Epidemiology and Trends of Cutaneous Fungal Infections (2019–2022) in Israel: A Single Tertiary-Center Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Baseline Characteristics and Clinical Features
3.2. Age and Sex Distribution
3.3. Infected Body Site and Positive Detection Rate
3.4. Epidemiology of Fungal Pathogens by Site and Age Group
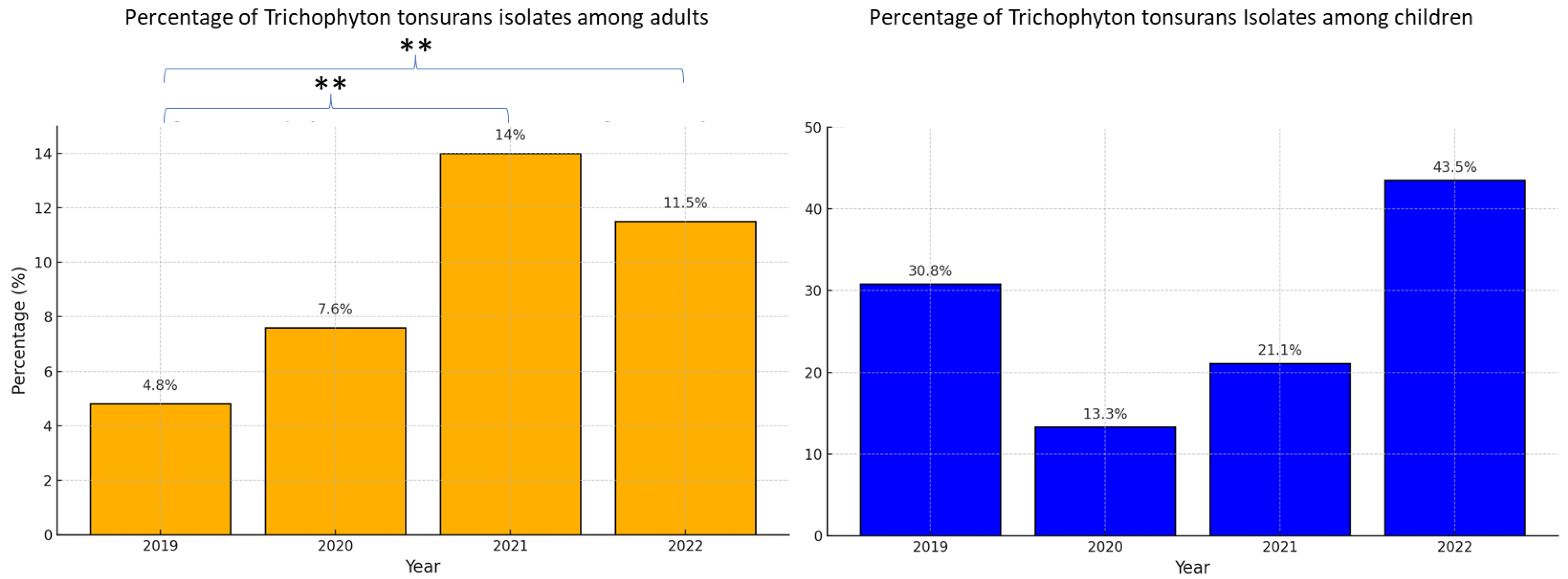
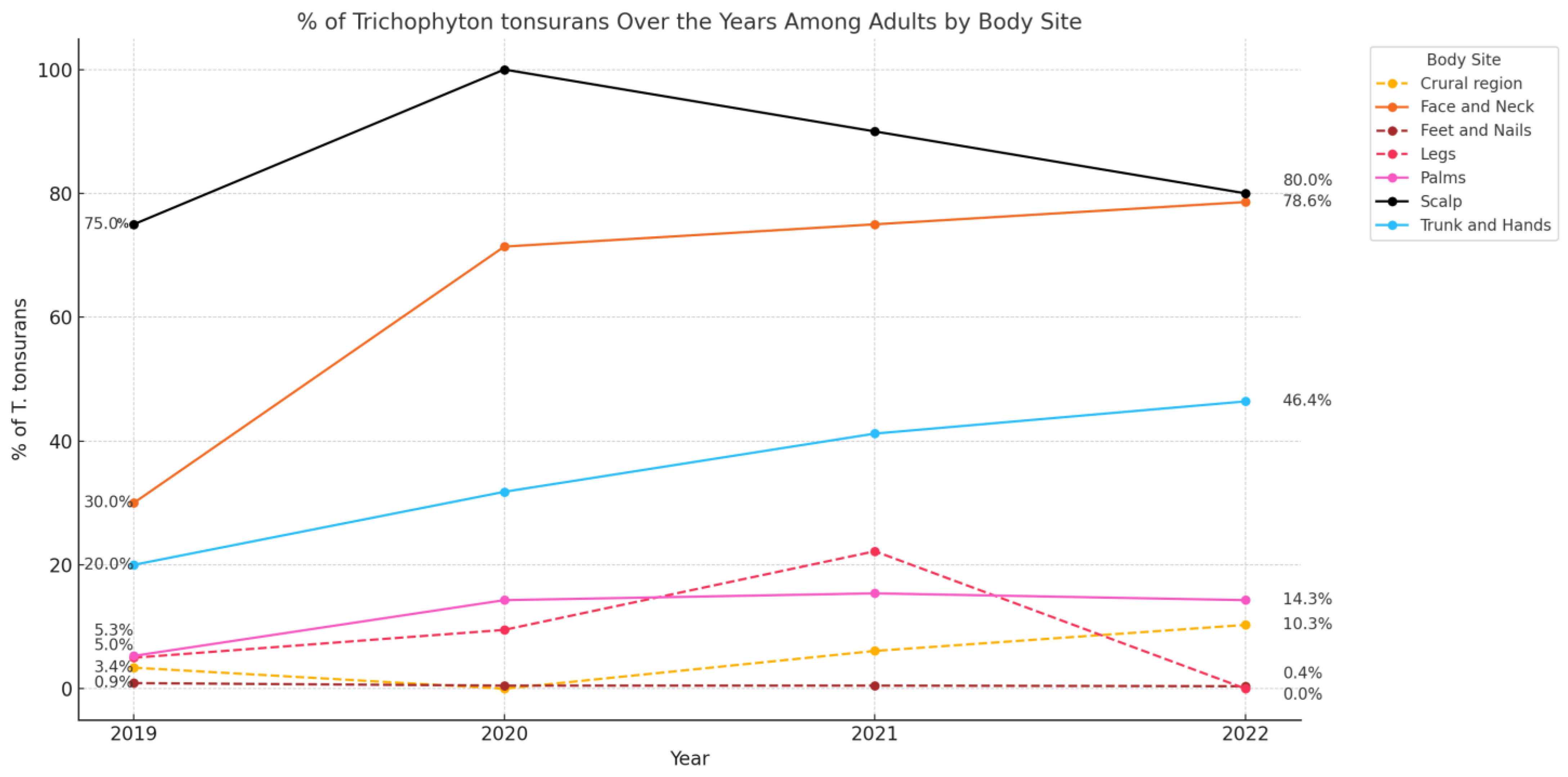
3.5. Trends over the Study Period
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C. | Candida |
| E. | Epidermophyton |
| M. | Microsporum |
| NDMs | Non-dermatophyte molds |
| PCR | Polymerase Chain Reaction |
| T. | Trichophyton |
References
- Urban, K.; Chu, S.; Scheufele, C.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G.R. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int. 2021, 2, 22–27. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, W. The changing face of dermatophytic infections worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Chanyachailert, P.; Leeyaphan, C.; Bunyaratavej, S. Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing. J. Fungi 2023, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Kershenovich, R.; Sherman, S.; Reiter, O.; Huss, S.R.; Didkovsky, E.; Mimouni, D.; Hodak, E.; Segal, R. A unique clinicopathological manifestation of fungal infection: A case series of deep dermatophytosis in immunosuppressed patients. Am. J. Clin. Dermatol. 2017, 18, 697–704. [Google Scholar] [CrossRef]
- Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe dermatophytosis and acquired or innate immunodeficiency: A review. J. Fungi 2015, 2, 4. [Google Scholar] [CrossRef]
- Lanternier, F.; Pathan, S.; Vincent, Q.B.; Liu, L.; Cypowyj, S.; Prando, C.; Migaud, M.; Taibi, L.; Ammar-Khodja, A.; Stambouli, O.B.; et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 2013, 369, 1704–1714. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Talukder, M. Onychomycosis in older adults: Prevalence, diagnosis, and management. Drugs Aging 2022, 39, 191–198. [Google Scholar] [CrossRef]
- Gupta, A.K.; Polla Ravi, S.; Wang, T.; Faour, S.; Bamimore, M.A.; Heath, C.R.; Friedlander, S.F. An update on tinea capitis in children. Pediatr. Dermatol. 2024, 41, 1030–1039. [Google Scholar] [CrossRef]
- Song, G.; Zhang, M.; Liu, W.; Liang, G. Changing face of epidemiology of dermatophytoses in Chinese Mainland: A 30 years nationwide retrospective study from 1991 to 2020. Mycoses 2022, 65, 440–448. [Google Scholar] [CrossRef]
- Hill, R.C.; Gold, J.A.W.; Lipner, S.R. Comprehensive review of tinea capitis in adults: Epidemiology, risk factors, clinical presentations, and management. J. Fungi 2024, 10, 357. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Szepietowski, J.C.; Pinto-Almazán, R.; Frías-De-León, M.G.; Espinosa-Hernández, V.M.; Chávez-Gutiérrez, E.; García-Salazar, E.; Vega-Sánchez, D.; Arenas, R.; et al. A systematic review of worldwide data on tinea capitis: Analysis of the last 20 years. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 844–883. [Google Scholar] [CrossRef]
- Friedland, R.; Reiss-Huss, S.; Sabbah, F.; Ben Amitai, D. Clinical clues and trends in epidemiology and pathogens in paediatric tinea capitis: A retrospective cohort study. Clin. Exp. Dermatol. 2022, 47, 50–56. [Google Scholar] [CrossRef]
- Shemer, A.; Gupta, A.K.; Galili, E.; Daniel, R.; Kassem, R.; Farhi, R.; Grunwald, H.; Bamimore, M.A. Management of tinea capitis in Israel: A comparative study. Pediatr. Dermatol. 2021, 38, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhi, H.; Xia, X.; Liu, Z. A survey of 701 cases of tinea faciei in Hangzhou, southeastern China, from 2018 to 2023. Mycoses 2024, 67, e13755. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Brasileiro, A.; Galhardas, C.; Apetato, M. Tinea faciei in a central Portuguese hospital: A 9-year survey. Mycoses 2018, 61, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Kieliger, S.; Glatz, M.; Cozzio, A.; Bosshard, P.P. Tinea capitis and tinea faciei in the Zurich area—An 8-year survey of trends in the epidemiology and treatment patterns. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1524–1529. [Google Scholar] [CrossRef]
- Khiewplueang, K.; Leeyaphan, C.; Bunyaratavej, S.; Jirawattanadon, P.; Saengthong-Aram, P.; Matthapan, L.; Prasong, W.; Panyawong, C.; Plengpanich, A. Tinea faciei clinical characteristics, causative agents, treatments and outcomes; a retrospective study in Thailand. Mycoses 2024, 67, e13754. [Google Scholar] [CrossRef]
- Zarzeka, D.; Benedict, K.; McCloskey, M.; Lockhart, S.R.; Lipner, S.R.; Gold, J.A.W. Current epidemiology of tinea corporis and tinea cruris causative species: Analysis of data from a major commercial laboratory, United States. J. Am. Acad. Dermatol. 2024, 91, 559–562. [Google Scholar] [CrossRef]
- Ferreira, C.B.; Lisboa, C. A systematic review on the emergence of terbinafine-resistant Trichophyton indotineae in Europe: Time to act? J. Eur. Acad. Dermatol. Venereol. 2024, 39, 364–376. [Google Scholar] [CrossRef]
- Chowdhary, A.; Singh, A.; Kaur, A.; Khurana, A. The emergence and worldwide spread of the species Trichophyton indotineae causing difficult-to-treat dermatophytosis: A new challenge in the management of dermatophytosis. PLoS Pathog. 2022, 18, e1010795. [Google Scholar] [CrossRef]
- Lin, B.B.; Pattle, N.; Kelley, P.; Jaksic, A.S. Multiplex RT-PCR provides improved diagnosis of skin and nail dermatophyte infections compared to microscopy and culture: A laboratory study and review of the literature. Diagn. Microbiol. Infect. Dis. 2021, 101, 115413. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Zaman, M.; Singh, J. Fast and sensitive detection of Trichophyton rubrum DNA from the nail samples of patients with onychomycosis by a double-round polymerase chain reaction-based assay. Br. J. Dermatol. 2007, 157, 698–703. [Google Scholar] [CrossRef]
- Pospischil, I.; Reinhardt, C.; Bontems, O.; Salamin, K.; Fratti, M.; Blanchard, G.; Chang, Y.-T.; Wagner, H.; Hermann, P.; Monod, M.; et al. Identification of Dermatophyte and Non-Dermatophyte Agents in Onychomycosis by PCR and DNA Sequencing-A Retrospective Comparison of Diagnostic Tools. J. Fungi 2022, 8, 1019. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.; Goshen, M.; Treigerman, O.; Ben-Zion, K.; Carp, M.-J.; Maisler, N.; Ehrenreich, I.B.; Kimchi, A.; Lifshitz, S.; Smollan, G.; et al. Evaluation of multiplex real-time PCR for identifying dermatophytes in clinical samples-A multicentre study. Mycoses 2018, 61, 119–126. [Google Scholar] [CrossRef]
- Alshawa, K.; Lacroix, C.; Benderdouche, M.; Mingui, A.; Derouin, F.; Feuilhade de Chauvin, M. Increasing incidence of Trichophyton tonsurans in Paris, France: A 15-year retrospective study. Br. J. Dermatol. 2012, 166, 1149–1150. [Google Scholar] [CrossRef]
- Hiruma, J.; Noguchi, H.; Hase, M.; Tokuhisa, Y.; Shimizu, T.; Ogawa, T.; Hiruma, M.; Harada, K.; Kano, R. Epidemiological study of terbinafine-resistant dermatophytes isolated from Japanese patients. J. Dermatol. 2021, 48, 564–567. [Google Scholar] [CrossRef]
- Pilz, J.F.; Köberle, M.; Kain, A.; Seidl, P.; Zink, A.; Biedermann, T.; Pilz, A.C. Increasing incidence of Trichophyton tonsurans in Munich-A single-centre observation. Mycoses 2023, 66, 441–447. [Google Scholar] [CrossRef]
- Ziegler, W.; Lempert, S.; Goebeler, M.; Kolb-Mäurer, A. Tinea capitis: Temporal shift in pathogens and epidemiology. J. Dtsch. Dermatol. Ges. 2016, 14, 818–825. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R.; Versteeg, S.G.; Piraccini, B.M.; Shear, N.H.; Piguet, V.; Tosti, A.; Friedlander, S. Tinea capitis in children: A systematic review of management. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2264–2274. [Google Scholar] [CrossRef]
- Poisson, D.M.; Rousseau, D.; Defo, D.; Estève, E. Outbreak of tinea corporis gladiatorum, a fungal skin infection due to Trichophyton tonsurans, in a French high level judo team. Euro Surveill. 2005, 10, 187–190. [Google Scholar] [CrossRef]
- Hryncewicz-Gwóźdź, A.; Beck-Jendroschek, V.; Brasch, J.; Kalinowska, K.; Jagielski, T. Tinea Capitis and Tinea Corporis with a Severe Inflammatory Response due to Trichophyton tonsurans. Acta Derm. Venereol. 2011, 91, 708–710. [Google Scholar] [CrossRef]
- Gray, R.M.; Champagne, C.; Waghorn, D.; Ong, E.; Grabczynska, S.A.; Morris, J. Management of a Trichophyton tonsurans outbreak in a day-care center. Pediatr. Dermatol. 2015, 32, 91–96. [Google Scholar] [CrossRef]
- Müller, V.L.; Kappa-Markovi, K.; Hyun, J.; Georgas, D.; Silberfarb, G.; Paasch, U.; Uhrlaß, S.; Nenoff, P.; Schaller, J. Tinea capitis et barbae caused by Trichophyton tonsurans: A retrospective cohort study of an infection chain after shavings in barber shops. Mycoses 2021, 64, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, Y.; Hiruma, M.; Hirose, N.; Ikeda, S. Commonly affected body sites in 92 Japanese combat sports participants with Trichophyton tonsurans infection. Mycoses 2009, 52, 339–342. [Google Scholar] [CrossRef]
- Galili, E.; Goldsmith, T.; Khanimov, I.; Arbel, C.; Sharvit, S.; Lyakhovitsky, A.; Shemer, A.; Barzilai, A.; Astman, N. Tinea capitis caused by Trichophyton tonsurans among adults: Clinical characteristics and treatment response. Mycoses 2023, 66, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Aharaz, A.; Jemec, G.B.E.; Hay, R.J.; Saunte, D.M.L. Tinea capitis asymptomatic carriers: What is the evidence behind treatment? J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2199–2207. [Google Scholar] [CrossRef]
- Cuétara, M.S.; Del Palacio, A.; Pereiro, M.; Noriega, A.R. Prevalence of undetected tinea capitis in a prospective school survey in Madrid: Emergence of new causative fungi. Br. J. Dermatol. 1998, 138, 658–660. [Google Scholar] [CrossRef]
| Nails | Feet | Scalp | Crural Region | Face and Neck | Palm | Trunk and Limbs | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), years | 46.6 (20.4) | 46.5 (20.3) | 19.8 (13.3) | 49.8 (22.0) | 32.4 (18.7) | 47.6 (22.3) | 48.3 (24.0) |
| Sex | |||||||
| %Male | 60.1% | 63.2% | 85.9% | 71.9% | 58.6% | 79.2% | 64.5% |
| %Female | 39.9% | 36.8% | 14.1% | 28.1% | 41.4% | 20.8% | 35.5% |
| Positive detection rate a, No. (%) | 671 (61.4%) | 397 (70.8%) | 71 (39.4%) | 114 (47.5%) | 58 (38.7%) | 48 (34.3%) | 183 (33.8%) |
| % adults | 97.2% | 97.5% | 67.7% | 98.2% | 87.9% | 95.8% | 96.2% |
| % children | 2.8% | 2.5% | 32.4% | 1.8% | 12.1% | 4.2% | 3.8% |
| Overall (n = 1472) | Onyco-Mycosis (n = 652) | Feet (n = 387) | Crural Region (n = 112) | Scalp (n = 48) | Face and Neck (n = 51) | Palms (n = 46) | Trunk and Limbs (n = 176) | |
|---|---|---|---|---|---|---|---|---|
| Dermatophytes, n (%) | 1401 (95.2%) | 607 (93.1%) | 378 (97.7%) | 105 (93.7%) | 48 (100%) | 51 (100%) | 38 (82.6%) | 175 (99.4%) |
| Epidermophyton Fluccosum | 36 (2.4%) | 16 (2.5%) | 14 (3.6%) | 2 (1.8%) | 1 (2.1%) | 1 (2.0%) | 0 | 2 (1.1%) |
| Microsporum Canis | 17 (1.2%) | 5 (0.8%) | 1 (0.3%) | 3 (2.7%) | 0 | 2 (3.9%) | 1 (2.2%) | 5 (2.8%) |
| M. gypseum | 4 (0.3%) | 1 (0.2%) | 0 | 1 (0.9%) | 0 | 0 | 1 (2.2%) | 1 (0.6%) |
| Trichophyton Rubrum | 1183 (80.4%) | 570 (87.4%) | 357 (92.2%) | 90 (80.4%) | 4 (8.3%) | 13 (25.5%) | 30 (65.2%) | 119 (67.6%) |
| T. mentagrophytes/ interdigitale | 20 (1.4%) | 10 (1.5%) | 3 (0.8%) | 3 (2.7%) | 0 | 1 (2.0%) | 1 (2.2%) | 2 (1.1%) |
| T. tonsurans | 136 (9.2%) | 4 (0.6%) | 2 (0.5%) | 6 (5.4%) | 40 (83.3%) | 35 (66.7%) | 5 (10.9%) | 45 (25.6%) |
| T. violaceum | 5 (0.3%) | 0 | 1 (0.3%) | 0 | 3 (6.3%) | 0 | 0 | 1 (0.6%) |
| Candida, n (%) | 48 (3.3%) | 30 (4.8%) | 3 (0.8%) | 7 (6.3%) | 0 | 0 | 8 (17.4%) | 0 |
| Albicans | 9 (0.6%) | 1 (0.2%) | 1 (0.3%) | 6 (5.4%) | 0 | 0 | 1 (2.2%) | 0 |
| Parapsilosis | 36 (2.4%) | 28 (4.3%) | 2 (0.5%) | 1 (0.9%) | 0 | 0 | 5 (10.9%) | 0 |
| Other | 3 (0.2%) | 1 (0.2%) | 0 | 0 | 0 | 0 | 2 (4.3%) | 0 |
| Non-dermatophyte molds, n (%) | 23 (1.6%) | 16 (2.5%) | 6 (1.6%) | 0 | 0 | 0 | 0 | 1 (0.6%) |
| Overall (n = 70) | Onyco-Mycosis (n = 19) | Feet (n = 10) | Crural Region (n = 2) | Scalp (n = 23) | Face and Neck (n = 8) | Palms (n = 2) | Trunk and Limbs (n = 7) | |
|---|---|---|---|---|---|---|---|---|
| Dermatophyte | 69 (98.6%) | 19 (100%) | 10 (100%) | 1 (50%) | 23 (100%) | 7 (100%) | 2 (100%) | 7 (100%) |
| Epidermophyton Fluccosum | 1 (1.4%) | 0 | 0 | 1 (50%) | 0 | 0 | 0 | 0 |
| Microsporum Canis | 11 (15.7%) | 0 | 0 | 0 | 9 (39.1%) | 1(14.3%) | 0 | 1 (14.3%) |
| Trichophyton Rubrum | 36 (51.4%) | 19 (100%) | 10 (100%) | 0 | 2 (8.7%) | 2 (28.6%) | 2 (100%) | 1 (14.3%) |
| T. tonsurans | 20 (28.6%) | 0 | 0 | 0 | 11 (47.8%) | 4 (57.1%) | 0 | 5 (71.4%) |
| T. violaceum | 1 (1.4%) | 0 | 0 | 0 | 1 (4.3%) | 0 | 0 | 0 |
| Candida | 1 (1.4%) | 0 | 0 | 1 (50%) | 0 | 0 | 0 | 0 |
| Albicans | 1 (1.4%) | 0 | 0 | 1 (50%) | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galili, E.; Taieb, A.; Shemer, A.; Leor, G.; Lyakhovitsky, A.; Barzilai, A.; Baum, S. Epidemiology and Trends of Cutaneous Fungal Infections (2019–2022) in Israel: A Single Tertiary-Center Study. J. Fungi 2025, 11, 320. https://doi.org/10.3390/jof11040320
Galili E, Taieb A, Shemer A, Leor G, Lyakhovitsky A, Barzilai A, Baum S. Epidemiology and Trends of Cutaneous Fungal Infections (2019–2022) in Israel: A Single Tertiary-Center Study. Journal of Fungi. 2025; 11(4):320. https://doi.org/10.3390/jof11040320
Chicago/Turabian StyleGalili, Eran, Auriella Taieb, Avner Shemer, Gil Leor, Anna Lyakhovitsky, Aviv Barzilai, and Sharon Baum. 2025. "Epidemiology and Trends of Cutaneous Fungal Infections (2019–2022) in Israel: A Single Tertiary-Center Study" Journal of Fungi 11, no. 4: 320. https://doi.org/10.3390/jof11040320
APA StyleGalili, E., Taieb, A., Shemer, A., Leor, G., Lyakhovitsky, A., Barzilai, A., & Baum, S. (2025). Epidemiology and Trends of Cutaneous Fungal Infections (2019–2022) in Israel: A Single Tertiary-Center Study. Journal of Fungi, 11(4), 320. https://doi.org/10.3390/jof11040320







