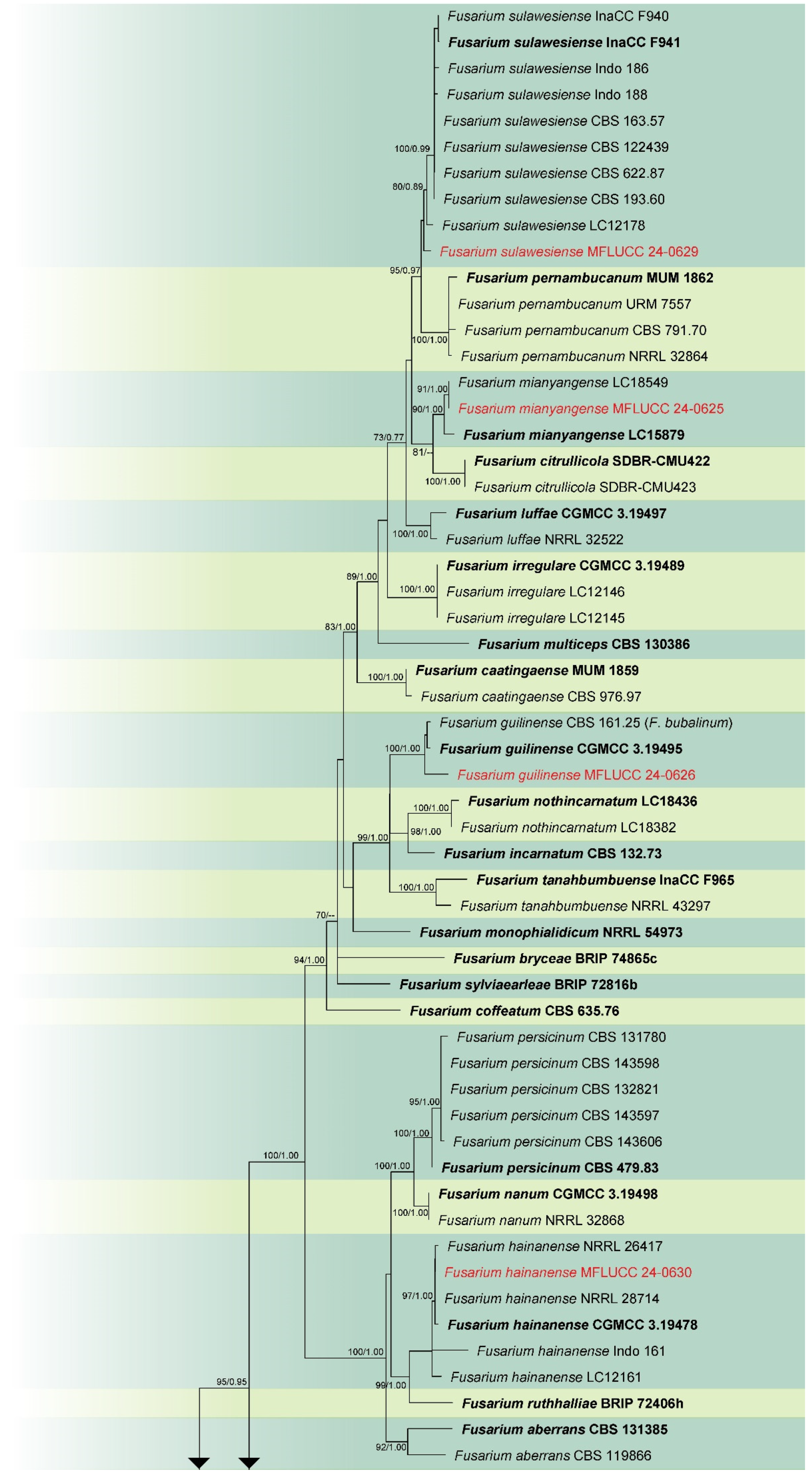
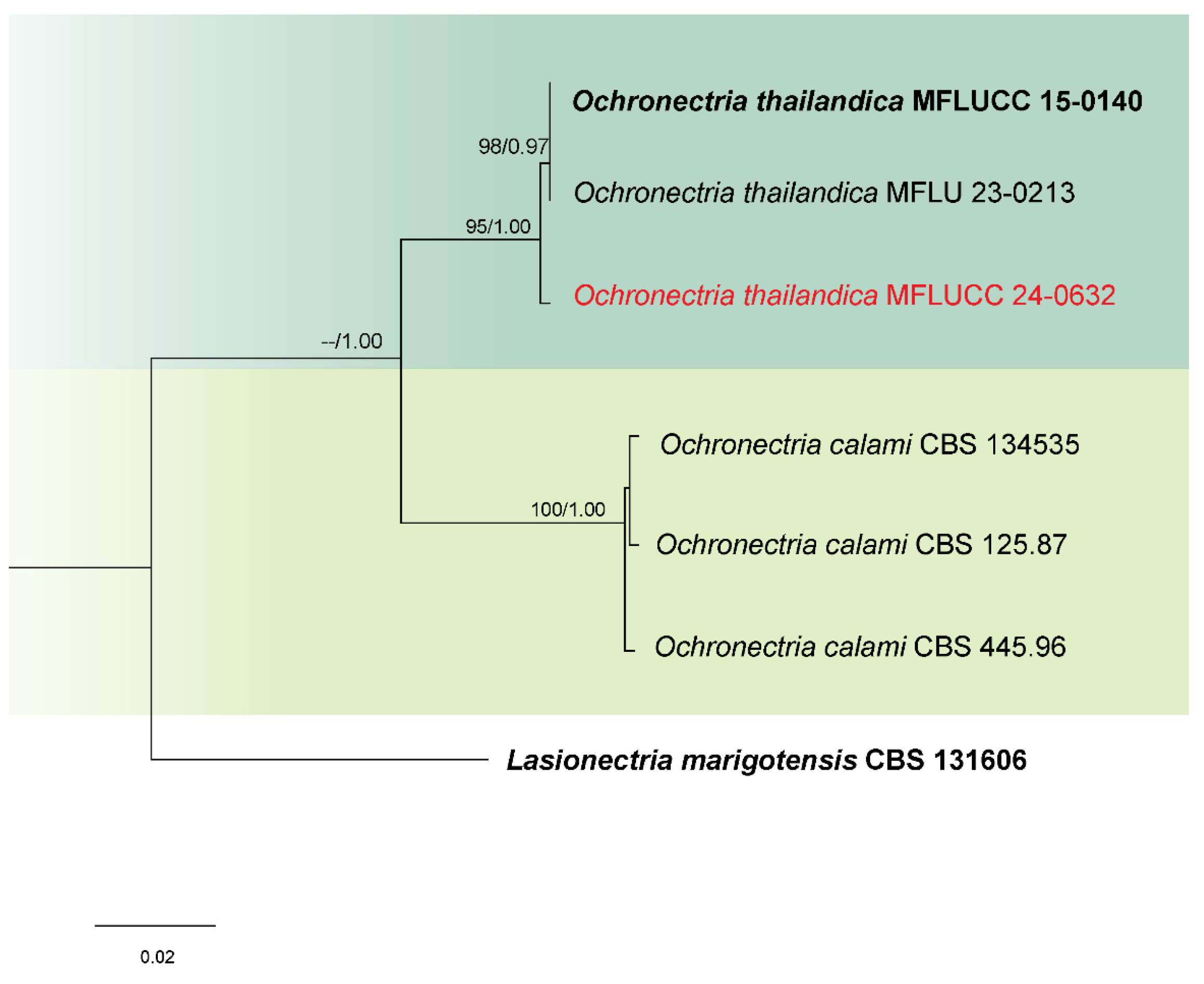
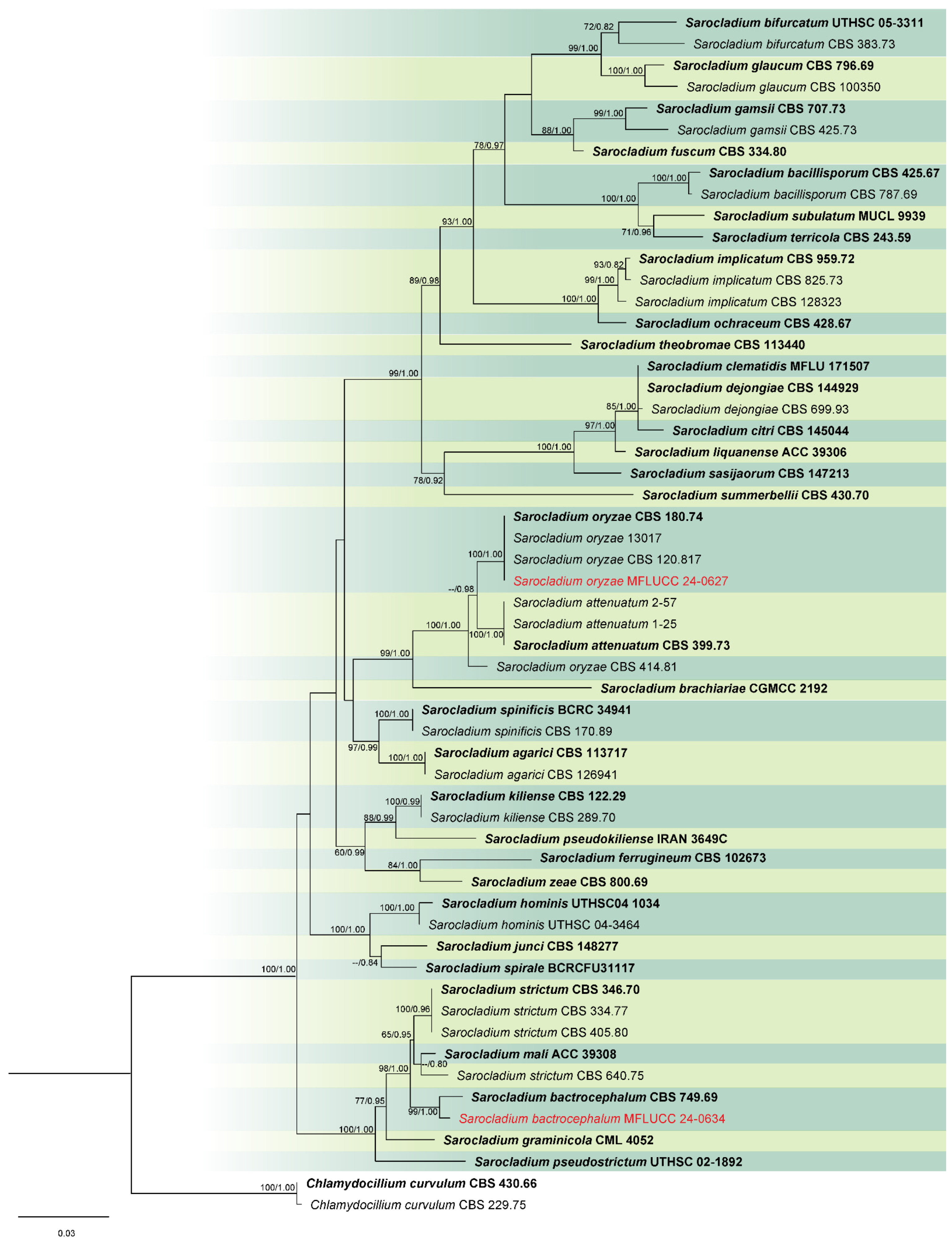
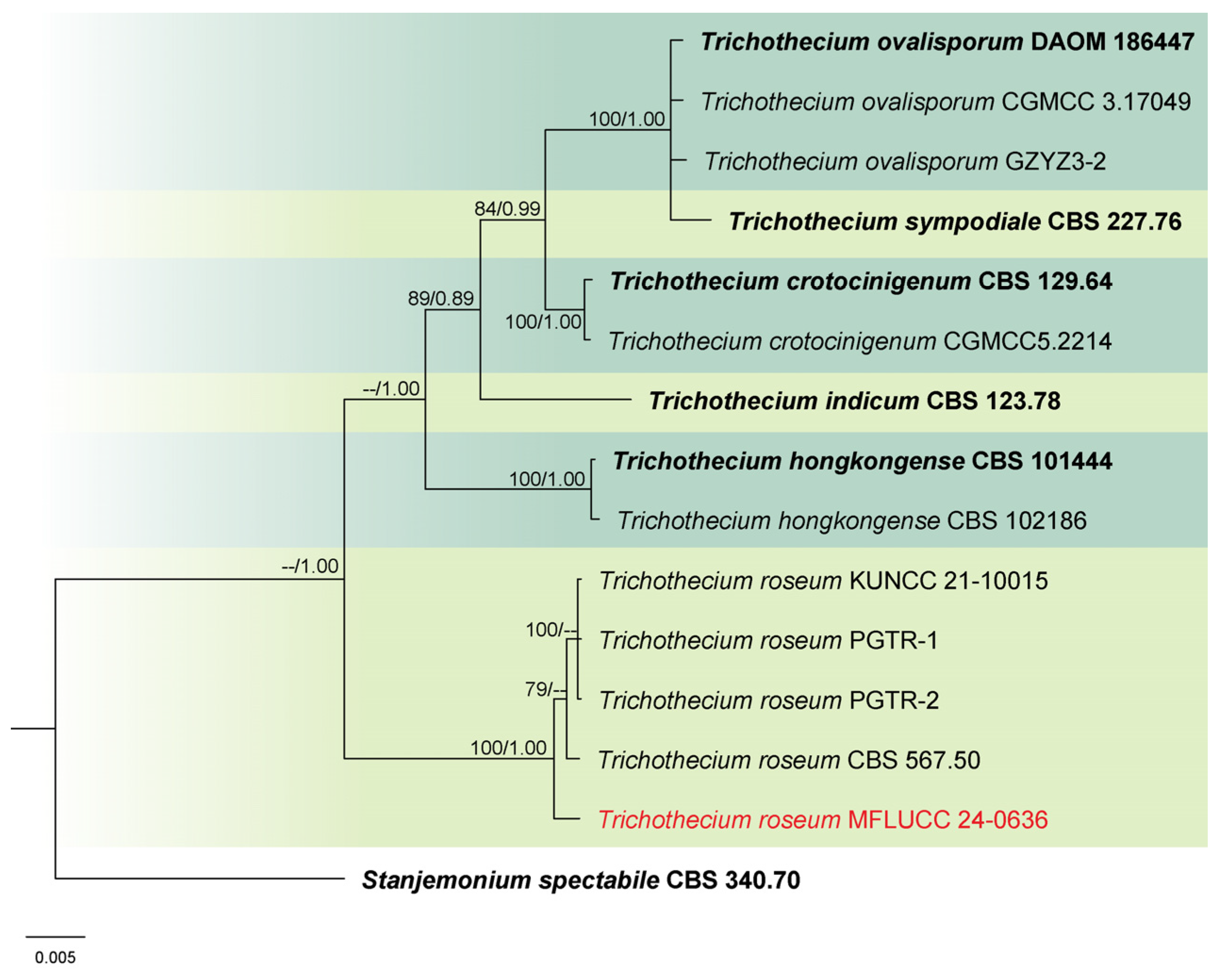
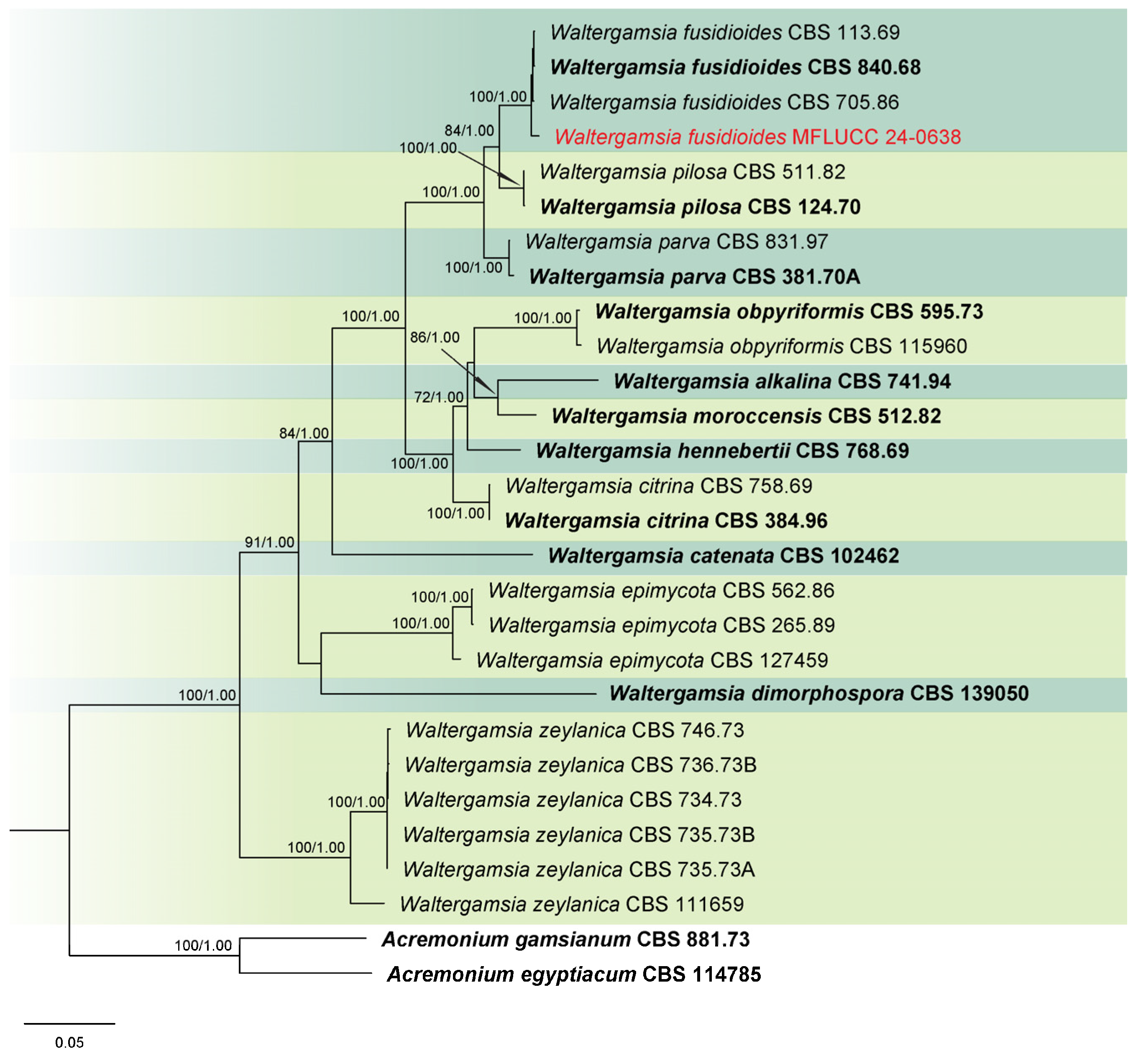
3.1. Phylogenetic Analysis
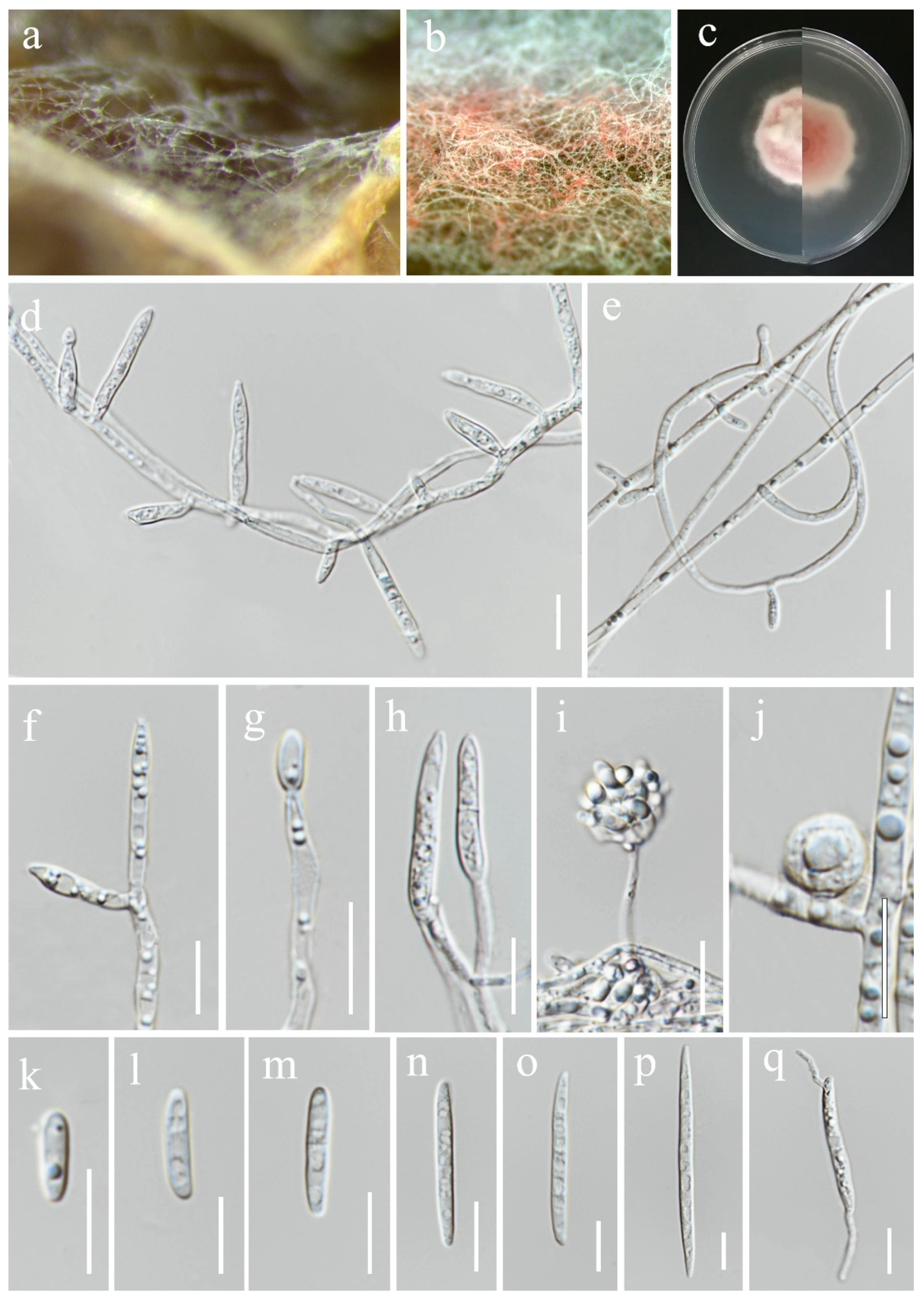
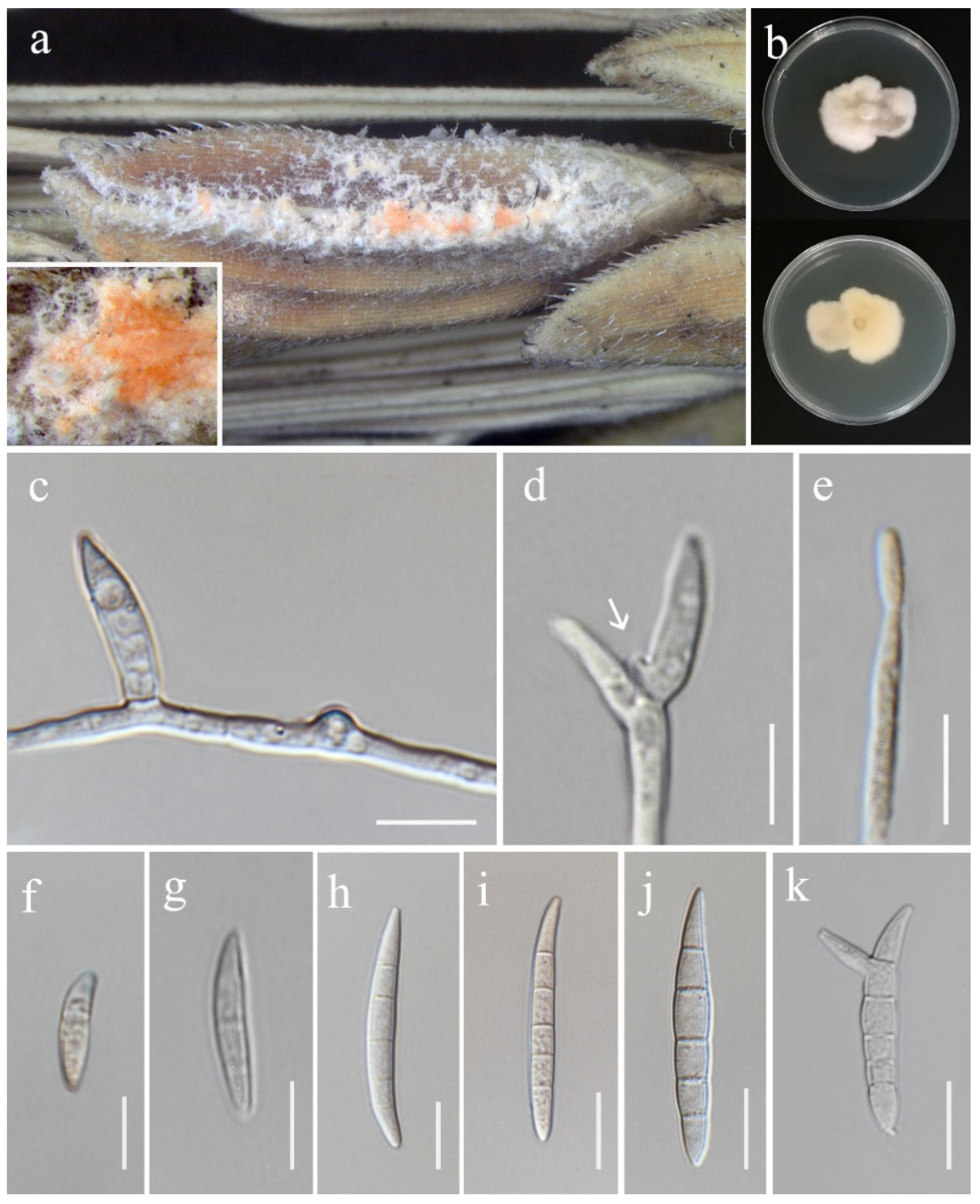
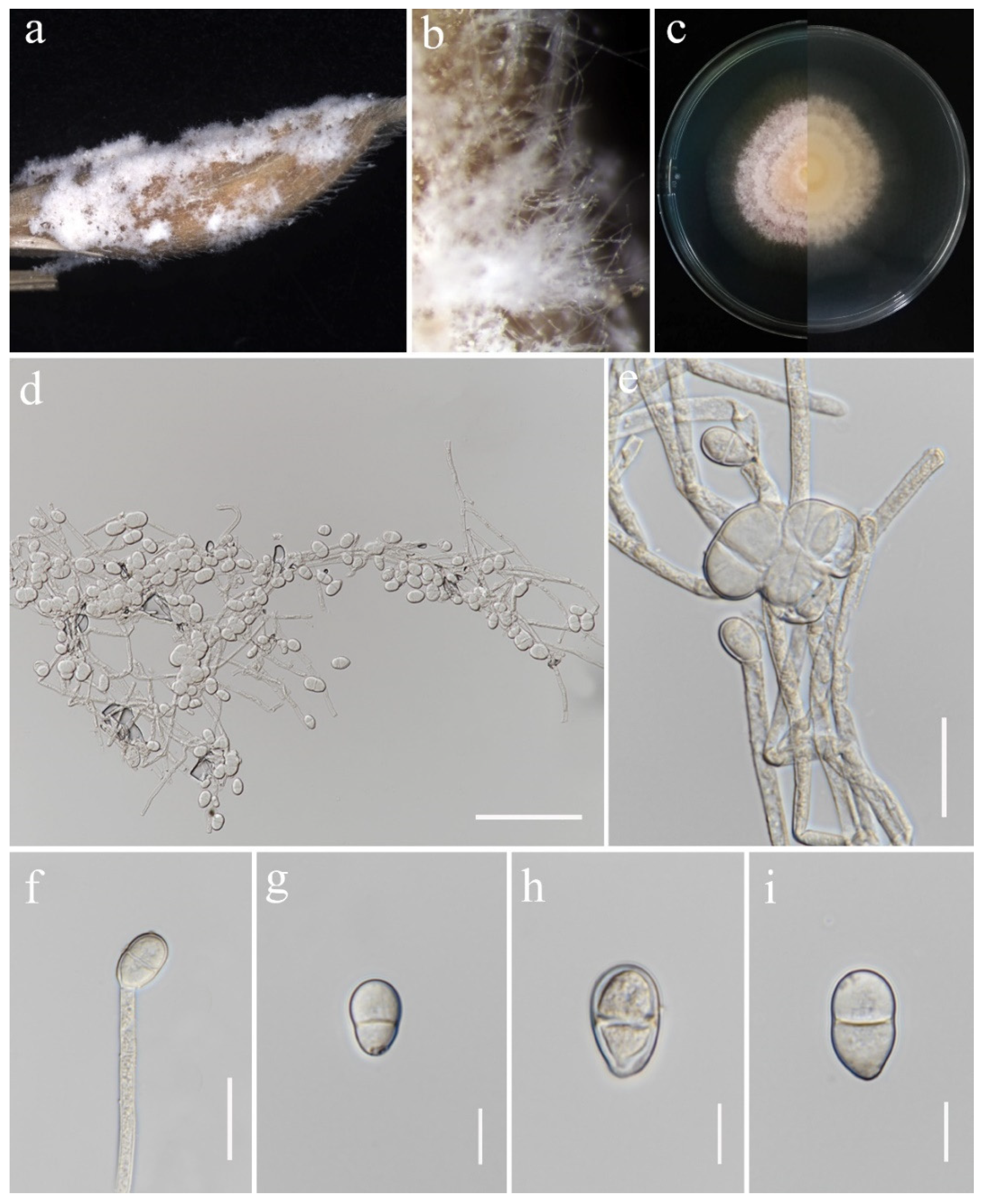
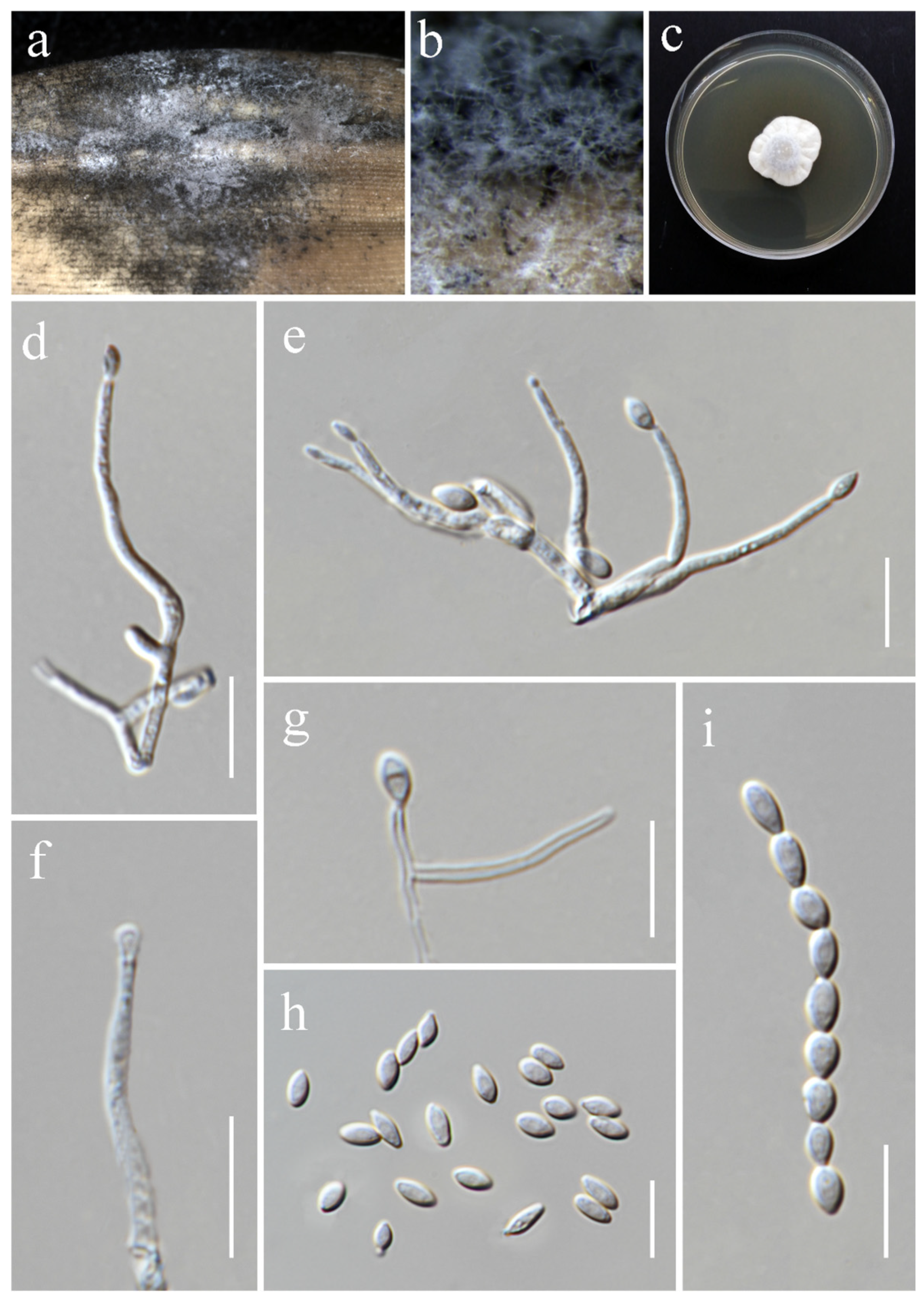
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juliano, B.O. Rice in Human Nutrition; FAO Food and Nutrition Series, No.26; The International Rice Research Institute (IRRI): Los Baños, Philippines, 1993; pp. 43–44. [Google Scholar]
- Zeigler, R.S.; Barclay, A. The relevance of rice. Rice 2008, 1, 3–10. [Google Scholar] [CrossRef]
- Johnston, D.B. Rice cultivation in Thailand: The development of an export economy by indigenous capital and labor. Mod. Asian Stud. 1981, 15, 107–126. [Google Scholar] [CrossRef]
- Poonyarat, C. Development-Thailand: Rice Culture Withering Away, Warn Experts. 2003. Available online: http://www.ipsnews.net/2003/01/development-thailand-rice-culture-withering-away-warn-experts/ (accessed on 15 September 2024).
- Kukusamude, C.; Sricharoen, P.; Limchoowong, N.; Kongsri, S. Heavy metals and probabilistic risk assessment via rice consumption in Thailand. Food Chem. 2021, 334, 127402. [Google Scholar] [CrossRef] [PubMed]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef]
- Naik, B.S.; Shashikala, J.; Krishnamurthy, Y.L.A. Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiol. Res. 2009, 164, 290–296. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, G.; Park, D.S.; Yang, Y. Inoculation and scoring methods for rice sheath blight disease. Methods Mol. Biol. 2013, 956, 257–268. [Google Scholar] [CrossRef]
- Laha, G.S.; Singh, R.; Ladhalakshmi, D.; Sunder, S.; Prasad, M.S.; Dagar, C.S.; Babu, V.R. Importance and management of rice diseases: A global perspective. In Rice Production Worldwide; Springer: Cham, Switzerland, 2017; pp. 303–360. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Huhndorf, S.M. Outline of Ascomycota-2007. Myconet 2007, 13, 1–58. [Google Scholar]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Hyde, K.D.; Noorabadi, M.T.; Thiyagaraja, V.; He, M.Q.; Johnston, P.R.; Wijesinghe, S.N.; Armand, A.; Biketova, A.Y.; Chethana, K.W.T.; Erdoğdu, M.; et al. The 2024 Outline of Fungi and fungus-like taxa. Mycosphere 2024, 15, 5146–6239. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Blackwell, M. Molecular systematics of unitunicate perithecial ascomycetes: The Clavicipitales-Hypocreales connection. Mycologia 1993, 85, 912–922. [Google Scholar] [CrossRef]
- Glenn, A.E.; Bacon, C.W.; Price, R.; Hanlin, R.T. Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 1996, 88, 369–383. [Google Scholar] [CrossRef]
- Sung, G.-H.; Hywel-Jones, N.L.; Sung, J.-M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Schumacher, R.K.; Wingfield, M.J.; Lombard, L.; Giraldo, A.; Christensen, M.; Gardiennet, A.; Nakashima, C.; Pereira, O.L.; Smith, A.; et al. Fungal systematics and evolution: FUSE 1. Sydowia 2015, 67, 118. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Le Roux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.J.; Heykoop, M.; et al. Fungal planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef]
- Lombard, L.; Houbraken, J.; Decock, C.; Samson, R.A.; Meijer, M.; Réblová, M.; Groenewald, J.Z.; Crous, P.W. Generic hyper-diversity in Stachybotriaceae. Persoonia 2016, 36, 156–246. [Google Scholar] [CrossRef]
- Aiello, D.; Polizzi, G.; Crous, P.W.; Lombard, L. Pleiocarpon gen. nov. and a new species of Ilyonectria causing basal rot of Strelitzia reginae in Italy. IMA Fungus 2017, 8, 65–76. [Google Scholar] [CrossRef]
- González, C.D.; Chaverri, P. Corinectria, a new genus to accommodate Neonectria fuckeliana and C. constricta sp. nov. from Pinus radiata in Chile. Mycol. Prog. 2017, 16, 1015–1027. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J. Clonostachys spinulosispora (Hypocreales, Bionectriaceae), a new species on palm from French Guiana. Ascomycete.org 2018, 10, 127–130. [Google Scholar]
- Perera, R.H.; Hyde, K.D.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Bundhun, D.; Camporesi, E.; Akulov, A.; Liu, J.K.; Liu, Z.Y. Profile of Bionectriaceae, Calcarisporiaceae, Hypocreaceae, Nectriaceae, Tilachlidiaceae, Ijuhyaceae fam. nov., Stromatonectriaceae fam. nov. and Xanthonectriaceae fam. nov. Fungal Divers. 2023, 118, 95–271. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, C.; Nie, L.T.; Maharachchikumbura, S.S.; Crous, P.W.; Hyde, K.D.; Xiang, M.M.; Al-Otibi, F.; Manawasinghe, I.S. Identification and characterization of Albonectria, Fusarium, and Neocosmospora species associated with ornamental plants in Southern China. Mycosphere 2024, 15, 6641–6717. [Google Scholar] [CrossRef]
- Rossman, A.Y. Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 1996, 88, 1–19. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Samuels, G.J.; Rogerson, C.T.; Lowen, R. Genera of Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales, Ascomycetes). Stud. Mycol. 1999, 42, 1–248. [Google Scholar]
- Kamata, N.; Sato, H.; Shimazu, M. Seasonal changes in the infection of pupae of the beech caterpillar, Quadricalcarifera punctatella (Motsch.) (Lep., Notodontidae), by Cordyceps militaris Link (Clavicipitales, Clavicipitaceae) in the soil of the Japanese beech forest. J. Appl. Entomol. 1997, 121, 17–21. [Google Scholar] [CrossRef]
- Kamata, N. Population dynamics of the beech caterpillar, Syntypistis punctatella, and biotic and abiotic factors. Popul. Ecol. 2000, 42, 267–278. [Google Scholar] [CrossRef]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Chapter VII—Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 189–253. [Google Scholar]
- Garrido-Jurado, I.; Fernandez-Bravo, M.; Campos, C.; Quesada-Moraga, E. Diversity of entomopathogenic Hypocreales in soil and phylloplanes of five Mediterranean cropping systems. J. Invertebr. Pathol. 2015, 130, 97–106. [Google Scholar] [CrossRef]
- Muthumeenakshi, S.; Mills, P.R.; Brown, A.E.; Seaby, D.A. Intraspecific molecular variation among Trichoderma harzianum isolates colonizing mushroom compost in the British Isles. Microbiology 1994, 140, 769–777. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef]
- Savoie, J.; Iapicco, R.; Largeteau-Mamoun, M. Factors influencing the competitive saprophytic ability of Trichoderma harzianum Th2 in mushroom (Agaricus bisporus) compost. Mycol. Res. 2001, 105, 1348–1356. [Google Scholar] [CrossRef]
- Tedersoo, L.; Partel, K.; Jairus, T.; Gates, G. Ascomycetes associated with ectomycorrhizas: Molecular diversity and ecology with particular reference to the Helotiales. Environ. Microbiol. 2009, 11, 3166–3178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cantero, A.; Guarro, J. Sarocladium and Acremonium infections: New faces of an old opportunistic fungus. Mycoses 2020, 63, 1203–1214. [Google Scholar] [CrossRef]
- Allaga, H.; Zhumakayev, A.; Buchner, R.; Kocsube, S.; Szucs, A.; Vagvolgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum species complex with mushroom pathogenic potential. Agronomy 2021, 11, 2434. [Google Scholar] [CrossRef]
- Isaka, M.; Kittakoop, P.; Thebtaranonth, Y. Secondary metabolites of Clavicipitalean fungi. In Clavicipitalean Fungi, 1st ed.; White, J.F., Bacon, C.W., Hywel-Jones, N.L., Spatafora, J.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 355–397. [Google Scholar]
- Summerbell, R.C.; Gueidan, C.; Schroers, H.J.; de Hoog, G.S.; Starink, M.; Rosete, A.Y.; Guarro, J.; Scott, J.A. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud. Mycol. 2011, 68, 139–162. [Google Scholar] [CrossRef]
- Zheng, P.; Xia, Y.; Xiao, G.; Xiong, C.; Hu, X.; Zhanh, S.; Zheng, H.; Huang, Y.; Zhou, Y.; Wang, S.; et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2012, 12, R116. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Fu, X. The pathogen of rice purple sheath disease Sarocladium synense sp. nov. Acta Mycol. Sin. Suppl. 1986, 1, 318–327. [Google Scholar]
- Pearce, D.A.; Bridge, P.D.; Hawksworth, D.L. Species concepts in Sarocladium, the causal agent of sheath rot in rice and bamboo blight. In Major Fungal Diseases of Rice: Recent Advances; Sreenivasaprasad, S., Johnson, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 285–292. [Google Scholar] [CrossRef]
- Ayyadurai, N.; Kirubakaran, S.I.; Srisha, S.; Sakthivel, N. Biological and molecular variability of Sarocladium oryzae, the sheath rot pathogen of rice (Oryza sativa L.). Curr. Microbiol. 2005, 50, 319–323. [Google Scholar] [CrossRef]
- Lee, J.; Chang, I.; Kim, H.; Yun, S.; Leslie, J.F.; Lee, Y. Genetic diversity and fitness of Fusarium graminearum populations from rice in Korea. Appl. Environ. Microbiol. 2009, 84, 3285–3295. [Google Scholar] [CrossRef]
- Amatulli, M.T.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol. 2010, 59, 839–844. [Google Scholar] [CrossRef]
- Wulff, E.G.; Sørensen, J.L.; Lübeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusarium spp. associated with rice Bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L.; Yaakop, A.S.; Salleh, B.; Zakaria, M. Endophytic fungi from paddy. Trop. Life. Sci. Res. 2010, 21, 101–107. [Google Scholar] [PubMed]
- Suada, I.K.; Suhartini, D.M.W.Y.; Sunariasih, N.P.L.; Wirawan, I.G.P.; Chun, K.W.; Cha, J.Y.; Ohga, S. Ability of endophytic fungi isolated from rice to inhibit Pyricularia oryzae–induced rice blast in Indonesia. J. Fac. Agric. Kyushu Univ. 2012, 57, 51–53. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Aggarwal, R.; Banerjee, S.; Gupta, S.; Sharma, S. Pathogenicity, ecology and genetic diversity of the Fusarium spp. associated with an emerging bakanae disease of rice (Oryza sativa L.) in India. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R., Eds.; Springer: New Delhi, India, 2014; pp. 307–314. [Google Scholar]
- Atugala, D.; Deshappriya, N. Effect of Endophytic fungi on plant growth and blast disease incidence of two traditional rice varieties. J. Natl. Sci. Found. Sri Lanka 2015, 43, 173. [Google Scholar] [CrossRef]
- Leewijit, T.; Pongnak, W.; Soytong, K.; Poeaim, S. Isolation of soil and endophytic fungi from rice (Oryza sativa L.). Int. J. Agric. Technol. 2016, 12, 2191–2202. [Google Scholar]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Seephueak, P.; Preecha, C.; Seephueak, W. The diversity of fungi associated with rice (Oryza sativa L.) from Nakhon Si Thammarat, Thailand. Int. J. Agric. Technol. 2019, 15, 485–500. [Google Scholar]
- Roy, S.; Mili, C.; Talukdar, R.; Wary, S.; Tayung, K. Seed borne endophytic fungi associated with some indigenous rice varieties of North East India and their growth promotion and antifungal potential. Indian J. Agric. Res. 2021, 55, 603–608. [Google Scholar] [CrossRef]
- Tian, X.G.; Bao, D.F.; Karunarathna, S.C.; Jayawardena, R.S.; Hyde, K.D.; Bhat, D.J.; Luo, Z.L.; Elgorban, A.M.; Hongsanan, S.; Maharachchikumbura, S.S.N.; et al. Taxonomy and phylogeny of ascomycetes associated with selected economically important monocotyledons in China and Thailand. Mycosphere 2024, 15, 1–274. [Google Scholar] [CrossRef]
- Senanayake, I.; Rathnayaka, A.; Marasinghe, D.; Calabon, M.; Gentekaki, E.; Lee, H.; Hurdeal, V.; Pem, D.; Dissanayake, S.; Wijesinghe, S.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Fisher, N.L.; Burgess, L.; Toussoun, T.; Nelson, P.E. Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 1982, 72, 151–153. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Crytococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Tacke, B.K.; Casper, H.C. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. USA 2000, 97, 7905–7910. [Google Scholar] [CrossRef]
- Dissanayake, L.S.; Wijayawardene, N.N.; Samarakoon, M.C.; Hyde, K.D.; Kang, J.-C. The taxonomy and phylogeny of Austropleospora ochracea sp. nov. (Didymosphaeriaceae) from Guizhou, China. Phytotaxa 2021, 491, 217–229. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinf. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Glez-Peña, D.; Gómez-Blanco, D.; Reboiro-Jato, M.; Fdez-Riverola, F.; Posada, D. ALTER, program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010, 38, 14–18. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree: Tree Figure Drawing Tool, Version 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Rheeder, J.P.; Marasas, W.F.O.; Nelson, P.E. Fusarium globosum, a new species from corn in southern Africa. Mycologia 1996, 88, 509–513. [Google Scholar] [CrossRef]
- Aoki, T.; Nirenberg, H.I. Fusarium globosum from subtropical Japan and the effect of different light conditions on its conidiogenesis. Mycoscience 1999, 40, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Q.; Diao, Y.; Duan, W.; Cai, L. Fusarium incarnatum-equiseti Complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names—Restyling the Fusarium incarnatum-equiseti species complex. Persoonia 2019, 43, 186–221. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 1–184. [Google Scholar] [CrossRef]
- Maryani, N.; Sandoval-Denis, M.; Lombard, L.; Crous, P.W.; Kema, G.H.J. New endemic Fusarium species hitch-hiking with pathogenic Fusarium strains causing Panama disease in small-holder banana plots in Indonesia. Persoonia 2019, 43, 48–69. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Rheeder, J.P.; Lamprecht, S.C.; Zeller, K.A.; Leslie, J.F. Fusarium andiyazi sp. nov., a new species from sorghum. Mycologia 2001, 93, 1203–1210. [Google Scholar] [CrossRef]
- Wang, J.H.; Peng, X.D.; Lin, S.H.; Wu, A.; Huang, S. First report of Fusarium head blight of wheat caused by Fusarium sacchari in China. Plant Dis. 2015, 99, 160. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Aggarwal, R.; Sharma, S.; Gupta, S.; Singh, U.B. Single and combined effects of three Fusarium species associated with rice seeds on the severity of bakanae disease of rice. J. Plant Pathol. 2016, 98, 405–412. [Google Scholar] [CrossRef]
- Duan, C.X.; Du, Q.; Wang, B.B. First report of maize ear rot caused by Fusarium sacchari in China. Plant Dis. 2019, 103, 2674. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. 2024. Available online: https://fungi.ars.usda.gov/ (accessed on 13 November 2024).
- Li, G.J.; Hyde, K.D.; Zhao, R.L.; Hongsanan, S.; Abdel-Aziz, F.A.; Abdel-Wahab, M.A.; Alvarado, P.; Alves-Silva, G.; Ammirati, J.F.; Ariyawansa, H.A.; et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 1–237. [Google Scholar] [CrossRef]
- Gams, W. Cephalosporium-Artige Schimmelpilze (Hyphomycetes); Gustav Fischer: Stuttgart, Germany, 1971; pp. 38–130. [Google Scholar]
- Sawada, K. Descriptive Catalogue of the Formosan Fungi II; Report of the Department of Agriculture; Government Research Institute: Taipei, Taiwan, 1922; pp. 139–164. [Google Scholar]
- Singh, R.; Basu, M. Trichothecium roseum Link on Punica granatum—A new record from India. Indian Phytopathol. 2006, 59, 532. [Google Scholar]
- Bello, D.G. First report of Trichothecium roseum causing postharvest fruit rot of tomato in Argentina. Aust. Plant Dis. Notes 2008, 3, 103–104. [Google Scholar] [CrossRef]
- Tang, Q.; Fang, L.; Liu, Y.J. First report of Trichothecium roseum causing trunk disease of Acer buergerianum in China. Plant Dis. 2015, 99, 1864. [Google Scholar] [CrossRef]
- Xue, C.S.; Xu, J.N.; Sun, J.Y.; Lu, Y.Y.; Li, G.F.; Xiao, S.Q. First report of Trichothecium roseum causing maize (Zea mays) ear rot in Northern China. Plant Dis. 2016, 100, 2324. [Google Scholar] [CrossRef]
- Li, Y.B.; Zhang, Z.P.; Luo, L.X.; Li, J.Q.; Liu, Y.X.; Hao, J.J. First report of Trichothecium roseum causing pink fruit rot of Prunus davidiana in China. Plant Dis. 2020, 104, 2520. [Google Scholar] [CrossRef]
- Ashraf, N.; Bhat, M.Y.; Wani, A.H. First report of foliicolus fungus Trichothecium roseum (Pers.) Link on sweet cherry Prunus avium L. from Kashmir Valley. Braz. J. Biol. Sci. 2020, 7, 225–228. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.; Wang, J.; Wang, X.; Xiang, W.; Zhao, J. First report of Trichothecium roseum causing postharvest fruit rot on purple passion fruit in China. Plant Dis. 2022, 106, 3212. [Google Scholar] [CrossRef]
- Harishchandra, D.L.; Zhang, W.; Wedaralalage, T.C.K. Identification of the postharvest pink mold rot fungus (Trichothecium roseum) on grapes in China. Chiang Mai J. Sci. 2023, 50, e2023040. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D.; Bhudhasamai, K.; Miscamble, B.F.; Wheeler, K.A.; Tanboon-Ek, P. The normal mycoflora of commodities from Thailand. 2. Beans, rice, small grains and other commodities. Int. J. Food Microbiol. 1994, 23, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Surekha, M.; Saini, K.; Reddy, V.K.; Reddy, A.R.; Reddy, S.M. Fungal succession in stored rice (Oryza sativa Linn.) fodder and mycotoxin production. Afr. J. Biotechnol. 2011, 10, 550–555. [Google Scholar]
- Hou, L.; Giraldo, A.; Groenewald, J.Z.; Raemae, T.; Summerbell, R.C.; Huang, G.; Cai, L.; Crous, P.W. Redisposition of acremonium-like fungi in Hypocreales. Stud. Mycol. 2023, 105, 23–203. [Google Scholar] [CrossRef] [PubMed]
- Izzati, N.A.M.; Salleh, B. Variability of Fusarium species associated with Bakanae disease of rice based on their virulence, vegetative and biological compatibilities. Sydowia 2010, 62, 89–104. [Google Scholar]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef]
- Petrovic, T.; Burgess, L.W.; Cowie, I.; Warren, R.A.; Harvey, P.R. Diversity and fertility of Fusarium sacchari from wild rice (Oryza australiensis) in Northern Australia, and pathogenicity tests with wild rice, rice, sorghum and maize. Eur. J. Plant Pathol. 2013, 136, 773–788. [Google Scholar] [CrossRef]
- Pak, D.; You, M.P.; Lanoiselet, V.; Barbetti, M.J. Reservoir of cultivated rice pathogens in wild rice in Australia. Eur. J. Plant Pathol. 2017, 147, 295–311. [Google Scholar] [CrossRef]
- Pramunadipta, S.; Widiastuti, A.; Wibowo, A.; Suga, H.; Priyatmojo, A. Identification and pathogenicity of Fusarium spp. associated with the sheath rot disease of rice (Oryza sativa) in Indonesia. J. Plant Pathol. 2022, 104, 251–267. [Google Scholar] [CrossRef]
- Mohamed Nor, N.M.I.; Salleh, B.; Leslie, J.F. Fusarium species from sorghum in Thailand. Plant Pathol. J. 2019, 35, 301–312. [Google Scholar] [CrossRef]
- Dekham, K.; Kanchanawatee, K. The first report of Fusarium sacchari causing yellow leaf spot disease on Rhynchostylis gigantea orchids in Thailand. Am. J. Agric. Biol. Sci. 2020, 15, 68–74. [Google Scholar] [CrossRef]
- Han, S.L.; Wang, M.M.; Ma, Z.Y.; Raza, M.; Zhao, P.; Liang, J.M.; Gao, M.; Li, Y.J.; Wang, J.W.; Hu, D.M. Fusarium diversity associated with diseased cereals in China, with an updated phylogenomic assessment of the genus. Stud. Mycol. 2023, 104, 87–148. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Gueidan, C.; Crous, P.W.; Geiser, D.M. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J. Clin. Microbiol. 2009, 47, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.; Li, Q.; Huang, S.; Tang, L.; Chen, X.; Guo, T.; Mo, J.; Ma, L. First report of leaf blight caused by Fusarium pernambucanum and Fusarium sulawesiense on plum in Sichuan, China. Plant Dis. 2022, 106, 2759. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.H.; Lian, T.; Su, J.J.; Chen, J. First report of internal black rot on Carica papaya fruit caused by Fusarium sulawesiense in China. Plant Dis. 2022, 106, 319. [Google Scholar] [CrossRef]
- Lima, E.N.; Oster, A.H.; Bordallo, P.N.; Araújo, A.A.C.; Silva, D.E.M.; Lima, C.S. A novel lineage in the Fusarium incarnatum-equiseti species complex is one of the causal agents of fusarium rot on melon fruits in Northeast Brazil. Plant Pathol. 2021, 70, 133–143. [Google Scholar] [CrossRef]
- Suwannarach, N.; Khuna, S.; Thitla, T.; Senwanna, C.; Nuangmek, W.; Kumla, J.; Lumyong, S. Morpho-phylogenetic identification and characterization of new causal agents of Fusarium species for postharvest fruit rot disease of muskmelon in northern Thailand and their sensitivity to fungicides. Front. Plant Sci. 2024, 15, 1459759. [Google Scholar] [CrossRef]
- Skovgaard, K.; Rosendahl, S.; O’Donnell, K.; Nirenberg, H.I. Fusarium commune is a new species identified by morphological and molecular phylogenetic data. Mycologia 2003, 95, 630–636. [Google Scholar] [CrossRef]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef]
- Hamini-Kadar, N.; Edel-Hermann, V.; Gautheron, N.; Steinberg, C. First report of Fusarium commune and Fusarium redolens causing crown and root rot on tomato in Algeria. New Dis. Rep. 2010, 22, 3. [Google Scholar] [CrossRef]
- Ellis, M.L.; Diaz Arias, M.M.; Cruz Jimenez, D.R.; Munkvold, G.P.; Leandro, L.F. First report of Fusarium commune causing damping-off, seed rot, and seedling root rot on soybean (Glycine max) in the United States. Plant Dis. 2013, 97, 284. [Google Scholar] [CrossRef]
- Yu, J.; Babadoost, M. Occurrence of Fusarium commune and F. oxysporum in horseradish roots. Plant Dis. 2013, 97, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chai, Z.; Bao, Y.; Wang, H.; Li, Y.; Rao, G.P.; Zhang, M.Q. First report of Fusarium commune causing root rot disease of sugarcane (var. Badila) in China. Plant Dis. 2018, 102, 1660. [Google Scholar] [CrossRef]
- Choi, H.-W.; Hong, S.; Lee, Y.K.; Kim, W.G.; Chun, S. Taxonomy of Fusarium fujikuroi species complex associated with bakanae on rice in Korea. Australas. Plant Pathol. 2018, 47, 23–34. [Google Scholar] [CrossRef]
- Husna, A.; Zakaria, L.; Nor, N.M.I.M. Fusarium commune associated with wilt and root rot disease in rice. Plant Pathol. 2020, 70, 123–132. [Google Scholar] [CrossRef]
- Giraldo, A.; Gené, J.; Sutton, D.; Madrid, H.; De Hoog, G.; Cano, J.; Decock, C.; Crous, P.W.; Guarro, J. Phylogeny of Sarocladium (Hypocreales). Persoonia. Mol. Phylogeny Evol. Fungi 2015, 34, 10. [Google Scholar] [CrossRef]
- Ou, J.H.; Lin, G.C.; Chen, C.Y. Sarocladium species associated with rice in Taiwan. Mycol. Prog. 2020, 19, 67–80. [Google Scholar] [CrossRef]
- Jones, M.P.; Jeutong, F.; Tchatchoua, J. A survey of rice diseases in Cameroon. Plant Dis. 1993, 77, 133–136. [Google Scholar] [CrossRef]
- Sakthivel, N.; Amudha, R.; Muthukrishnan, S. Production of phytotoxic metabolites by Sarocladium oryzae. Mycol. Res. 2002, 106, 609–614. [Google Scholar] [CrossRef]
- Lanoiselet, V.; You, M.P.; Li, Y.P.; Wang, C.P.; Shivas, R.G.; Barbetti, M.J. First report of Sarocladium oryzae causing sheath rot on rice (Oryza sativa) in Western Australia. Plant Dis. 2012, 96, 1382. [Google Scholar] [CrossRef]
- Giatgong, P. Host Index of Plant Diseases in Thailand, 2nd ed.; Mycology Branch, Plant Pathology and Microbiology Division, Department of Agriculture and Cooperatives: Bangkok, Thailand, 1980; p. 124. [Google Scholar]
- Unartngam, J.; Kopmoo, N.; Pinruan, U.; Kosawang, C.; Jørgensen, H.J.L. Molecular and morphological identification of Sarocladium species causing sheath rot of rice in Thailand and their division into physiological races. J. Fungi 2024, 10, 535. [Google Scholar] [CrossRef]
- Anjos, R.M.; Moreira, S.I.; Costa, S.S.; Abreu, L.M.; Alves, E.; Cardoso, P.G. Sarocladium graminicola, a new endophytic species from tropical grasses. Mycol. Prog. 2020, 19, 605–614. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: http://www.indexfungorum.org/Names/Names.asp (accessed on 25 November 2024).
- Fisher, P.J.; Petrini, O. Fungal saprobes and pathogens as endophytes of rice (Oryza sativa L.). New Phytol. 1992, 120, 137–143. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; McKenzie, E.H.C.; Hyde, K.D. Are some endophytes of Musa acuminata latent pathogens. Fungal Divers. 2004, 16, 131–140. [Google Scholar]
- Weisberg, A.J.; Grünwald, N.J.; Savory, E.A.; Putnam, M.L.; Chang, J.H. Genomic approaches; to plant-pathogen epidemiology and diagnostics. Annu. Rev. Phytopathol. 2021, 59, 311–332. [Google Scholar] [CrossRef]
- O’Hanlon, R. Fungi in the Environment. In Fungi: Biology and Applications, 3rd ed.; Kavanagh, K., Ed.; John Wiley & Sons, Inc.: Chichester, UK, 2017; pp. 333–353. [Google Scholar] [CrossRef]
- Mortimer, P.; Wanasinghe, D.N.; Mortimer, P.E. The impact of land use change on the diversity and emergence of fungal pathogens. Authorea 2023, 341, 34155158. [Google Scholar] [CrossRef]
- Mew, T.W.; Leung, H.; Savary, S.; Vera Cruz, C.M.; Leach, J.E. Looking ahead in rice disease research and management. Crit. Rev. Plant Sci. 2004, 23, 103–127. [Google Scholar] [CrossRef]
- Savary, S.; Horgan, F.; Willocquet, L.; Heong, K.L. A review of principals for sustainable pest management in rice. Crop Prot. 2012, 32, 54–63. [Google Scholar] [CrossRef]












| Locus | Primers | PCR Conditions | References |
|---|---|---|---|
| ITS | ITS5/ITS4 | 94 °C 3 min; 35 cycles of 94 °C 45 s, 56 °C 1 min, 72 °C 1 min; 72 °C 10 min | [60] |
| LSU | LROR/LR5 | 94 °C 3 min; 35 cycles of 94 °C 30 s, 55 °C 50 s, 72 °C 90 s; 72 °C 10 min | [59] |
| tef1-α | EF-1/EF-2 | 94 °C 90 s; 35 cycles of 94 °C 45 s, 55 °C 45 s, 72 °C 1 min; 72 °C 10 min | [61] |
| rpb2 | RPB2–5F2/RPB2–7cR | 94 °C 90 s; 40 cycles of 94 °C 30 s, 55 °C 30 s, 72 °C 2 min; 72 °C 10 min | [62] |
| cmdA | CAL-CL1/CAL-CL2A | 94 °C 3 min; 35 cycles of 94 °C 30 s, 57 °C 30 s, 72 °C 1 min; 72 °C 10 min | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Absalan, S.; Armand, A.; Jayawardena, R.S.; Suwannarach, N.; Monkai, J.; Jungkhun Gomes de Farias, N.; Lumyong, S.; Hyde, K.D. Morpho-Molecular Characterization of Hypocrealean Fungi Isolated from Rice in Northern Thailand. J. Fungi 2025, 11, 321. https://doi.org/10.3390/jof11040321
Absalan S, Armand A, Jayawardena RS, Suwannarach N, Monkai J, Jungkhun Gomes de Farias N, Lumyong S, Hyde KD. Morpho-Molecular Characterization of Hypocrealean Fungi Isolated from Rice in Northern Thailand. Journal of Fungi. 2025; 11(4):321. https://doi.org/10.3390/jof11040321
Chicago/Turabian StyleAbsalan, Sahar, Alireza Armand, Ruvishika S. Jayawardena, Nakarin Suwannarach, Jutamart Monkai, Nootjarin Jungkhun Gomes de Farias, Saisamorn Lumyong, and Kevin D. Hyde. 2025. "Morpho-Molecular Characterization of Hypocrealean Fungi Isolated from Rice in Northern Thailand" Journal of Fungi 11, no. 4: 321. https://doi.org/10.3390/jof11040321
APA StyleAbsalan, S., Armand, A., Jayawardena, R. S., Suwannarach, N., Monkai, J., Jungkhun Gomes de Farias, N., Lumyong, S., & Hyde, K. D. (2025). Morpho-Molecular Characterization of Hypocrealean Fungi Isolated from Rice in Northern Thailand. Journal of Fungi, 11(4), 321. https://doi.org/10.3390/jof11040321







