Abstract
Hypocreales is one of the largest orders within the class Sordariomycetes and is renowned for its diversity of lifestyles, encompassing plant, insect, and human pathogens, as well as endophytes, parasites, and saprobes. In this study, we focused on saprobic hypocrealean fungi isolated from rice in northern Thailand. Species identification was conducted using morphological characteristics and multilocus phylogenetic analyses, including the internal transcribed spacer region (ITS), 28S large subunit nuclear ribosomal DNA (LSU), translation elongation factor 1–alpha (tef1-α), RNA polymerase II second-largest subunit (rpb2), and calmodulin (cmdA). This research confirmed the presence of 14 species of hypocrealean taxa, viz. Fusarium (9), Ochronectria (1), Sarocladium (2), Trichothecium (1), and Waltergamsia (1). Among these were two new species (Fusarium chiangraiense and F. oryzigenum), four new host records (Fusarium kotabaruense, Ochronectria thailandica, Sarocladium bactrocephalum, and Waltergamsia fusidioides), and three new geographical records (Fusarium commune, F. guilinense, and F. hainanese).
1. Introduction
Oryza sativa L., commonly known as rice, is a crucial cereal crop and a vital source of fiber, minerals, proteins, and vitamins, promoting good health for more than half of the world’s population [1,2]. In Thailand, rice is a major economic crop cultivated in all regions of the country, making Thailand one of the leading producers and exporters of rice [3,4,5]. However, many factors, especially microorganisms such as fungi, can significantly impact rice supply [6]. Rice-associated fungi, which exhibit diverse nutritional modes, play key roles in rice production, ranging from causing severe diseases to providing beneficial effects [7,8,9].
Hypocreales ranks among the largest orders within the class Sordariomycetes, comprising 358 genera across 29 families, with some genera incertae sedis [10,11,12,13]. The taxonomy of families in this order has been fairly well studied [14,15,16,17,18,19,20,21,22,23,24]. They have worldwide distribution across a wide range of hosts and habitats [25]. Hypocreales are considered the richest source of biocontrol agents within the Ascomycota, containing species with significant ecological and economic roles [25,26,27,28,29,30,31]. They are also found as crop pathogens, saprobes, entomopathogens, and mycoparasites [23,25,32,33,34,35,36,37,38]. In general, families within this order exhibit distinct associations with specific hosts. For instance, Nectriaceae encompasses many important plant pathogens, including Fusarium; Cordycipitaceae consists of well-known insect pathogens and medicinal fungi; and Clavicipitaceae includes grass endophytes that provide benefits to plants [16,39,40,41]. The association between hypocrealean fungi and the family Poaceae, particularly rice, has been documented in many studies. Certain genera, including Acremonium, Fusarium, and Sarocladium, have been most frequently observed and isolated from rice [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. The present study aims to investigate saprobic hypocrealean taxa associated with rice, with an emphasis on Fusarium in northern Thailand.
2. Materials and Methods
2.1. Sample Collection, Isolation, and Examination
Rice specimens, predominantly consisting of the Thai Jasmine rice cultivars RD15 and KDML105, were collected mainly during the dry season (November and December) from paddy fields in different districts of Chiang Rai Province, Thailand. Samples were placed in plastic bags with labels detailing their collection information and transferred to the laboratory. External observations and examinations were conducted using a stereomicroscope (Motic SMZ 168), and microscopic images were captured using a digital camera attached to a compound microscope (Nikon DS-Ri2). The images featured in the figures were edited with Adobe Photoshop version 21.1.3 (Adobe Systems, San Jose, CA, USA) and measured using the Tarosoft® Image Framework (version 0.9.7).
The fungal isolation and purification of obtained strains were carried out using the hyphal tip technique as described by Senanayake et al. [57]. Some of the isolates were transferred onto carnation leaf agar (CLA) plates and incubated at 27 °C for 7–14 days to induce sporulation [58]. Morphological characteristics were assessed either directly from the host or after a two-week incubation period of the obtained cultures. Specimens were deposited at Mae Fah Luang University Herbarium (Herb. MFLU) and living cultures on PDA were preserved in the Culture Collection of Mae Fah Luang University (MFLUCC). New taxa were assigned numbers from Index Fungorum (https://www.indexfungorum.org/names/names.asp, accessed on 25 November 2024).
2.2. DNA Extraction, PCR Amplification, and Sequencing
Fresh mycelia from axenic cultures were scraped for DNA extraction using the OMEGA E.Z.N.A.® Forensic DNA Kit (Norcross, GA, USA) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) amplifications were performed for internal transcribed spacer region (ITS), 28S large subunit nuclear ribosomal DNA (LSU), translation elongation factor 1–alpha (tef1-α), RNA polymerase II second largest subunit (rpb2), and calmodulin (cmdA) using related forward and reverse primer pairs [59,60,61,62,63]. The PCR thermal cycle program and primers used in this study are listed in Table 1. The total volume of PCR mixtures was 25 µL, which contained double-distilled water (ddH2O), 12.5 μL of 2 × Power Taq PCR MasterMix (Taq DNA polymerase, dNTPs, MgCl2, and optimized reaction buffers), 1 μL each of the primers (10 pM), and 1 μL of genomic DNA. All of the PCR products were assessed using 1.5% agarose gel electrophoresis and viewed on a Cybergreen-stained agarose gel using ultraviolet light with a gel imaging system. DNA fragments with positive results in the PCR analysis were subsequently shipped to SolGent Co. (Daejon, Republic of Korea) for purification and sequencing.

Table 1.
Primers and PCR conditions used in this study.
2.3. Phylogenetic Analyses
Phylogenetic analyses followed the procedures described by Dissanayake et al. [64]. Sequence data for all loci were subjected to a BLASTn search (Basic Local Alignment Search Tool, https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 17 July 2024) to identify the most closely related taxa from the NCBI (National Center for Biotechnology Information) database. Each selected sequence was aligned using the server version of MAFFT v.7.036 [65], and the alignments were manually edited using BioEdit v.7.0.5.2 before conducting phylogenetic analyses [66]. The FASTA files of combined alignment were converted to PHYLIP and NEXUS formats using the Alignment Transformation Environment (ALTER) online tool (https://www.sing-group.org/ALTER, accessed on 17 July 2024) [67] for Maximum Likelihood (ML) and Bayesian analysis, respectively. The ML trees were constructed using IQ-TREE on XSEDE v.2.3.2 [68] via the CIPRES Science Gateway platform [69] implementing the ultrafast bootstrap with 1000 replicates. Bayesian posterior probability (PP) was calculated using MrBayes version 3.1.2 [70] with Markov Chain Monte Carlo sampling (MCMC). Four simultaneous Markov chains were run for 1,000,000 generations, with sampling every 100th generation. The first 25% of trees from the burn-in phase were discarded, while the remaining 75% were used to compute the posterior probability (PP). Phylogenetic trees were visualized using FigTree v.1.4.0 [71] and edited with Adobe Illustrator CC 22.0.0 (Adobe Systems, San Jose, CA, USA).
3. Results
3.1. Phylogenetic Analysis
Analysis 1: A multilocus phylogenetic analysis of the Fusarium fujikuroi species complex (FFSC) was carried out using cmdA, rpb2, and tef1-α gene sequences, comprising 99 ingroup taxa. Fusarium curvatum (CBS 238.94) and F. inflexum (CBS 716.74) were used as outgroups. The aligned dataset included 1855 positions: cmdA (1–800), rpb2 (801–1330), and tef1-α (1331–1855), with gaps considered. The best ML tree yielded a final likelihood score of −11755.683 (Figure 1). The alignment showed 683 distinct patterns, 418 parsimony-informative sites, 171 singleton sites, and 1295 constant sites. The Bayesian tree converged at the 1,000,000th generation with an average standard deviation of split frequencies of 0.195795. This phylogeny resolved four regional lineages: African clades A and B, and American and Asian clades. Strains MFLUCC 24-0637 and MFLUCC 24-0628 were placed within African clade A and the Asian clade, respectively.
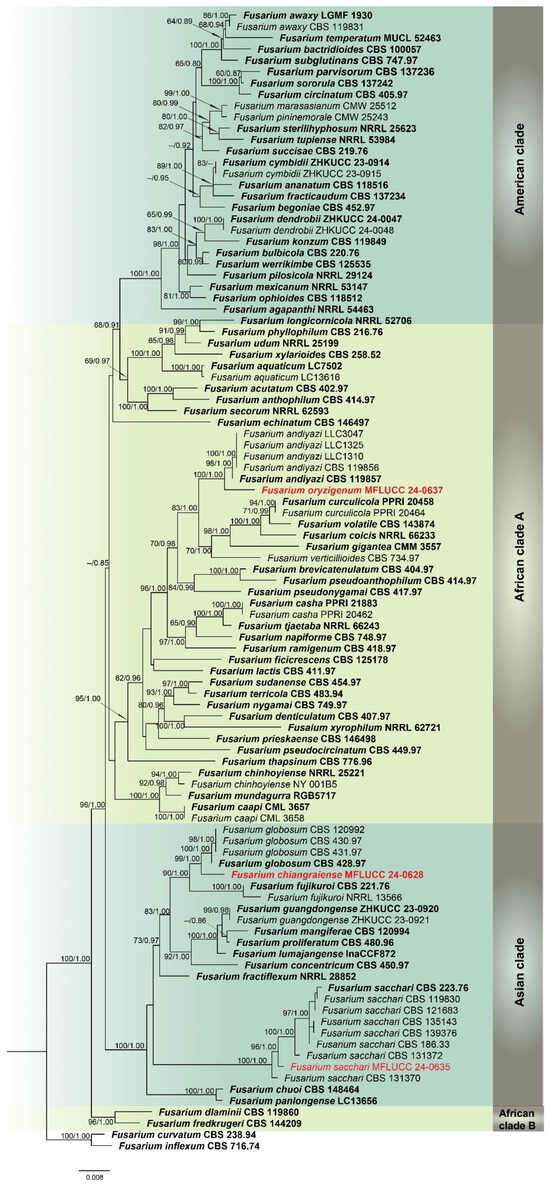
Figure 1.
Combined phylogeny of cmdA, rpb2, and tef1-α gene regions of the Fusarium fujikuroi species complex (FFSC). The tree is rooted to Fusarium curvatum (CBS 238.94) and F. inflexum (CBS 716.74). Maximum likelihood bootstrap support values (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.80, are given at the nodes (ML/PP). The isolate from the current study is highlighted in red, and type strains are indicated in bold black.
Analysis 2: A phylogenetic reconstruction of the Fusarium nisikadoi species complex (FNSC) was conducted using a combined dataset of rpb2 and tef1-α from 13 ingroup isolates, with F. udum (BBA 65058) as the outgroup. The alignment spanned 1467 characters: rpb2 (1–901) and tef1-α (902–1330), with gaps included. The optimal IQ-TREE yielded a final likelihood score of −2874.566 (Figure 2). The matrix comprised 118 distinct patterns, 60 parsimony-informative and singleton sites each, alongside 1343 constant sites. The Bayesian tree converged at the 1,000,000th generation with an average standard deviation of split frequencies of 0.001498.
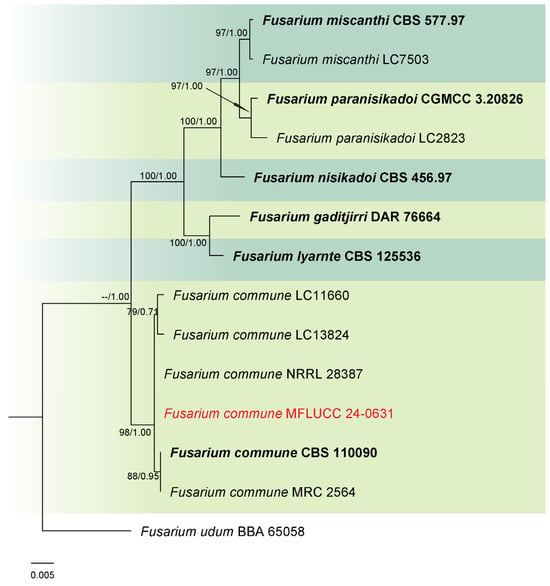
Figure 2.
Combined phylogeny of rpb2 and tef1-α gene regions of the Fusarium nisikadoi species complex (FNSC). The tree is rooted to Fusarium udum (BBA 65058). Maximum likelihood bootstrap support values (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.80, are given at the nodes (ML/PP). The isolate from the current study is highlighted in red, and type strains are indicated in bold black.
Analysis 3: The combined phylogenetic analysis of cmdA, rpb2, and tef1-α conducted on Fusarium incarnatum-equiseti species complex (FIESC) isolates included 101 ingroup taxa, with Fusarium concolor (CBS 961.87) as the outgroup. The dataset after alignment consisted of 2153 characters: 1–648 for cmdA, 649–1525 for rpb2, and 1526–2153 for tef1-α, including gaps. The best scoring IQ-TREE, with a value of −12,330.550 as a final ML optimization likelihood, is presented in Figure 3. The matrix comprised 796 distinct patterns, 490 parsimony-informative sites, 287 singleton sites, and 1376 constant sites. The Bayesian tree converged at the 1,000,000th generation with an average standard deviation of split frequencies of 0.038027.

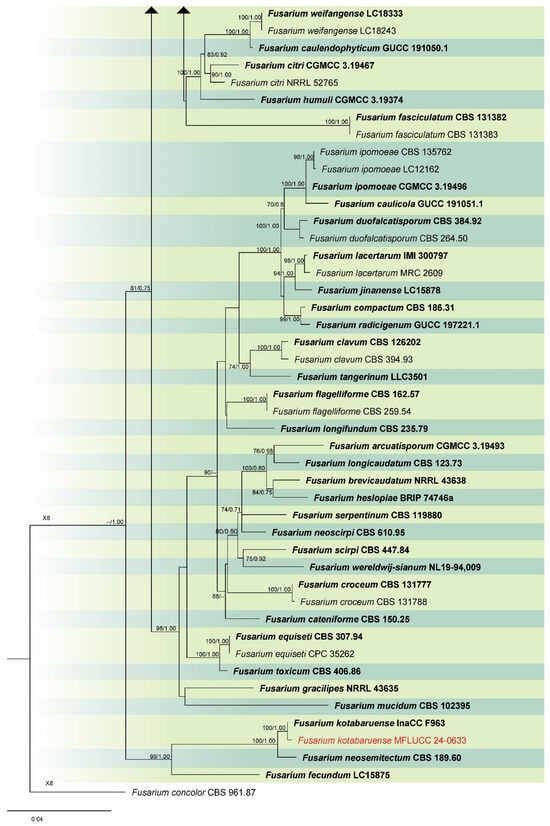
Figure 3.
Combined phylogeny of cmdA, rpb2, and tef1 gene regions of the Fusarium incarnatum-equiseti species complex (FIESC). The tree is rooted to Fusarium concolor (CBS 961.87). Maximum likelihood bootstrap support values (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.80, are given at the nodes (ML/PP). The isolate from the current study is highlighted in red, and type strains are indicated in bold black.
Analysis 4: Phylogenetic analysis of Ochronectria isolates was performed using a combined dataset from ITS, LSU, rpb2, and tef1-α gene regions, involving six ingroup taxa. Lasionectria marigotensis (CBS 131606) was designated as the outgroup. The sequence alignment totaled 2808 characters: 1–493 for ITS, 494–1270 for LSU, 1271–2005 for rpb2, and 2006–2808 for tef1-α, including gaps. The maximum likelihood tree yielded a final score of −5572.845 (Figure 4). This alignment comprised 144 distinct patterns, 104 parsimony-informative sites, 228 singleton sites, and 2476 constant sites. The Bayesian tree converged at the 1,000,000th generation with an average standard deviation of split frequencies of 0.002432.
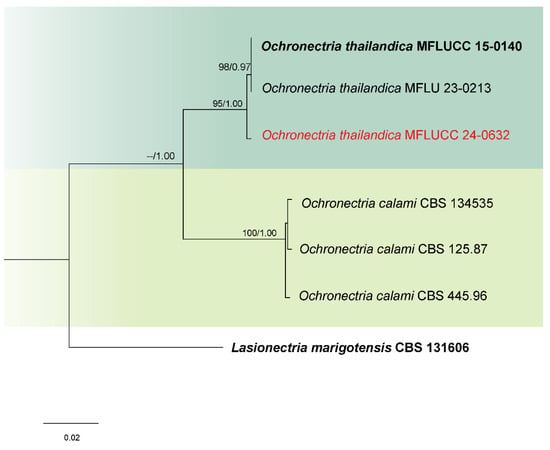
Figure 4.
Combined phylogeny of ITS, LSU, rpb2, and tef1-α gene regions of the Ochronectria species. The tree is rooted to Lasionectria marigotensis (CBS 131606). Maximum likelihood bootstrap support values (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.80, are given at the nodes (ML/PP). The isolate from the current study is highlighted in red, and type strains are indicated in bold black.
Analysis 5: For Sarocladium species, a multi-gene phylogeny was conducted using ITS, LSU, and act loci, with 54 ingroup taxa. Chlamydocillium curvulum (CBS 430.66 and CBS 229.75) were used as outgroups. The aligned dataset consisted of 1944 characters: 1–425 for ITS, 426–1202 for LSU, and 1203–1944 for act, including gaps. The ML analysis returned a best tree with a likelihood score of −9784.272 (Figure 5). The matrix comprised 491 distinct patterns, 375 parsimony-informative sites, 65 singleton sites, and 1504 constant sites. The Bayesian tree converged at the 1,000,000th generation with an average standard deviation of split frequencies of 0.007275.
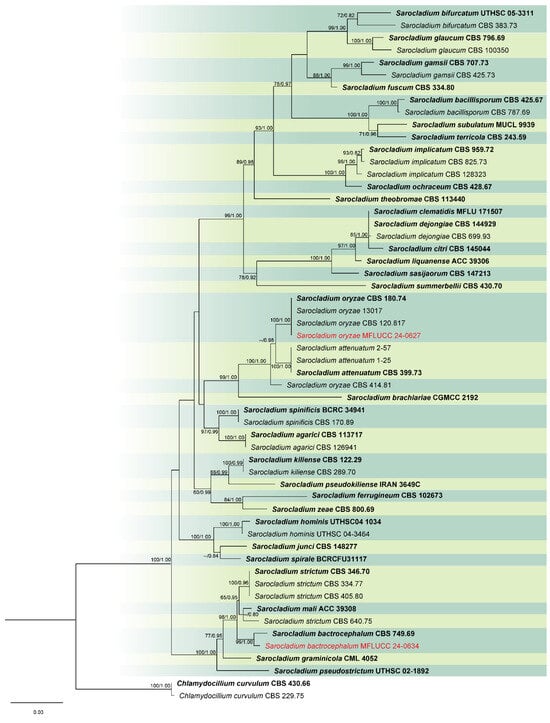
Figure 5.
Combined phylogeny of ITS, LSU, and act gene regions of the Sarocladium species. The tree is rooted to Chlamydocillium curvulum (CBS 430.66) and C. curvulum (CBS 229.75). Maximum likelihood bootstrap support values (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.80, are given at the nodes (ML/PP). The isolate from the current study is highlighted in red, and type strains are indicated in bold black.
Analysis 6: The combined phylogenetic analysis of ITS, LSU, and tef1-α conducted on Trichothecium isolates included 14 ingroup taxa, with Stanjemonium spectabile (CBS 340.70) as an outgroup. The dataset after alignment consisted of 2077 characters: 1–491 for ITS, 492–1269 for LSU, and 1270–2077 for tef1-α, including gaps. The best scoring IQ-TREE, with a value of −4281.827 as a final ML optimization likelihood, is presented in Figure 6. The matrix had 144 distinct patterns, 164 parsimony-informative sites, 91 singleton sites, and 1873 constant sites. The Bayesian tree converged at the 1,000,000th generation with an average standard deviation of split frequencies of 0.002635.
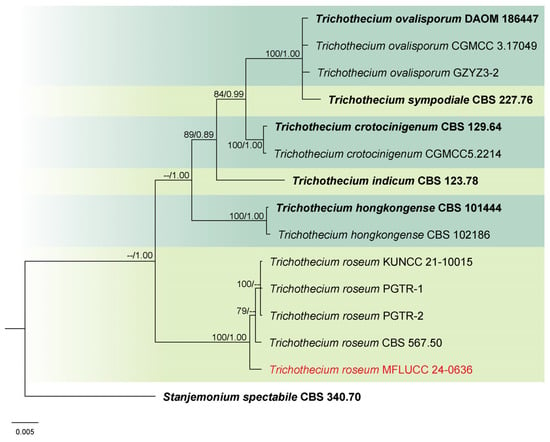
Figure 6.
Combined phylogeny of ITS, LSU, and tef1-α gene regions of the Trichothecium species. The tree is rooted to Stanjemonium spectabile (CBS 340.70). Maximum likelihood bootstrap support values (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.80, are given at the nodes (ML/PP). The isolate from the current study is highlighted in red, and type strains are indicated in bold black.
Analysis 7: Phylogenetic relationships of Waltergamsia species were inferred using a combined ITS, LSU, rpb2, and tef1-α dataset with 26 ingroup taxa. Outgroup taxa included Acremonium egyptiacum (CBS 114785) and A. gamsianum (CBS 881.73). The alignment comprised 2840 characters: 1–501 for ITS, 502–1278 for LSU, 1279–2032 for rpb2, and 2033–2840 for tef1-α, including gaps. The best scoring IQ-TREE, with a value of −13,746.021 as a final ML optimization likelihood, is presented in Figure 7. The matrix comprised 720 distinct patterns, 660 parsimony-informative sites, 169 singleton sites, and 2011 constant sites. The Bayesian tree converged at the 1,000,000th generation with an average standard deviation of split frequencies of 0.002520.
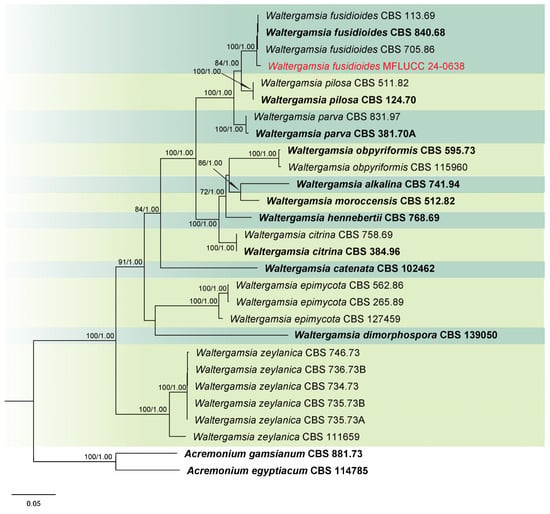
Figure 7.
Combined phylogeny of ITS, LSU, rpb2, and tef1-α gene regions of the Waltergamsia species. The tree is rooted to Acremonium egyptiacum (CBS 114785) and A. gamsianum (CBS 881.73). Maximum likelihood bootstrap support values (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.80, are given at the nodes (ML/PP). The isolate from the current study is highlighted in red, and type strains are indicated in bold black.
3.2. Taxonomy
Fusarium chiangraiense S. Absalan, S. Lumyong & K. D. Hyde, sp. nov. (Figure 8).
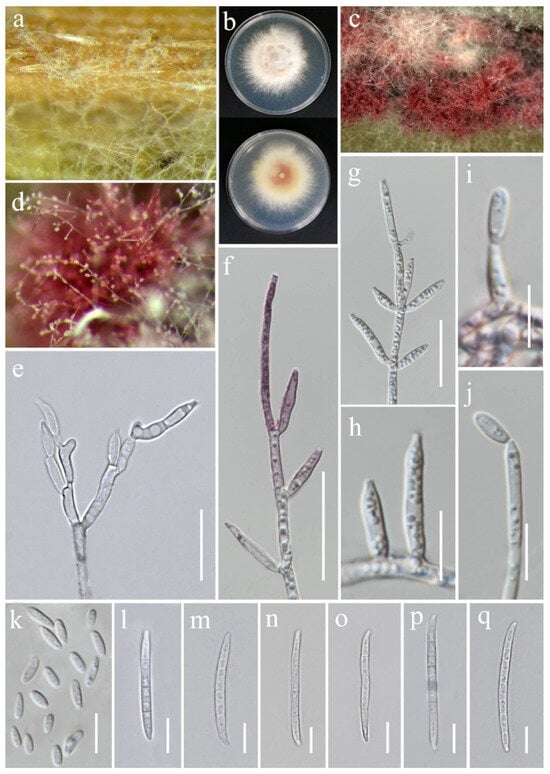
Figure 8.
Fusarium chiangraiense (MFLUCC 24-0628, ex-type). (a) Appearance of mycelia on the panicle of Oryza sativa; (b) front and reverse of colony on PDA; (c,d) conidiophores and conidia formed on the carnation leaf; (e–j) mono- and polyphialides on aerial mycelium; (k) aerial microconidia; (l–q) aerial macroconidia. Scale bars: (e,f) = 20 μm; (g–q) = 10 μm.
Index Fungorum Number: IF903755
Etymology: Named after Chiang Rai Province, from where it was collected.
Saprobic on Oryza sativa. Conidiophores 30–95 μm tall, borne on aerial mycelia, septate, proliferating percurrently, bearing terminal or lateral phialides, sympodial and branched, smooth- and thin-walled, pink to hyaline. Conidiogenous cells 9.5–17 × 2.5–3.5 μm ( = 12 × 3 μm, n = 20), mono- and polyphialidic, subulate to subcylindrical, smooth and thin-walled, hyaline. Microconidia 6–9 × 2.5–3 μm ( = 7 × 3 μm, n = 30), single on the tips of phialides, ovoid to ellipsoidal, aseptate, smooth- and thin-walled, hyaline. Macroconidia 37–52 × 3.5–4.5 μm ( = 46 × 4 μm, n = 30), straight to falcate, slender and sometimes slightly curved, tapering toward the basal part, apical cells papillate, basal cells indistinct or foot-shaped, 3–4-septate, hyaline. Sporodochia and Chlamydospores not observed.
Culture characteristics: Colonies on PDA reaching 67–70 mm diameter after a week at 28 °C, pale greyish rose, cottony to velvety, raised, aerial mycelia medium-dense, filiform margin. Reverse pale orange with pale red at the center.
Material examined: Thailand, Chiang Rai Province, Phan District, Mueang Phan Sub-district (19.484667° N, 99.720012° E), on the panicle of Oryza sativa, 9 November 2021, Nootjarin Jungkhun, (NS27-1 = MFLU 25-0033); (ex-type, living culture MFLUCC 24-0628).
GenBank numbers: cmdA = PV297810, rpb2 = PV394842, tef1-α = PV394838.
Notes: Based on the morphological and molecular data, Fusarium chiangraiense has been confirmed as a new species, supported by 99% ML and 1.00 PP (Figure 1). The phylogram indicates that F. chiangraiense is closely related to F. globosum in the FFSC; however, it diverges by 17 base pairs across the combined three-locus dataset (cmdA+rpb2+tef1-α). Fusarium globosum is characterized by two types of microconidia: globose and ellipsoidal or clavate, with the latter sometimes forming chains or false heads. It also typically produces macroconidia on sporodochia [72,73]. In contrast, F. chiangraiense lacks both globose microconidia and sporodochia. It can also be differentiated by the red mycelium that it forms on carnation leaves.
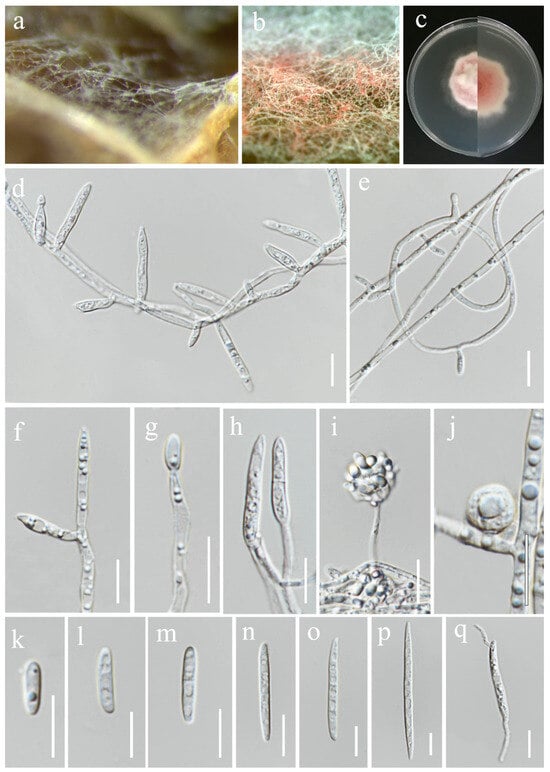
Figure 9.
Fusarium commune (MFLUCC 24-0631). (a,b) Appearance of mycelia on the flag leaf of Oryza sativa and carnation leaves, respectively; (c) front and reverse of colony on PDA; (d–h) monophialides and conidia on aerial mycelium; (i) microconidia on false head; (j) chlamydospores; (k–p) aerial conidia; (q) microcyclic conidiation. Scale bars: (d–q) = 10 μm.
Index Fungorum Number: IF489435
Saprobic on Oryza sativa. Conidiophores 20–50 μm tall or reduced to conidiogenous cells borne terminally or laterally on aerial mycelia, unbranched, smooth- and thin-walled, hyaline. Conidiogenous cells 5.5–30 × 2.5–3.5 μm ( = 22 × 3.5 μm, n = 20), monophialidic (polyphialidic conidiogenous cells were not observed), subulate to subcylindrical, smooth- and thin-walled, hyaline. Aerial conidia 6.5–65 × 2.5–4.5 μm ( = 33 × 4 μm, n = 40), cylindrical, straight to slightly curved, smooth- and thin-walled, 0–3-septate, microcyclic conidiogenesis observed, hyaline. Sporodochia not observed. Chlamydospores 7.5–10 mm diameter, intercalary or terminal, smooth, hyaline.
Culture characteristics: Colonies on PDA reaching 45–53 mm diameter after a week at 28 °C, pink to pale violet, dense, floccose to fluffy, slightly undulate margin. Reverse coral pink with white margin.
Material examined: Thailand, Chiang Rai Province, Phan District, Mueang Phan Sub-district (19.528477° N, 99.74594° E), on the flag leaf of Oryza sativa, 3 December 2021, Nootjarin Jungkhun, (NS40-1 = MFLU 25-0036); (living culture MFLUCC 24-0631).
GenBank numbers: cmdA = N/A, rpb2 = PV394843, tef1-α = PV394839.
Notes: Phylogenetic analysis revealed that our strain clustered with the ex-type strain (CBS 110090) and other Fusarium commune isolates within the FNSC, with 98% ML and 1.00 PP support (Figure 2). Combining morphological characteristics and molecular data, strain MFLUCC 24-0631 was confirmed as F. commune. While most morphological features were similar between the ex-type strain and our collection, we did not observe polyphialidic conidiogenous cells in our sample.
Fusarium guilinense M.M. Wang, Qian Chen & L. Cai, Persoonia 43: 80 (2019). (Figure 10).
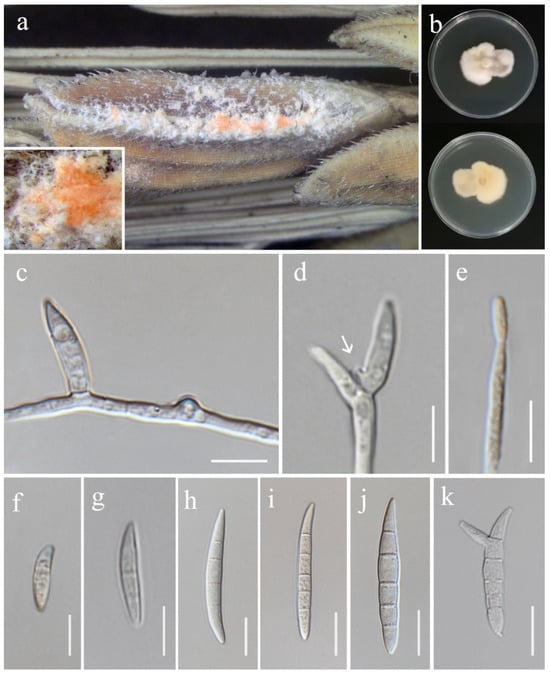
Figure 10.
Fusarium guilinense (MFLU 25-0032, new geographical record). (a) Appearance of mycelia on the panicle of Oryza sativa; (b) front and reverse of colony on PDA; (c) lateral monophialide on aerial mycelium; (d,e) mono- and polyphialides on aerial mycelium (arrow pointing at phialid); (f–j) aerial conidia; (k) microcyclic conidiation. Scale bars: (c–k) = 10 μm.
Index Fungorum Number: IF829535
Saprobic on Oryza sativa. Conidiophores 46–75 × 3.5–5 ( = 60 × 4.5 μm, n = 8), borne on aerial mycelia, unbranched, bearing terminal or lateral phialides. Conidiogenous cells mono- and polyphialidic, slightly cylindrical, smooth- and thin-walled, hyaline. Aerial conidia 12–58 × 4–6 μm ( = 45 × 5.5 μm, n = 30), ellipsoid to falcate, slender, smooth- and thin-walled, slightly curved, tapering at both apex and base, 1–5-septate, microcyclic conidiogenesis observed, hyaline. Sporodochia and Chlamydospores not observed.
Culture characteristics: Colonies on PDA reaching 46–48 mm diameter after a week at 28 °C, white, velvety to felty, irregular margin, aerial mycelium scant in the center. Reverse white to pale salmon.
Material examined: Thailand, Chiang Rai Province, Mueang Chiang Rai District, on the panicle of Oryza sativa, 10 November 2021, Ruvishika Jayawardena, (RS14-1 = MFLU 25-0032); (living culture MFLUCC 24-0626).
GenBank numbers: cmdA = PV297811, rpb2 = PV394827, tef1-α = PV394837.
Notes: Our isolate grouped with Fusarium guilinense (CGMCC 3.19495) in the phylogenetic analysis, with 100% ML and 1.00 PP support (Figure 3). Both isolates are morphologically similar, however, our isolate predominantly produced 3–5-septate conidia, whereas Wang et al. [74] described conidia in their strain as mostly 3-septate. In the present study, we documented F. guilinense as the first geographical record for Thailand.
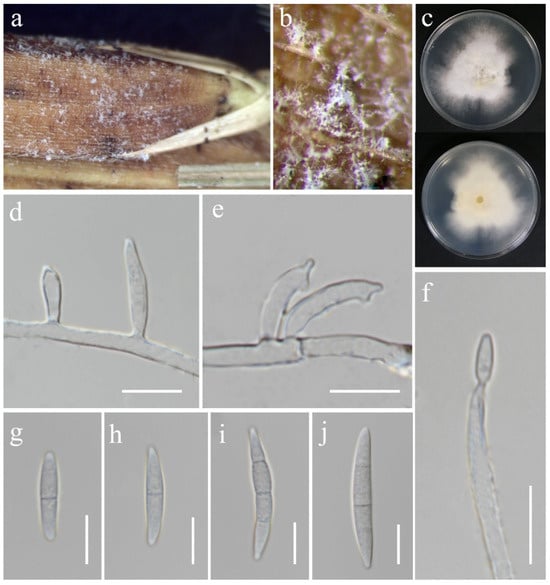
Figure 11.
Fusarium hainanense (MFLU 25-0035). (a,b) Appearance of mycelia on the panicle of Oryza sativa; (c) front and reverse of colony on PDA; (d) monophialides on aerial mycelium; (e) polyphialides on aerial mycelium; (f) conidium produced on monophialide; (g–j) aerial conidia. Scale bars: (d–j) = 10 μm.
Index Fungorum Number: IF829536
Saprobic on Oryza sativa. Conidiophores borne on aerial mycelia, septate, bearing terminal or lateral phialides, smooth- and thin-walled, hyaline. Conidiogenous cells 11–22 × 2.5–3.5 μm ( = 18 × 3 μm, n = 15), mono- and polyphialidic, subulate to subcylindrical, smooth- and thin-walled, hyaline. Aerial conidia 18–36 × 3–5 μm ( = 28 × 4 μm, n = 20), fusiform, straight to slightly curved, sometimes with constricted septa, apical cell blunt to papillate, basal cell barely to distinctly notched, 1–3-septate, hyaline. Sporodochia, Chlamydospores and Microconidia not observed.
Culture characteristics: Colonies on PDA reaching 67–74 mm diameter after a week at 28 °C, white with pale yellow in the center, cottony to floccose, aerial mycelia dense, lobate margin. Reverse white cream.
Material examined: Thailand, Chiang Rai Province, Phan District, Mueang Phan Sub-district (19.48468° N, 99.719868° E), on the panicle of Oryza sativa, 9 November 2021, Nootjarin Jungkhun, (NS29-1 = MFLU 25-0035); (living culture MFLUCC 24-0630).
GenBank numbers: cmdA = PV297812, rpb2 = PV394828, tef1-α = PV394833.
Notes: Fusarium hainanense was first described by Wang et al. [74], obtained from a stem of Oryza sp., and represents a phylo-species within the FIESC. In this study, the isolate MFLUCC 24-0630 has been identified as F. hainanense according to both morphology and phylogeny analysis. This species has been reported in several tropical and subtropical countries [74]. However, it has not been previously recorded in Thailand. Hence, we introduce F. hainanense as a new geographical record for Thailand.
Fusarium kotabaruense Maryani, Sand. Den., L. Lombard, Kema & Crous, Persoonia 43: 65 (2019). (Figure 12).
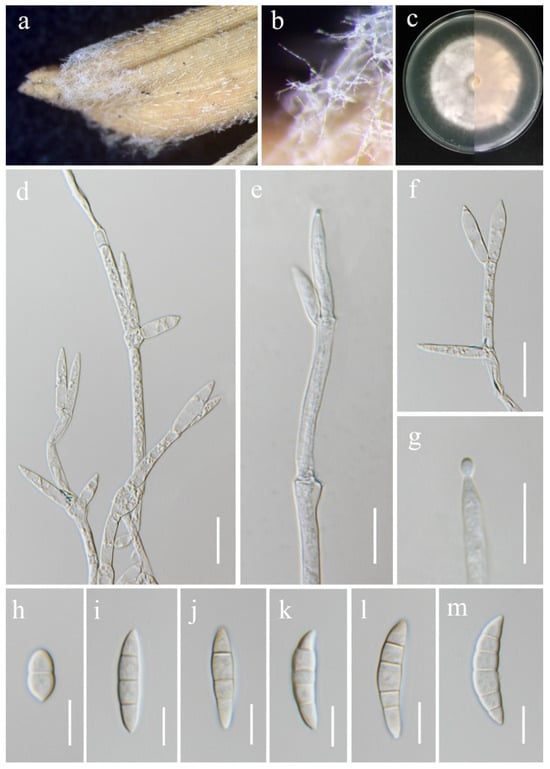
Figure 12.
Fusarium kotabaruense (MFLU 25-0037, new host and geographical record). (a,b) Appearance of mycelia on the panicle of Oryza sativa; (c) front and reverse of colony on PDA; (d–f) conidiophores and conidiogenous cells; (g) aerial conidium produced on a monophialide; (h–m) aerial conidia. Scale bars: (d–g) = 20 μm; (h–m) = 10 μm.
Index Fungorum Number: IF828964
Saprobic on Oryza sativa. Conidiophores borne on aerial mycelia, septate, proliferating percurrently, usually bearing terminal phialides, irregularly branched, smooth- and thin-walled, hyaline. Conidiogenous cells 17.5–30 × 4–7 μm ( = 22 × 4.5 μm, n = 20), monophialidic, subulate to subcylindrical, smooth- and thin-walled, hyaline. Aerial conidia 12–36 × 5.5–8 μm ( = 29 × 7 μm, n = 30), straight to slightly curved, slightly falcate, apical cells gently blunt, basal cells indistinct or foot-shaped, 1–5-septate, hyaline. Sporodochia and Chlamydospores not observed.
Culture characteristics: Colonies on PDA reaching 67–70 mm diameter after a week at 28 °C, dull white to rose buff, medium-dense, cottony, radiate. Reverse yellowish white with pale orange in the center.
Material examined: Thailand, Chiang Rai Province, Mueang Chiang Rai District, Huai Sak Sub-district (19.781206° N, 99.921459° E), on the panicle of Oryza sativa, 17 December 2021, Sahar Absalan, (HS69-1 = MFLU 25-0037); (living culture MFLUCC 24-0633).
GenBank numbers: cmdA = PV297813, rpb2 = PV394831, tef1-α = PV394836.
Notes: According to the phylogram (Figure 3), isolate MFLUCC 24-0633 was identified as Fusarium kotabaruense, positioned basally to the FIESC. Subsequent studies, however, indicated that this species is more appropriately placed within the Fusarium camptoceras species complex (FCSC) [75,76]. Fusarium kotabaruense was first reported from the infected pseudostem of Musa sp. var. Pisang Hawa in Indonesia [77]. This study documents the first known case of F. kotabaruense on rice and a new geographical record for Thailand.
Fusarium mianyangense S.L. Han, M.M. Wang & L. Cai, Stud. Mycol. 104: 131 (2023). (Figure 13).
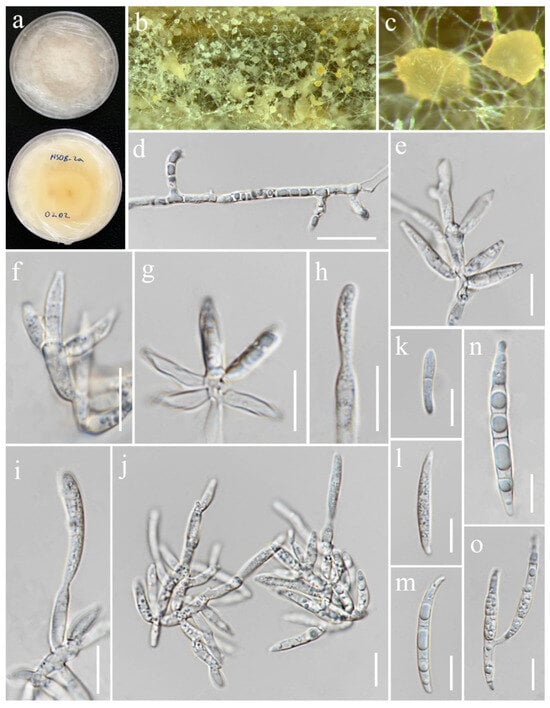
Figure 13.
Fusarium mianyangense (MFLUCC 24-0625). (a) Front and reverse of colony on PDA; (b,c) sporodochia formed on the carnation leaf; (d) lateral monophialides on aerial mycelium; (e–j) monophialides and conidia formed on sporodochia; (k–n) sporodochial conidia; (o) microcyclic conidiation. Scale bars: (d) = 20 μm; (e–o) = 10 μm.
Index Fungorum Number: IF847026
Saprobic on Oryza sativa. Conidiophores borne on sporodochia, verticillately branched, proliferating percurrently, smooth- and thin-walled, hyaline. Conidiogenous cells 8–17.5 × 3–3.5 μm ( = 11 × 3.5 μm, n = 20), monophialidic, subulate to subcylindrical, smooth- and thin-walled, hyaline. Sporodochial conidia 18–46.5 × 3–5 μm ( = 26 × 4 μm, n = 30), straight to slightly curved, falcate, apical cell blunt and sometimes papillate, basal cell barely notched, mostly 1–4-septate, hyaline. Sporodochia pale orange, formed on the surface of carnation leaves. Chlamydospores not observed.
Culture characteristics: Colonies on PDA reaching 86–90 mm diameter after a week at 28 °C, yellowish white, velvety to floccose, slightly convex, dense, entire margin. Reverse yellowish white with pale orange in the center.
Material examined: Thailand, Chiang Rai Province, Phan District, Mueang Phan Sub-district (19.528477° N, 99.74594° E), on the seedling of Oryza sativa, 26 November 2021, Nootjarin Jungkhun, (NS08-2a = MFLU 25-0031); (living culture MFLUCC 24-0625).
GenBank numbers: cmdA = PV297814, rpb2 = PV394829, tef1-α = PV394834.
Notes: According to the results of phylogenetic analyses, the isolate MFLUCC 24-0625 is closely related to Fusarium mianyangense, with 90% ML and 1.00 PP support (Figure 3). Morphologically, our isolate is similar to the ex-type strain in the size and shape of conidiogenous cells and conidia, with the exception of the chlamydospores, which were not observed in our study. Consequently, based on both morphological and phylogenetic evidence, the isolate MFLUCC 24-0625 was identified as F. mianyangense.
Fusarium oryzigenum S. Absalan, S. Lumyong & K. D. Hyde, sp. nov. (Figure 14)
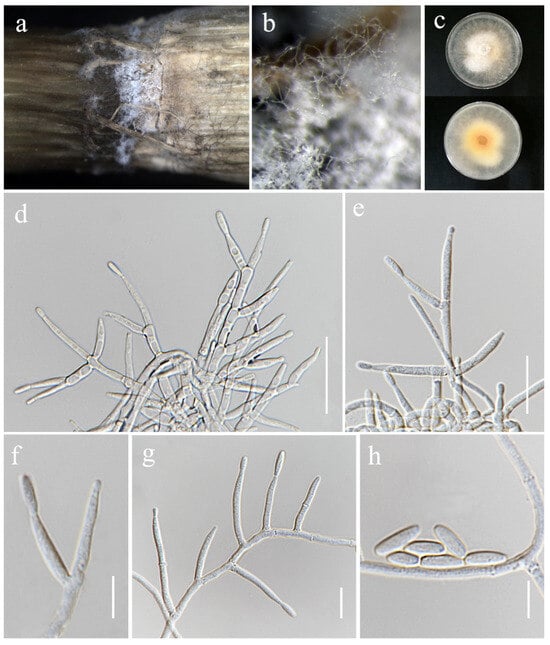
Figure 14.
Fusarium oryzigenum (MFLU 25-0039, holotype). (a,b) Appearance of mycelia on the stem of Oryza sativa; (c) front and reverse of colony on PDA; (d–g) conidiophores and conidiogenous cells; (h) aerial conidia. Scale bars: (d–h) = 10 μm.
Index Fungorum Number: IF903756
Etymology: Name refers to the host genus Oryza, from which it was isolated.
Saprobic on Oryza sativa. Conidiophores borne on aerial mycelia, septate, proliferating percurrently, bearing terminal or lateral phialides, irregularly and verticillately branched, smooth- and thin-walled, hyaline. Conidiogenous cells 15–27 × 2.5–3.5 μm ( = 20 × 3 μm, n = 20), monophialidic, subulate to subcylindrical, periclinal thickening inconspicuous, smooth- and thin-walled, hyaline. Aerial conidia 6–10 × 2.5–3.5 μm ( = 8 × 3 μm, n = 20), ellipsoidal to clavate, usually single on the tips of phialides, aseptate, hyaline. Sporodochia, Chlamydospores and Macroconidia not observed.
Culture characteristics: Colonies on PDA reaching 54–58 mm diameter after a week at 28 °C, white with pale saffron in the center, floccose, radiate, aerial mycelia dense, margin irregular or entire, filiform. Reverse pale orange.
Material examined: Thailand, Chiang Rai Province, Phan District, Mueang Phan Sub-district (19.528477° N, 99.74594° E), on the stem of Oryza sativa, 28 June 2022, Sahar Absalan, (PA159 = MFLU 25-0039); (ex-type, living culture MFLUCC 24-0637).
GenBank numbers: cmdA = PV297815, rpb2 = PV394844, tef1-α = PV394840.
Notes: Our phylogenetic analyses (Figure 1) revealed that Fusarium oryzigenum is located at a distinct branch, forming a well-supported lineage (100% ML and 1.00 PP) from the clade of Fusarium andiyazi. Fusarium oryzigenum is closely related to F. andiyazi (CBS 119857), but it differs by 15 base pairs in the three-locus combined dataset (cmdA+rpb2+tef1-α). Morphologically, this species can be distinguished from F. andiyazi by the absence of macroconidia and chlamydospores (not observed in our collection), the length of monophialides (15–27 × 2.5–3.5 μm in Fusarium oryzigenum vs. 14–42 × 2–3.5 μm in F. andiyazi), and the proliferation of microconidia (borne singly on the tips of phialides in Fusarium oryzigenum vs. borne in chains or false heads in F. andiyazi) [78]. Therefore, herein we introduce Fusarium oryzigenum as a new species, based on morphological examination and phylogenetic analysis.
Fusarium sacchari (E.J. Butler) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 218 (1971). (Figure 15).
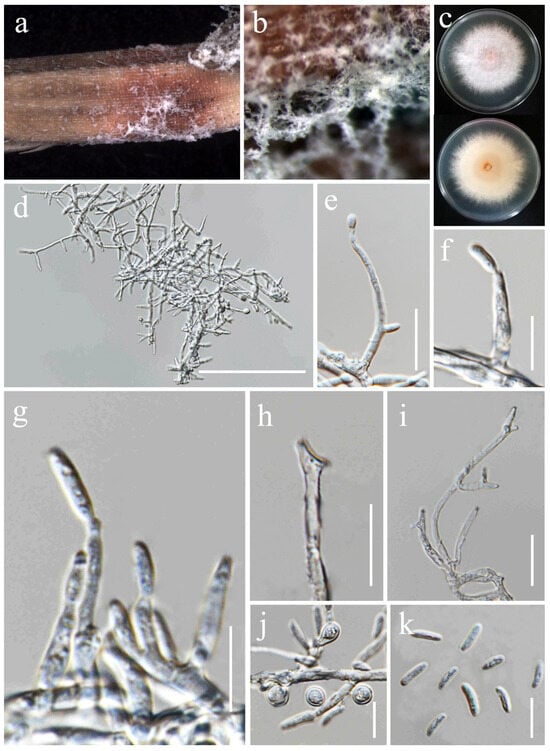
Figure 15.
Fusarium sacchari (MFLU 25-0038). (a,b) Appearance of mycelia on the panicle of Oryza sativa; (c) front and reverse of colony on PDA; (d) conidiophores and conidiogenous cells; (e–g) aerial conidia produced on monophialides; (h,i) polyphialieds; (j) chlamydospores; (k) aerial conidia. Scale bars: (d) = 100 μm; (e,i) = 20 μm; (f–h,j–k) = 10 μm.
Index Fungorum Number: IF314221
Saprobic on Oryza sativa. Conidiophores borne on aerial mycelia, septate, proliferating percurrently, bearing terminal or lateral phialides, irregularly branched, smooth- and thin-walled, hyaline. Conidiogenous cells 6.5–27 × 2.5–4 μm ( = 19 × 3.5 μm, n = 20), mono- and polyphialidic, subulate to subcylindrical, smooth- and thin-walled, hyaline. Aerial conidia 6–10 × 2.5–3 μm ( = 7 × 2.7 μm, n = 30), ovoid to ellipsoid, straight to slightly curved, hyaline. Sporodochia and Macroconidia not observed. Chlamydospores 5.2–7 μm diameter, abundant, subglobose to ovoid, smooth- or rough-walled, terminal or intercalary, solitary, hyaline.
Culture characteristics: Colonies on PDA reaching 80–83 mm diameter after a week at 28 °C, white with pale pink in the center, medium-dense, cottony. Reverse pink-white in the center, white at the margin.
Material examined: Thailand, Chiang Rai Province, Mueang Chiang Rai District, Tha Sut Sub-district (20.060626° N, 99.850604° E), on the panicle of Oryza sativa, 17 December 2021, Sahar Absalan, (TS108a = MFLU 25-0038); (living culture MFLUCC 24-0635).
GenBank numbers: cmdA = N/A, rpb2 = PV394832, tef1-α = PV394841.
Notes: Based on the morphological and molecular data from our study, the isolate MFLUCC 24-0635 was identified as a representative of Fusarium sacchari, with 96% ML and 1.00 PP support (Figure 1). According to previous studies, F. sacchari is one of the most prevalent pathogens affecting a variety of crops globally, including rice, in which it causes bakanae and spikelet rot diseases [79,80,81,82].
Fusarium sulawesiense Maryani, Sand.-Den., L. Lombard, Kema & Crous [as “sulawense”], Persoonia 43: 65 (2019). (Figure 16).
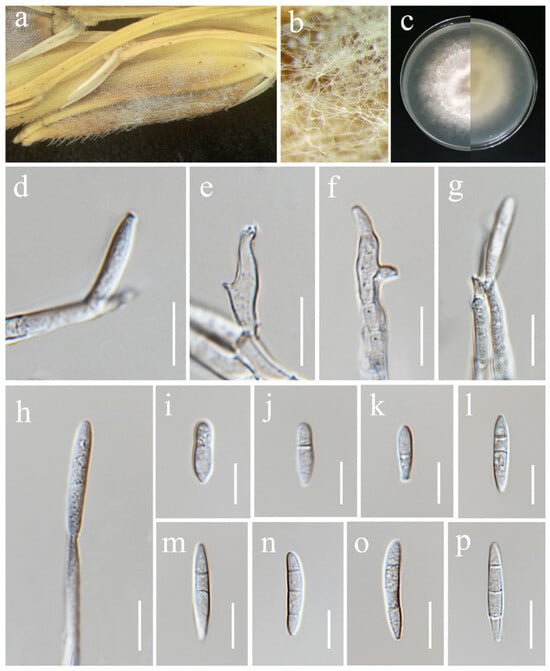
Figure 16.
Fusarium sulawesiense (MFLU 25-0034). (a,b) Appearance of mycelia on the panicle of Oryza sativa; (c) front and reverse of colony on PDA; (d,f) monophialides; (e) polyphialides; (g,h) aerial conidia produced on mono- and polyphialides; (i–p) aerial conidia. Scale bars: (d–p) = 10 μm.
Index Fungorum Number: IF314221
Saprobic on Oryza sativa. Conidiophores borne on aerial mycelia, septate, proliferating percurrently, bearing terminal or lateral phialides, irregularly branched, smooth- and thin-walled, hyaline. Conidiogenous cells 14–20 × 2.5–4 μm ( = 16 × 3 μm, n = 15), mono- and polyphialidic, subulate to subcylindrical, smooth- and thin-walled, hyaline. Conidia 10–28 × 3.5–5 μm ( = 20 × 4.5 μm, n = 20), sometimes obovoid to ellipsoidal, straight to slightly curved, apical cells papillate, basal cells indistinct or foot-shaped, 1–3-septate, hyaline. Sporodochia and Chlamydospores not observed.
Culture characteristics: Colonies on PDA reaching 69–72 mm diameter after a week at 28 °C, pale buff, aerial mycelia medium-dense, immersed mycelia at the margin, radiate, cottony. Reverse pale yellow.
Material examined: Thailand, Chiang Rai Province, Phan District, Mueang Phan Sub-district (19.48468° N, 99.719868° E), on the panicle of Oryza sativa, 9 November 2021, Nootjarin Jungkhun, (NS28-4 = MFLU 25-0034); (living culture MFLUCC 24-0629).
GenBank numbers: cmdA = PV297816, rpb2 = PV394830, tef1-α = PV394835.
Notes: Our isolate MFLUCC 24-0629 clustered with the type (InaCC F940) and other strains of Fusarium sulawesiense, with 89% ML and 0.87 PP support in the phylogenetic analysis of the FIESC (Figure 3). Infected pseudostem of Musa acuminata var. Pisang Cere, collected in Indonesia, was documented as the type substrate of F. sulawesiense [77]. The first association of this species with rice was reported in China by Wang et al. [74]
Ochronectria thailandica Q.J. Shang & K.D. Hyde, Fungal Diversity 78: (84) (2016). (Figure 17).
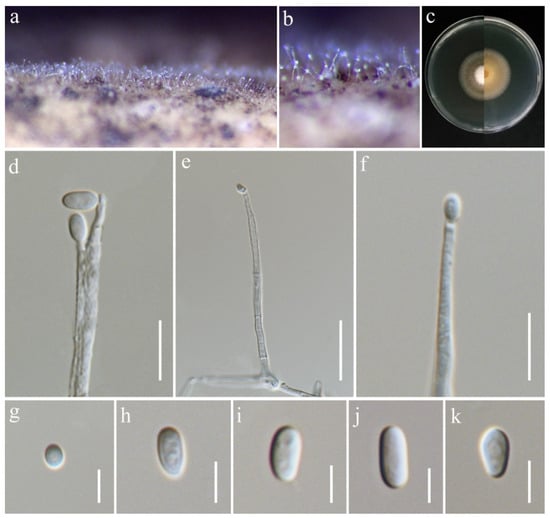
Figure 17.
Ochronectria thailandica (MFLU 25-0042, new host record). (a,b) Appearance of conidiophores on the stem of Oryza sativa; (c) front and reverse of colony on PDA; (d–f) conidiophores; (g–k) conidia. Scale bars: (d,e) = 20 μm; (f) = 10 μm; (g–k) = 5 μm.
Index Fungorum Number: IF551918
Saprobic on Oryza sativa. Conidiophores 36–84 × 2–3.5 μm ( = 63 × 3 μm, n = 15), erect, straight, unbranched, septate, slightly inflated at base, smooth-walled, hyaline. Conidiogenous cells monophialidic, terminally proliferating, subulate or cylindrical, producing a single conidium, smooth-walled, hyaline. Conidia 3.8–7.5 × 3–4 μm ( = 6 × 3.5 μm, n = 25), aseptate, subglobose to ellipsoidal, thin- and smooth-walled, hyaline.
Culture characteristics: Colonies on PDA reaching 40–45 mm diameter after a week at 28 °C, greyish salmon with white center, cottony to felty, aerial mycelia moderate, margin entire. Reverse pale orange with pale greyish white margin.
Material examined: Thailand, Chiang Rai Province, Mueang Chiang Rai District, Huai Sak Sub-district (19.781206° N, 99.921459° E), on the stem of Oryza sativa, 17 December 2021, Sahar Absalan, (HS59-1 = MFLU 25-0042); (living culture MFLUCC 24-0632).
GenBank numbers: ITS = PV243865, LSU = PV243885, rpb2 = PV297809, tef1-α = PV297807.
Notes: Based on the phylogenetic analyses of concatenated ITS, LSU, rpb2, and tef1-α sequence data, our strain (MFLUCC 24-0632) clustered with Ochronectria thailandica, with 95% ML and 1.00 PP support (Figure 4). It is worth noting that previously reported strains of O. thailandica have been identified in their sexual form [56,83]. This study, however, is the first to document its asexual morph. Additionally, it marks the first recorded association of O. thailandica with rice.
Sarocladium bactrocephalum (W. Gams) Summerb., Stud. Mycol. 68: 158 (2011). (Figure 18).
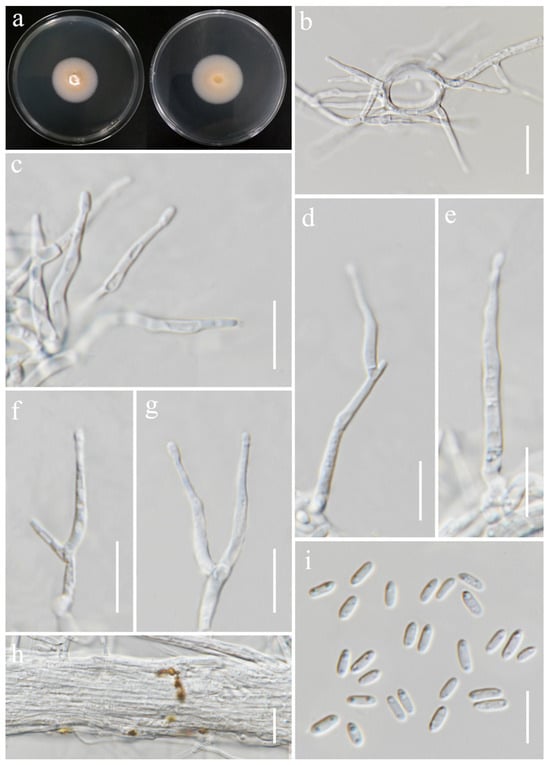
Figure 18.
Sarocladium bactrocephalum (MFLUCC 24-0634, new host record). (a) Front and reverse of colony on PDA; (b) conidiophores radiating out from coils; (c–g) conidiophores; (h) rope of hyphae-containing crystals; (i) conidia. Scale bars: (b–i) = 20 μm.
Index Fungorum Number: IF519590
Saprobic on Oryza sativa. Conidiophores 23–40 × 2–3 μm ( = 36 × 2.5 μm, n = 20), erect, straight to flexuous, sometimes slightly bent, arising from aerial mycelium or radiating out from the mycelial coils, unbranched or basitonously branched, 1–2-septa in base, smooth-walled, hyaline. Phialides 12–28 × 1–2.5 μm ( = 19 × 1.5 μm, n = 20), lateral or terminal, subulate or cylindrical, sometimes constricted at the base, smooth-walled, hyaline. Conidia 4–6.5 × 1.5–2.5 μm ( = 5 × 2 μm, n = 40), aseptate, straight, short cylindrical, rounded at both ends, thin- and smooth-walled, 2-guttulate, hyaline. Crystals present. Chlamydospores not observed.
Culture characteristics: Colonies on PDA reaching 35–37 mm diameter after a week at 28 °C, pale salmon, slow growing, hairy, flat, aerial mycelia sparse, margin entire. Reverse white with pale orange in the center.
Material examined: Thailand, Chiang Rai Province, Mueang Chiang Rai District, Mae Yao Sub-district (19.968012° N, 99.772583° E), on the panicle of Oryza sativa, 17 December 2021, Sahar Absalan, (MS89 = MFLU 25-0041); (living culture MFLUCC 24-0634).
GenBank numbers: ITS = PV243886, LSU = PV243904.
Notes: Based on the results of phylogenetic analyses, our isolate was grouped together with the type strain of Sarocladium bactrocephalum (CBS 749.69), with 99% ML and 1.00 PP support (Figure 5). This species was formerly known as Acremonium bactrocephalum and was later transferred to Sarocladium by Summerbell et al. [40]. According to Gams [84], S. bactrocephalum shares a close relationship with S. strictum but can be differentiated by its distinctively long and narrow conidia. Additionally, it is molecularly distinguishable through LSU sequence analysis. In this study, we provided the first association of this species with rice as a new host record.
Sarocladium oryzae (Sawada) W. Gams & D. Hawksw., Kavaka 3: 58 (1976) [1975]. (Figure 19).
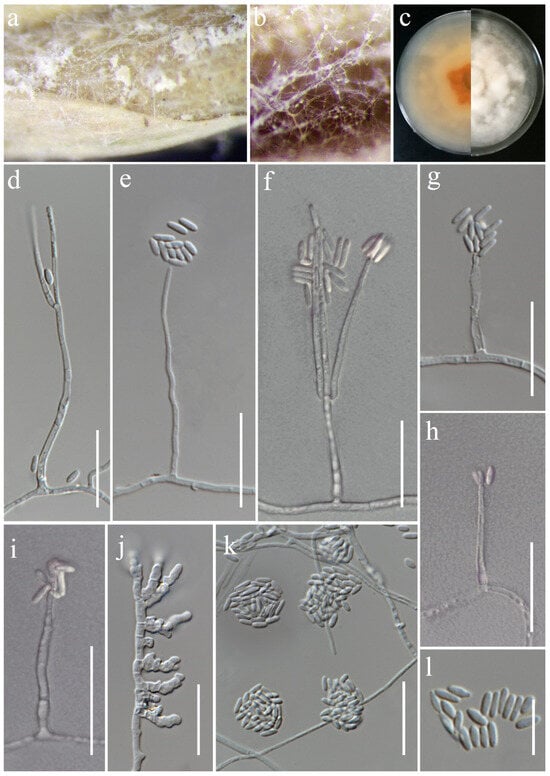
Figure 19.
Sarocladium oryzae (MFLUCC 24-0627). (a,b) Appearance of mycelia on the panicle of Oryza sativa; (c) front and reverse of colony on PDA; (d–i) conidiophores; (j) inflated hyphae; (k) conidia in slimy heads; (l) conidia. Scale bars: (d–l) = 20 μm.
Index Fungorum Number: IF323106
Saprobic on Oryza sativa. Conidiophores 30–80 × 1.5–3 μm ( = 43 × 2 μm, n = 25), erect, straight or irregularly curved and flexuose, arising from aerial mycelium, unbranched or sometimes basitonously or verticillately branched, occasionally warted near the base, 1–2-septa in base or middle, smooth-walled, hyaline. Phialides 20–45 × 1–2 μm ( = 43 × 1.5 μm, n = 30), lateral or terminal, subulate or cylindrical, thick- and smooth-walled, hyaline. Conidia 4.5–7 × 1.5–2.5 μm ( = 5.5 × 2 μm, n = 40), aseptate, straight, cylindrical, with rounded ends, thin- and smooth-walled, arranged in slimy heads, hyaline. Chlamydospores not observed.
Culture characteristics: Colonies on PDA reaching 90 mm diameter after a week at 28 °C, dull white to pale orange, cottony to fluffy, arised, aerial mycelia dense, margin entire. Reverse pale orange with apricot orange in the center.
Material examined: Thailand, Chiang Rai Province, Phan District, Mueang Phan Sub-district (19.528477° N, 99.74594° E), on the panicle of Oryza sativa, 26 November 2021, Nootjarin Jungkhun, (NS02-1 = MFLU 25-0040); (living culture MFLUCC 24-0627).
GenBank numbers: ITS = PV243887, LSU = PV243905.
Notes: Sarocladium oryzae, initially identified as Acrocylindrium oryzae [85], is the type species of Sarocladium. In this study, the strain MFLUCC 24-0627 was identified as Sarocladium oryzae, based on the similarity in the morphological characteristics and phylogenetic analysis (Figure 5).
Trichothecium roseum (Pers.) Link, Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 18 (1809). (Figure 20).
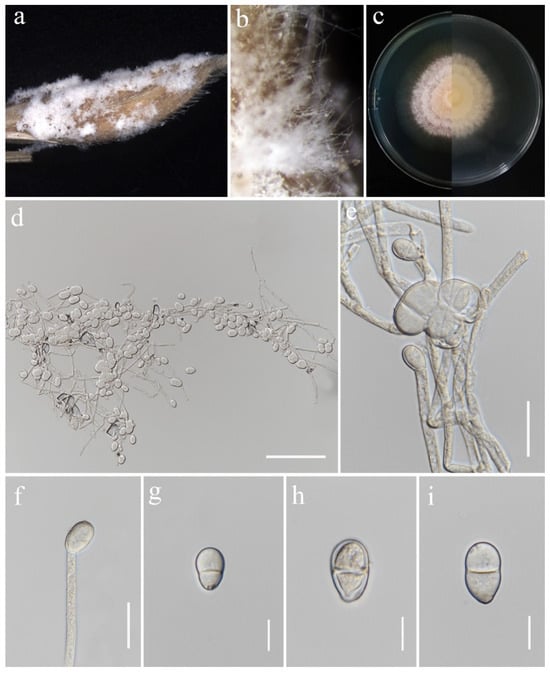
Figure 20.
Trichothecium roseum (MFLU 25-0043). (a,b) Appearance of mycelia on the panicle of Oryza sativa; (c) front and reverse of colony on PDA; (d–f) conidiophores; (g–i) conidia. Scale bars: (d) = 100 μm; (e,f) = 20 μm; (g–i) = 10 μm.
Index Fungorum Number: IF152448
Saprobic on Oryza sativa. Conidiophores 67–117 × 4–5.5 μm ( = 85 × 5 μm, n = 15), erect, straight, occasionally branched, septate, rough-walled, hyaline. Conidiogenous cells monoblastic, terminally proliferating, hyaline. Conidia 10–23.5 × 7–13.5 μm ( = 16.5 × 11 μm, n = 30), initially aseptate, two-celled in mature conidia, sometimes with constricted septa, ellipsoid to pyriform, apical cell rounded, basal cell blunt, smooth-walled, hyaline.
Culture characteristics: Colonies on PDA reaching 64–66 mm diameter after a week at 28 °C, initially white, becoming rose buff, with concentric rings, powdery or granular, flat, margin entire. Reverse pale buff.
Material examined: Thailand, Chiang Rai Province, Mueang Chiang Rai District, Tha Sut Sub-district (20.060626° N, 99.850604° E), on the panicle of Oryza sativa, 17 December 2021, Sahar Absalan, (TS101 = MFLU 25-0043); (living culture MFLUCC 24-0636).
GenBank numbers: ITS = PV243909, tef1-α = PV297805.
Notes: Morphology and molecular analyses confirmed that strain MFLUCC 24-0636 belongs to Trichothecium roseum, with 100% ML and 1.00 PP support (Figure 6). Trichothecium roseum is a significant postharvest pathogen with a global presence, responsible for pink rot and white stain diseases in various fruit crops. This fungus commonly inhabits soil and can become pathogenic under favorable conditions [86,87,88,89,90,91,92,93]. In rice, T. roseum has been reported during storage, and previous studies in Thailand have documented its occurrence in both rice and sorghum [94,95].
Waltergamsia fusidioides (Nicot) L.W. Hou, L. Cai & Crous, in Hou, Giraldo, Groenewald, Rämä, Summerbell, Huang, Cai & Crous, Stud. Mycol. 105: 141 (2023) (Figure 21).
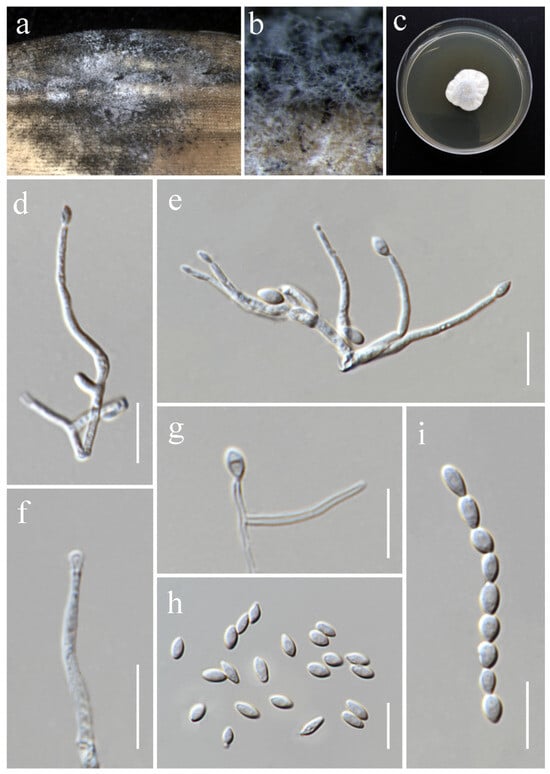
Figure 21.
Waltergamsia fusidioides (MFLU 25-0044, new host and geographical record). (a,b) Appearance of mycelia on the panicle of Oryza sativa; (c) colony on PDA; (d–g) conidiophores; (h,i) conidia. Scale bars: (d–i) = 10 μm.
Index Fungorum Number: IF845886
Saprobic on Oryza sativa. Conidiophores 22–47 × 1.4–2 μm ( = 31 × 1.5 μm, n = 10), solitary, erect, straight or curved, aseptate, usually reduced to single phialides, smooth-walled, hyaline. Phialides lateral or terminal, subulate, smooth-walled, hyaline. Conidia 4.2–6.7 × 2.5–3.5 μm ( = 5.7 × 3 μm, n = 30), aseptate, thin- and smooth-walled, ovoid or fusoid, sometimes arranged in long chains, hyaline.
Culture characteristics: Colonies on PDA reaching 30–33 mm diameter after a week at 28 °C, white, felty, slightly raised, radially folded, margin crenate. Reverse greyish white.
Material examined: Thailand, Chiang Rai Province, Mueang Chiang Rai District, Tha Sut Sub-district (20.060626° N, 99.850604° E), on the panicle of Oryza sativa, 17 December 2021, Sahar Absalan, (M2S224 = MFLU 25-0044); (living culture MFLUCC 24-0638).
GenBank numbers: ITS = PV243954, LSU = PV243955, rpb2 = PV297808, tef1-α = PV297806.
Notes: Based on the molecular data, the strain MFLUCC 24-0638 was identified as Waltergamsia fusidioides, with 100% ML and 1.00 PP support (Figure 7). Waltergamsia fusidioides was recently reclassified by Hou et al. [96] as a new combination for the ex-type culture of Paecilomyces fusidioides, now considered its basionym. Morphological examination revealed that this species typically produces two types of conidia: globose and fusoid [84]. In our study, however, only one type of conidia was observed, which may be attributed to differences in substrate. This study reports the first documentation of W. fusidioides associated with rice and represents its new geographical record for Thailand.
4. Discussion
This study reports on nine Fusarium species along with five species in four other genera from rice. Nine Fusarium species were collected in total, belonging to three species complexes: the Fusarium fujikoroi species complex (FFSC), the Fusarium incarnatum-equiseti species complex (FIESC), and the Fusarium nisikadoi species complex (FNSC). Within the FFSC, three species of Fusarium were identified, including two newly described species (F. chiangraiense and F. oryzigenum) and one known species (F. sacchari). Fusarium sacchari has previously been reported from various grains, including rice (Oryza sativa) and wild rice (Oryza australiensis), in Australia, India, Indonesia, and Malaysia [80,97,98,99,100,101]. Although this species has been isolated from sorghum (Sorghum bicolor) and orchid (Rhynchostylis gigantea) in Thailand, there has been no previous report of the species associated with rice [102,103].
Within the FIESC, five species were identified, including new host and geographical records. Fusarium guilinense was originally isolated from the leaf of Musa nana in China and was later reported from rice glumes as well [74,104]. Multilocus phylogenetic analysis by Han et al. [104] determined F. bubalinum to be synonymous with F. guilinense, which was first reported by Xia et al. [75] from an unknown host. Although Han et al. [104] did not record F. guilinense as a pathogen on rice, Pramunadipta et al. [101] reported that F. guilinense (synonym: F. bubalinum) causes sheath rot in rice. In the present study, we isolated this fungus as a saprobe from a dead rice panicle, representing a new geographical record for Thailand. Fusarium hainanense also represents a new geographical record for Thailand. This species has previously been found on various hosts, including Acacia sp., Musa acuminata, Musa nana, Oryza australiensis, Oryza sativa, and Zea mays [74,75,101,104]. Fusarium hainanense is known to cause two diseases in rice: sheath rot and spikelet rot [101,104]. Similarly, F. mianyangense, originally isolated from rice in China, has been documented to cause rice spikelet rot [104]. Fusarium sulawesiense is a fungus with a wide range of hosts and substrates, including plants and humans [74,75,77,104,105,106,107]. Its association with rice has been recorded in China, India, Indonesia, and Brazil [74,101,108], and it is confirmed as a causal agent of rice sheath rot disease [101]. In a recent study, both F. mianyangense and F. sulawesiense were isolated in Thailand as causal agents of fruit rot in muskmelon (Cucumis melo) [109]; thus, the current study is the second report of these species in Thailand. Another species of Fusarium within the FIESC identified in this study is Fusarium kotabaruense, previously reported from a single host, Musa sp. [77]. This study marks the second global record of the species and its first association with rice.
Fusarium commune was initially isolated from soil and peas in Denmark, as well as from a variety of other substrates, such as white pine, Douglas fir, carnation, corn, carrot, and barley, in multiple countries across the northern hemisphere [110]. Morphologically, F. commune closely resembles F. oxysporum, leading to its misidentification in earlier studies. It was later classified as a new species within the FNSC [110,111]. Fusarium commune is known as a pathogen that causes fusarium wilt and root rot in various plants, such as soybean, sugarcane, horseradish, and tomato [112,113,114,115]. This species has also been reported from rice in Bangladesh and Korea, where it was associated with diseases such as wilt, root rot, and bakanae [116,117]. However, in this study, it was isolated as a saprobic fungus.
Many Sarocladium species are commonly associated with poaceous hosts, particularly rice [42,118,119]. Among them, S. oryzae, the type species of the genus, has been widely reported as the causal agent of sheath rot in rice in various countries [44,119,120,121,122]. In Thailand, several studies have documented the association between S. oryzae and rice in different regions [54,123,124]. This study further contributes to the documentation of S. oryzae occurrence in rice in the northern part of Thailand. Sarocladium bactrocephalum has previously been isolated from Ustilago sp., Brachiaria ruziziensis, Panicum maximum, and the human eye [118,125]. In this study, we report it as a new host record from rice and a new geographical record for Thailand.
Ochronectria is a small genus within the Hypocreales, comprising only three recognized species, all previously known solely from their sexual morphs [26,126]. These fungi typically exhibit a Nectria-like sexual morph with perithecial ascomata and an Acremonium-like asexual morph [96]. In this study, we report the asexual morph of Ochronectria thailandica for the first time. Until now, this species had only been observed in its sexual form on unidentified submerged wood and coconut substrates [56,83]. Our discovery provides new insight into the life cycle and morphology of this rare genus.
Another new host record documented here is Waltergamsia fusidioides, which was isolated from the dung of antelope, decaying leaf of Canna indica, and an unknown substrate in the Central African Republic, Italy, and France, respectively [96].
This study expands the database of fungi associated with rice, providing valuable insights for future research on plant health, disease management, and agricultural biosecurity. The identification of Fusarium spp. and other species as saprobes in dead rice tissues raises important questions about their ecological roles and potential risks to rice cultivation in Thailand. Given that many Fusarium species are well-known pathogens, their presence as saprobes suggests that, under favorable conditions, they could transition to a pathogenic lifestyle, posing a threat to rice production [127,128]. Future research should focus on monitoring these fungal species across different growth stages of rice to determine their potential pathogenicity. Genome-based studies could provide insights into their virulence factors, while controlled pathogenicity tests would help assess their ability to infect living plants [129]. Additionally, understanding environmental triggers that influence the shift from saprobic to pathogenic phases will be crucial in developing early detection strategies and preventive measures [130,131].
These findings highlight the importance of quarantine regulations and disease management strategies [132,133]. By enhancing our knowledge of fungal diversity in rice ecosystems, this research contributes to the development of protective measures against emerging pathogens, ensuring the sustainability of rice cultivation in Thailand.
Author Contributions
Conceptualization, S.A., R.S.J. and S.L.; methodology, S.A., A.A. and N.J.G.d.F.; software, S.A.; formal analysis, S.A. and A.A.; writing—original draft preparation, S.A.; writing—review and editing, A.A., N.S., J.M., N.J.G.d.F., R.S.J., K.D.H. and S.L.; supervision, S.L., R.S.J. and K.D.H.; validation, A.A., N.S., J.M., N.J.G.d.F., R.S.J., K.D.H. and S.L.; funding acquisition, S.L. and R.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Council of Thailand (NRCT) grant “Biodiversity, taxonomy, phylogeny and evolution of Colletotrichum in northern Thailand” (grant no. NRCT-TRG010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequence data are available in NCBI GenBank following the accession numbers in the manuscript.
Acknowledgments
Sahar Absalan is grateful for the Chiang Mai University Presidential Scholarship. The authors express their gratitude to Shaun Pennycook for his correction of Latin names for novelties. This research was partially supported by Chiang Mai University, Thailand. Sahar Absalan and Nootjarin Jungkhun Gomes de Farias would like to thank Nipaporn Wangmanow for her kind help in the sample collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Juliano, B.O. Rice in Human Nutrition; FAO Food and Nutrition Series, No.26; The International Rice Research Institute (IRRI): Los Baños, Philippines, 1993; pp. 43–44. [Google Scholar]
- Zeigler, R.S.; Barclay, A. The relevance of rice. Rice 2008, 1, 3–10. [Google Scholar] [CrossRef]
- Johnston, D.B. Rice cultivation in Thailand: The development of an export economy by indigenous capital and labor. Mod. Asian Stud. 1981, 15, 107–126. [Google Scholar] [CrossRef]
- Poonyarat, C. Development-Thailand: Rice Culture Withering Away, Warn Experts. 2003. Available online: http://www.ipsnews.net/2003/01/development-thailand-rice-culture-withering-away-warn-experts/ (accessed on 15 September 2024).
- Kukusamude, C.; Sricharoen, P.; Limchoowong, N.; Kongsri, S. Heavy metals and probabilistic risk assessment via rice consumption in Thailand. Food Chem. 2021, 334, 127402. [Google Scholar] [CrossRef] [PubMed]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef]
- Naik, B.S.; Shashikala, J.; Krishnamurthy, Y.L.A. Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiol. Res. 2009, 164, 290–296. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, G.; Park, D.S.; Yang, Y. Inoculation and scoring methods for rice sheath blight disease. Methods Mol. Biol. 2013, 956, 257–268. [Google Scholar] [CrossRef]
- Laha, G.S.; Singh, R.; Ladhalakshmi, D.; Sunder, S.; Prasad, M.S.; Dagar, C.S.; Babu, V.R. Importance and management of rice diseases: A global perspective. In Rice Production Worldwide; Springer: Cham, Switzerland, 2017; pp. 303–360. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Huhndorf, S.M. Outline of Ascomycota-2007. Myconet 2007, 13, 1–58. [Google Scholar]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Hyde, K.D.; Noorabadi, M.T.; Thiyagaraja, V.; He, M.Q.; Johnston, P.R.; Wijesinghe, S.N.; Armand, A.; Biketova, A.Y.; Chethana, K.W.T.; Erdoğdu, M.; et al. The 2024 Outline of Fungi and fungus-like taxa. Mycosphere 2024, 15, 5146–6239. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Blackwell, M. Molecular systematics of unitunicate perithecial ascomycetes: The Clavicipitales-Hypocreales connection. Mycologia 1993, 85, 912–922. [Google Scholar] [CrossRef]
- Glenn, A.E.; Bacon, C.W.; Price, R.; Hanlin, R.T. Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 1996, 88, 369–383. [Google Scholar] [CrossRef]
- Sung, G.-H.; Hywel-Jones, N.L.; Sung, J.-M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Schumacher, R.K.; Wingfield, M.J.; Lombard, L.; Giraldo, A.; Christensen, M.; Gardiennet, A.; Nakashima, C.; Pereira, O.L.; Smith, A.; et al. Fungal systematics and evolution: FUSE 1. Sydowia 2015, 67, 118. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Le Roux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.J.; Heykoop, M.; et al. Fungal planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef]
- Lombard, L.; Houbraken, J.; Decock, C.; Samson, R.A.; Meijer, M.; Réblová, M.; Groenewald, J.Z.; Crous, P.W. Generic hyper-diversity in Stachybotriaceae. Persoonia 2016, 36, 156–246. [Google Scholar] [CrossRef]
- Aiello, D.; Polizzi, G.; Crous, P.W.; Lombard, L. Pleiocarpon gen. nov. and a new species of Ilyonectria causing basal rot of Strelitzia reginae in Italy. IMA Fungus 2017, 8, 65–76. [Google Scholar] [CrossRef]
- González, C.D.; Chaverri, P. Corinectria, a new genus to accommodate Neonectria fuckeliana and C. constricta sp. nov. from Pinus radiata in Chile. Mycol. Prog. 2017, 16, 1015–1027. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J. Clonostachys spinulosispora (Hypocreales, Bionectriaceae), a new species on palm from French Guiana. Ascomycete.org 2018, 10, 127–130. [Google Scholar]
- Perera, R.H.; Hyde, K.D.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Bundhun, D.; Camporesi, E.; Akulov, A.; Liu, J.K.; Liu, Z.Y. Profile of Bionectriaceae, Calcarisporiaceae, Hypocreaceae, Nectriaceae, Tilachlidiaceae, Ijuhyaceae fam. nov., Stromatonectriaceae fam. nov. and Xanthonectriaceae fam. nov. Fungal Divers. 2023, 118, 95–271. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, C.; Nie, L.T.; Maharachchikumbura, S.S.; Crous, P.W.; Hyde, K.D.; Xiang, M.M.; Al-Otibi, F.; Manawasinghe, I.S. Identification and characterization of Albonectria, Fusarium, and Neocosmospora species associated with ornamental plants in Southern China. Mycosphere 2024, 15, 6641–6717. [Google Scholar] [CrossRef]
- Rossman, A.Y. Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 1996, 88, 1–19. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Samuels, G.J.; Rogerson, C.T.; Lowen, R. Genera of Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales, Ascomycetes). Stud. Mycol. 1999, 42, 1–248. [Google Scholar]
- Kamata, N.; Sato, H.; Shimazu, M. Seasonal changes in the infection of pupae of the beech caterpillar, Quadricalcarifera punctatella (Motsch.) (Lep., Notodontidae), by Cordyceps militaris Link (Clavicipitales, Clavicipitaceae) in the soil of the Japanese beech forest. J. Appl. Entomol. 1997, 121, 17–21. [Google Scholar] [CrossRef]
- Kamata, N. Population dynamics of the beech caterpillar, Syntypistis punctatella, and biotic and abiotic factors. Popul. Ecol. 2000, 42, 267–278. [Google Scholar] [CrossRef]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Chapter VII—Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 189–253. [Google Scholar]
- Garrido-Jurado, I.; Fernandez-Bravo, M.; Campos, C.; Quesada-Moraga, E. Diversity of entomopathogenic Hypocreales in soil and phylloplanes of five Mediterranean cropping systems. J. Invertebr. Pathol. 2015, 130, 97–106. [Google Scholar] [CrossRef]
- Muthumeenakshi, S.; Mills, P.R.; Brown, A.E.; Seaby, D.A. Intraspecific molecular variation among Trichoderma harzianum isolates colonizing mushroom compost in the British Isles. Microbiology 1994, 140, 769–777. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef]
- Savoie, J.; Iapicco, R.; Largeteau-Mamoun, M. Factors influencing the competitive saprophytic ability of Trichoderma harzianum Th2 in mushroom (Agaricus bisporus) compost. Mycol. Res. 2001, 105, 1348–1356. [Google Scholar] [CrossRef]
- Tedersoo, L.; Partel, K.; Jairus, T.; Gates, G. Ascomycetes associated with ectomycorrhizas: Molecular diversity and ecology with particular reference to the Helotiales. Environ. Microbiol. 2009, 11, 3166–3178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cantero, A.; Guarro, J. Sarocladium and Acremonium infections: New faces of an old opportunistic fungus. Mycoses 2020, 63, 1203–1214. [Google Scholar] [CrossRef]
- Allaga, H.; Zhumakayev, A.; Buchner, R.; Kocsube, S.; Szucs, A.; Vagvolgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum species complex with mushroom pathogenic potential. Agronomy 2021, 11, 2434. [Google Scholar] [CrossRef]
- Isaka, M.; Kittakoop, P.; Thebtaranonth, Y. Secondary metabolites of Clavicipitalean fungi. In Clavicipitalean Fungi, 1st ed.; White, J.F., Bacon, C.W., Hywel-Jones, N.L., Spatafora, J.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 355–397. [Google Scholar]
- Summerbell, R.C.; Gueidan, C.; Schroers, H.J.; de Hoog, G.S.; Starink, M.; Rosete, A.Y.; Guarro, J.; Scott, J.A. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud. Mycol. 2011, 68, 139–162. [Google Scholar] [CrossRef]
- Zheng, P.; Xia, Y.; Xiao, G.; Xiong, C.; Hu, X.; Zhanh, S.; Zheng, H.; Huang, Y.; Zhou, Y.; Wang, S.; et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2012, 12, R116. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Fu, X. The pathogen of rice purple sheath disease Sarocladium synense sp. nov. Acta Mycol. Sin. Suppl. 1986, 1, 318–327. [Google Scholar]
- Pearce, D.A.; Bridge, P.D.; Hawksworth, D.L. Species concepts in Sarocladium, the causal agent of sheath rot in rice and bamboo blight. In Major Fungal Diseases of Rice: Recent Advances; Sreenivasaprasad, S., Johnson, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 285–292. [Google Scholar] [CrossRef]
- Ayyadurai, N.; Kirubakaran, S.I.; Srisha, S.; Sakthivel, N. Biological and molecular variability of Sarocladium oryzae, the sheath rot pathogen of rice (Oryza sativa L.). Curr. Microbiol. 2005, 50, 319–323. [Google Scholar] [CrossRef]
- Lee, J.; Chang, I.; Kim, H.; Yun, S.; Leslie, J.F.; Lee, Y. Genetic diversity and fitness of Fusarium graminearum populations from rice in Korea. Appl. Environ. Microbiol. 2009, 84, 3285–3295. [Google Scholar] [CrossRef]
- Amatulli, M.T.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol. 2010, 59, 839–844. [Google Scholar] [CrossRef]
- Wulff, E.G.; Sørensen, J.L.; Lübeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusarium spp. associated with rice Bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L.; Yaakop, A.S.; Salleh, B.; Zakaria, M. Endophytic fungi from paddy. Trop. Life. Sci. Res. 2010, 21, 101–107. [Google Scholar] [PubMed]
- Suada, I.K.; Suhartini, D.M.W.Y.; Sunariasih, N.P.L.; Wirawan, I.G.P.; Chun, K.W.; Cha, J.Y.; Ohga, S. Ability of endophytic fungi isolated from rice to inhibit Pyricularia oryzae–induced rice blast in Indonesia. J. Fac. Agric. Kyushu Univ. 2012, 57, 51–53. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Aggarwal, R.; Banerjee, S.; Gupta, S.; Sharma, S. Pathogenicity, ecology and genetic diversity of the Fusarium spp. associated with an emerging bakanae disease of rice (Oryza sativa L.) in India. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R., Eds.; Springer: New Delhi, India, 2014; pp. 307–314. [Google Scholar]
- Atugala, D.; Deshappriya, N. Effect of Endophytic fungi on plant growth and blast disease incidence of two traditional rice varieties. J. Natl. Sci. Found. Sri Lanka 2015, 43, 173. [Google Scholar] [CrossRef]
- Leewijit, T.; Pongnak, W.; Soytong, K.; Poeaim, S. Isolation of soil and endophytic fungi from rice (Oryza sativa L.). Int. J. Agric. Technol. 2016, 12, 2191–2202. [Google Scholar]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Seephueak, P.; Preecha, C.; Seephueak, W. The diversity of fungi associated with rice (Oryza sativa L.) from Nakhon Si Thammarat, Thailand. Int. J. Agric. Technol. 2019, 15, 485–500. [Google Scholar]
- Roy, S.; Mili, C.; Talukdar, R.; Wary, S.; Tayung, K. Seed borne endophytic fungi associated with some indigenous rice varieties of North East India and their growth promotion and antifungal potential. Indian J. Agric. Res. 2021, 55, 603–608. [Google Scholar] [CrossRef]
- Tian, X.G.; Bao, D.F.; Karunarathna, S.C.; Jayawardena, R.S.; Hyde, K.D.; Bhat, D.J.; Luo, Z.L.; Elgorban, A.M.; Hongsanan, S.; Maharachchikumbura, S.S.N.; et al. Taxonomy and phylogeny of ascomycetes associated with selected economically important monocotyledons in China and Thailand. Mycosphere 2024, 15, 1–274. [Google Scholar] [CrossRef]
- Senanayake, I.; Rathnayaka, A.; Marasinghe, D.; Calabon, M.; Gentekaki, E.; Lee, H.; Hurdeal, V.; Pem, D.; Dissanayake, S.; Wijesinghe, S.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Fisher, N.L.; Burgess, L.; Toussoun, T.; Nelson, P.E. Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 1982, 72, 151–153. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Crytococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Tacke, B.K.; Casper, H.C. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. USA 2000, 97, 7905–7910. [Google Scholar] [CrossRef]
- Dissanayake, L.S.; Wijayawardene, N.N.; Samarakoon, M.C.; Hyde, K.D.; Kang, J.-C. The taxonomy and phylogeny of Austropleospora ochracea sp. nov. (Didymosphaeriaceae) from Guizhou, China. Phytotaxa 2021, 491, 217–229. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinf. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Glez-Peña, D.; Gómez-Blanco, D.; Reboiro-Jato, M.; Fdez-Riverola, F.; Posada, D. ALTER, program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010, 38, 14–18. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree: Tree Figure Drawing Tool, Version 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Rheeder, J.P.; Marasas, W.F.O.; Nelson, P.E. Fusarium globosum, a new species from corn in southern Africa. Mycologia 1996, 88, 509–513. [Google Scholar] [CrossRef]
- Aoki, T.; Nirenberg, H.I. Fusarium globosum from subtropical Japan and the effect of different light conditions on its conidiogenesis. Mycoscience 1999, 40, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Q.; Diao, Y.; Duan, W.; Cai, L. Fusarium incarnatum-equiseti Complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names—Restyling the Fusarium incarnatum-equiseti species complex. Persoonia 2019, 43, 186–221. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 1–184. [Google Scholar] [CrossRef]
- Maryani, N.; Sandoval-Denis, M.; Lombard, L.; Crous, P.W.; Kema, G.H.J. New endemic Fusarium species hitch-hiking with pathogenic Fusarium strains causing Panama disease in small-holder banana plots in Indonesia. Persoonia 2019, 43, 48–69. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Rheeder, J.P.; Lamprecht, S.C.; Zeller, K.A.; Leslie, J.F. Fusarium andiyazi sp. nov., a new species from sorghum. Mycologia 2001, 93, 1203–1210. [Google Scholar] [CrossRef]
- Wang, J.H.; Peng, X.D.; Lin, S.H.; Wu, A.; Huang, S. First report of Fusarium head blight of wheat caused by Fusarium sacchari in China. Plant Dis. 2015, 99, 160. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Aggarwal, R.; Sharma, S.; Gupta, S.; Singh, U.B. Single and combined effects of three Fusarium species associated with rice seeds on the severity of bakanae disease of rice. J. Plant Pathol. 2016, 98, 405–412. [Google Scholar] [CrossRef]
- Duan, C.X.; Du, Q.; Wang, B.B. First report of maize ear rot caused by Fusarium sacchari in China. Plant Dis. 2019, 103, 2674. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. 2024. Available online: https://fungi.ars.usda.gov/ (accessed on 13 November 2024).
- Li, G.J.; Hyde, K.D.; Zhao, R.L.; Hongsanan, S.; Abdel-Aziz, F.A.; Abdel-Wahab, M.A.; Alvarado, P.; Alves-Silva, G.; Ammirati, J.F.; Ariyawansa, H.A.; et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 1–237. [Google Scholar] [CrossRef]
- Gams, W. Cephalosporium-Artige Schimmelpilze (Hyphomycetes); Gustav Fischer: Stuttgart, Germany, 1971; pp. 38–130. [Google Scholar]
- Sawada, K. Descriptive Catalogue of the Formosan Fungi II; Report of the Department of Agriculture; Government Research Institute: Taipei, Taiwan, 1922; pp. 139–164. [Google Scholar]
- Singh, R.; Basu, M. Trichothecium roseum Link on Punica granatum—A new record from India. Indian Phytopathol. 2006, 59, 532. [Google Scholar]
- Bello, D.G. First report of Trichothecium roseum causing postharvest fruit rot of tomato in Argentina. Aust. Plant Dis. Notes 2008, 3, 103–104. [Google Scholar] [CrossRef]
- Tang, Q.; Fang, L.; Liu, Y.J. First report of Trichothecium roseum causing trunk disease of Acer buergerianum in China. Plant Dis. 2015, 99, 1864. [Google Scholar] [CrossRef]
- Xue, C.S.; Xu, J.N.; Sun, J.Y.; Lu, Y.Y.; Li, G.F.; Xiao, S.Q. First report of Trichothecium roseum causing maize (Zea mays) ear rot in Northern China. Plant Dis. 2016, 100, 2324. [Google Scholar] [CrossRef]
- Li, Y.B.; Zhang, Z.P.; Luo, L.X.; Li, J.Q.; Liu, Y.X.; Hao, J.J. First report of Trichothecium roseum causing pink fruit rot of Prunus davidiana in China. Plant Dis. 2020, 104, 2520. [Google Scholar] [CrossRef]
- Ashraf, N.; Bhat, M.Y.; Wani, A.H. First report of foliicolus fungus Trichothecium roseum (Pers.) Link on sweet cherry Prunus avium L. from Kashmir Valley. Braz. J. Biol. Sci. 2020, 7, 225–228. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.; Wang, J.; Wang, X.; Xiang, W.; Zhao, J. First report of Trichothecium roseum causing postharvest fruit rot on purple passion fruit in China. Plant Dis. 2022, 106, 3212. [Google Scholar] [CrossRef]
- Harishchandra, D.L.; Zhang, W.; Wedaralalage, T.C.K. Identification of the postharvest pink mold rot fungus (Trichothecium roseum) on grapes in China. Chiang Mai J. Sci. 2023, 50, e2023040. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D.; Bhudhasamai, K.; Miscamble, B.F.; Wheeler, K.A.; Tanboon-Ek, P. The normal mycoflora of commodities from Thailand. 2. Beans, rice, small grains and other commodities. Int. J. Food Microbiol. 1994, 23, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Surekha, M.; Saini, K.; Reddy, V.K.; Reddy, A.R.; Reddy, S.M. Fungal succession in stored rice (Oryza sativa Linn.) fodder and mycotoxin production. Afr. J. Biotechnol. 2011, 10, 550–555. [Google Scholar]
- Hou, L.; Giraldo, A.; Groenewald, J.Z.; Raemae, T.; Summerbell, R.C.; Huang, G.; Cai, L.; Crous, P.W. Redisposition of acremonium-like fungi in Hypocreales. Stud. Mycol. 2023, 105, 23–203. [Google Scholar] [CrossRef] [PubMed]
- Izzati, N.A.M.; Salleh, B. Variability of Fusarium species associated with Bakanae disease of rice based on their virulence, vegetative and biological compatibilities. Sydowia 2010, 62, 89–104. [Google Scholar]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef]
- Petrovic, T.; Burgess, L.W.; Cowie, I.; Warren, R.A.; Harvey, P.R. Diversity and fertility of Fusarium sacchari from wild rice (Oryza australiensis) in Northern Australia, and pathogenicity tests with wild rice, rice, sorghum and maize. Eur. J. Plant Pathol. 2013, 136, 773–788. [Google Scholar] [CrossRef]
- Pak, D.; You, M.P.; Lanoiselet, V.; Barbetti, M.J. Reservoir of cultivated rice pathogens in wild rice in Australia. Eur. J. Plant Pathol. 2017, 147, 295–311. [Google Scholar] [CrossRef]
- Pramunadipta, S.; Widiastuti, A.; Wibowo, A.; Suga, H.; Priyatmojo, A. Identification and pathogenicity of Fusarium spp. associated with the sheath rot disease of rice (Oryza sativa) in Indonesia. J. Plant Pathol. 2022, 104, 251–267. [Google Scholar] [CrossRef]
- Mohamed Nor, N.M.I.; Salleh, B.; Leslie, J.F. Fusarium species from sorghum in Thailand. Plant Pathol. J. 2019, 35, 301–312. [Google Scholar] [CrossRef]
- Dekham, K.; Kanchanawatee, K. The first report of Fusarium sacchari causing yellow leaf spot disease on Rhynchostylis gigantea orchids in Thailand. Am. J. Agric. Biol. Sci. 2020, 15, 68–74. [Google Scholar] [CrossRef]
- Han, S.L.; Wang, M.M.; Ma, Z.Y.; Raza, M.; Zhao, P.; Liang, J.M.; Gao, M.; Li, Y.J.; Wang, J.W.; Hu, D.M. Fusarium diversity associated with diseased cereals in China, with an updated phylogenomic assessment of the genus. Stud. Mycol. 2023, 104, 87–148. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Gueidan, C.; Crous, P.W.; Geiser, D.M. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J. Clin. Microbiol. 2009, 47, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.; Li, Q.; Huang, S.; Tang, L.; Chen, X.; Guo, T.; Mo, J.; Ma, L. First report of leaf blight caused by Fusarium pernambucanum and Fusarium sulawesiense on plum in Sichuan, China. Plant Dis. 2022, 106, 2759. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.H.; Lian, T.; Su, J.J.; Chen, J. First report of internal black rot on Carica papaya fruit caused by Fusarium sulawesiense in China. Plant Dis. 2022, 106, 319. [Google Scholar] [CrossRef]
- Lima, E.N.; Oster, A.H.; Bordallo, P.N.; Araújo, A.A.C.; Silva, D.E.M.; Lima, C.S. A novel lineage in the Fusarium incarnatum-equiseti species complex is one of the causal agents of fusarium rot on melon fruits in Northeast Brazil. Plant Pathol. 2021, 70, 133–143. [Google Scholar] [CrossRef]
- Suwannarach, N.; Khuna, S.; Thitla, T.; Senwanna, C.; Nuangmek, W.; Kumla, J.; Lumyong, S. Morpho-phylogenetic identification and characterization of new causal agents of Fusarium species for postharvest fruit rot disease of muskmelon in northern Thailand and their sensitivity to fungicides. Front. Plant Sci. 2024, 15, 1459759. [Google Scholar] [CrossRef]
- Skovgaard, K.; Rosendahl, S.; O’Donnell, K.; Nirenberg, H.I. Fusarium commune is a new species identified by morphological and molecular phylogenetic data. Mycologia 2003, 95, 630–636. [Google Scholar] [CrossRef]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef]
- Hamini-Kadar, N.; Edel-Hermann, V.; Gautheron, N.; Steinberg, C. First report of Fusarium commune and Fusarium redolens causing crown and root rot on tomato in Algeria. New Dis. Rep. 2010, 22, 3. [Google Scholar] [CrossRef]
- Ellis, M.L.; Diaz Arias, M.M.; Cruz Jimenez, D.R.; Munkvold, G.P.; Leandro, L.F. First report of Fusarium commune causing damping-off, seed rot, and seedling root rot on soybean (Glycine max) in the United States. Plant Dis. 2013, 97, 284. [Google Scholar] [CrossRef]
- Yu, J.; Babadoost, M. Occurrence of Fusarium commune and F. oxysporum in horseradish roots. Plant Dis. 2013, 97, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chai, Z.; Bao, Y.; Wang, H.; Li, Y.; Rao, G.P.; Zhang, M.Q. First report of Fusarium commune causing root rot disease of sugarcane (var. Badila) in China. Plant Dis. 2018, 102, 1660. [Google Scholar] [CrossRef]
- Choi, H.-W.; Hong, S.; Lee, Y.K.; Kim, W.G.; Chun, S. Taxonomy of Fusarium fujikuroi species complex associated with bakanae on rice in Korea. Australas. Plant Pathol. 2018, 47, 23–34. [Google Scholar] [CrossRef]
- Husna, A.; Zakaria, L.; Nor, N.M.I.M. Fusarium commune associated with wilt and root rot disease in rice. Plant Pathol. 2020, 70, 123–132. [Google Scholar] [CrossRef]
- Giraldo, A.; Gené, J.; Sutton, D.; Madrid, H.; De Hoog, G.; Cano, J.; Decock, C.; Crous, P.W.; Guarro, J. Phylogeny of Sarocladium (Hypocreales). Persoonia. Mol. Phylogeny Evol. Fungi 2015, 34, 10. [Google Scholar] [CrossRef]
- Ou, J.H.; Lin, G.C.; Chen, C.Y. Sarocladium species associated with rice in Taiwan. Mycol. Prog. 2020, 19, 67–80. [Google Scholar] [CrossRef]
- Jones, M.P.; Jeutong, F.; Tchatchoua, J. A survey of rice diseases in Cameroon. Plant Dis. 1993, 77, 133–136. [Google Scholar] [CrossRef]
- Sakthivel, N.; Amudha, R.; Muthukrishnan, S. Production of phytotoxic metabolites by Sarocladium oryzae. Mycol. Res. 2002, 106, 609–614. [Google Scholar] [CrossRef]
- Lanoiselet, V.; You, M.P.; Li, Y.P.; Wang, C.P.; Shivas, R.G.; Barbetti, M.J. First report of Sarocladium oryzae causing sheath rot on rice (Oryza sativa) in Western Australia. Plant Dis. 2012, 96, 1382. [Google Scholar] [CrossRef]
- Giatgong, P. Host Index of Plant Diseases in Thailand, 2nd ed.; Mycology Branch, Plant Pathology and Microbiology Division, Department of Agriculture and Cooperatives: Bangkok, Thailand, 1980; p. 124. [Google Scholar]
- Unartngam, J.; Kopmoo, N.; Pinruan, U.; Kosawang, C.; Jørgensen, H.J.L. Molecular and morphological identification of Sarocladium species causing sheath rot of rice in Thailand and their division into physiological races. J. Fungi 2024, 10, 535. [Google Scholar] [CrossRef]
- Anjos, R.M.; Moreira, S.I.; Costa, S.S.; Abreu, L.M.; Alves, E.; Cardoso, P.G. Sarocladium graminicola, a new endophytic species from tropical grasses. Mycol. Prog. 2020, 19, 605–614. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: http://www.indexfungorum.org/Names/Names.asp (accessed on 25 November 2024).
- Fisher, P.J.; Petrini, O. Fungal saprobes and pathogens as endophytes of rice (Oryza sativa L.). New Phytol. 1992, 120, 137–143. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; McKenzie, E.H.C.; Hyde, K.D. Are some endophytes of Musa acuminata latent pathogens. Fungal Divers. 2004, 16, 131–140. [Google Scholar]
- Weisberg, A.J.; Grünwald, N.J.; Savory, E.A.; Putnam, M.L.; Chang, J.H. Genomic approaches; to plant-pathogen epidemiology and diagnostics. Annu. Rev. Phytopathol. 2021, 59, 311–332. [Google Scholar] [CrossRef]
- O’Hanlon, R. Fungi in the Environment. In Fungi: Biology and Applications, 3rd ed.; Kavanagh, K., Ed.; John Wiley & Sons, Inc.: Chichester, UK, 2017; pp. 333–353. [Google Scholar] [CrossRef]
- Mortimer, P.; Wanasinghe, D.N.; Mortimer, P.E. The impact of land use change on the diversity and emergence of fungal pathogens. Authorea 2023, 341, 34155158. [Google Scholar] [CrossRef]
- Mew, T.W.; Leung, H.; Savary, S.; Vera Cruz, C.M.; Leach, J.E. Looking ahead in rice disease research and management. Crit. Rev. Plant Sci. 2004, 23, 103–127. [Google Scholar] [CrossRef]
- Savary, S.; Horgan, F.; Willocquet, L.; Heong, K.L. A review of principals for sustainable pest management in rice. Crop Prot. 2012, 32, 54–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).