Clinical Significance and Therapeutic Challenges of Scedosporium spp. and Lomentospora prolificans Isolates in a Single-Center Cohort of Lung Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Laboratory Fungal Isolation and Susceptibility Testing
2.3. Definitions
2.4. Statistical Analysis
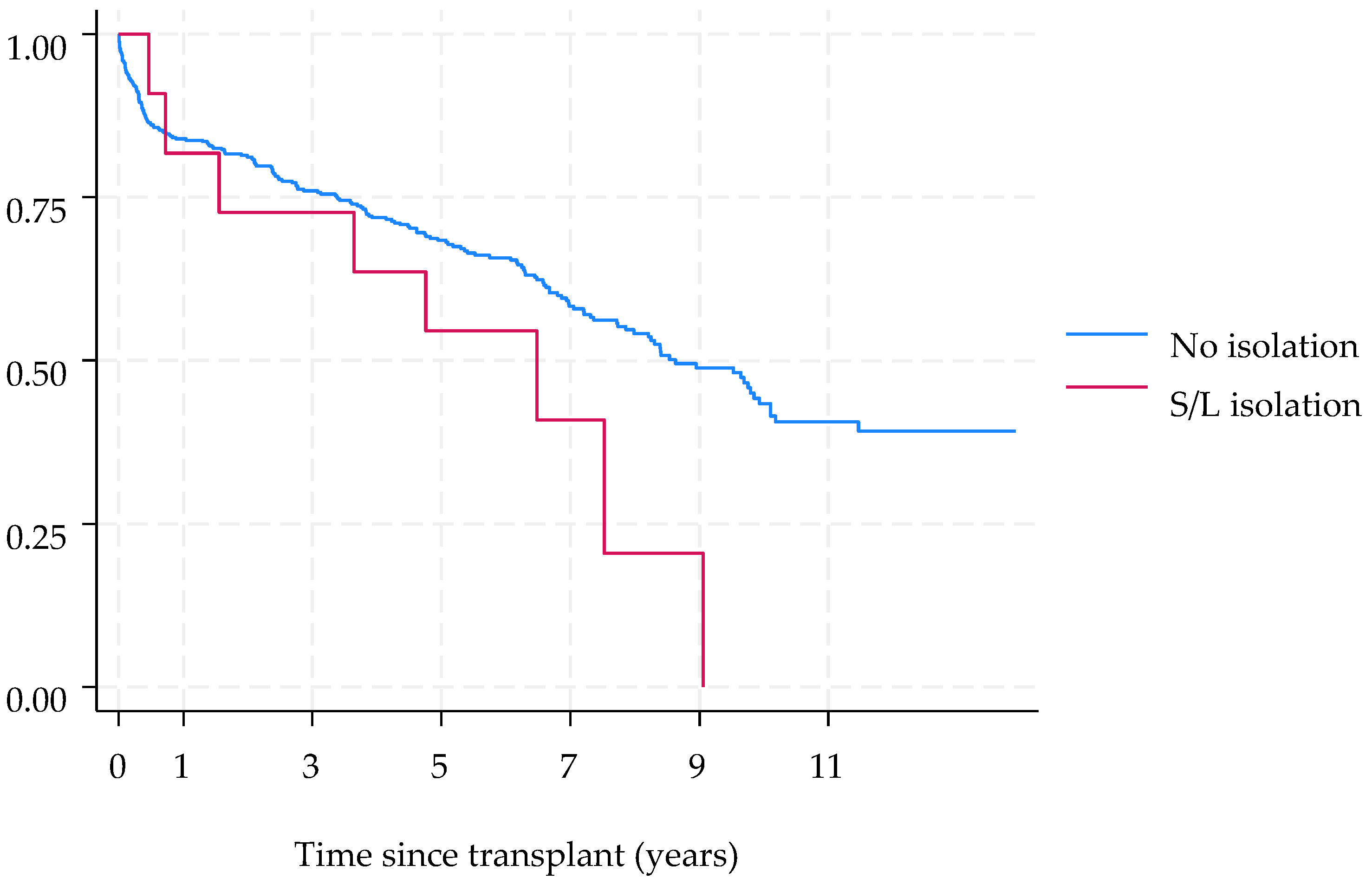
3. Results
3.1. Characteristics of the Study Population
3.2. Microbiological Findings
3.3. Type of Infection and Clinical Presentation
3.4. Antifungal Treatment and Outcomes
3.5. Antifungal Susceptibility Profiles
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samanta, P.; Clancy, C.J.; Nguyen, M.H. Fungal Infections in Lung Transplantation. J. Thorac. Dis. 2021, 13, 6695–6707. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Alcalde, P.; Garcia-Vidal, C. Non-Aspergillus Mould Lung Infections. Eur. Respir. Rev. 2022, 31, 220104. [Google Scholar] [CrossRef] [PubMed]
- Cobo, F.; Lara-Oya, A.; Rodríguez-Granger, J.; Sampedro, A.; Aliaga-Martínez, L.; Navarro-Marí, J.M. Infections Caused by Scedosporium/Lomentospora Species: Clinical and Microbiological Findings in 21 Cases. Med. Mycol. 2018, 56, 917–925. [Google Scholar] [CrossRef]
- Blyth, C.C.; Middleton, P.G.; Harun, A.; Sorrell, T.C.; Meyer, W.; Chen, S.C.-A. Clinical Associations and Prevalence of Scedosporium Spp. in Australian Cystic Fibrosis Patients: Identification of Novel Risk Factors? Med. Mycol. 2010, 48 (Suppl. S1), S37–S44. [Google Scholar] [CrossRef]
- Ravenel, K.; Guegan, H.; Gastebois, A.; Bouchara, J.-P.; Gangneux, J.-P.; Giraud, S. Fungal Colonization of the Airways of Patients with Cystic Fibrosis: The Role of the Environmental Reservoirs. Mycopathologia 2024, 189, 19. [Google Scholar] [CrossRef]
- Husain, S.; Sole, A.; Alexander, B.D.; Aslam, S.; Avery, R.; Benden, C.; Billaud, E.M.; Chambers, D.; Danziger-Isakov, L.; Fedson, S.; et al. The 2015 International Society for Heart and Lung Transplantation Guidelines for the Management of Fungal Infections in Mechanical Circulatory Support and Cardiothoracic Organ Transplant Recipients: Executive Summary. J. Heart Lung Transplant. 2016, 35, 261–282. [Google Scholar] [CrossRef]
- Cortez, K.J.; Roilides, E.; Quiroz-Telles, F.; Meletiadis, J.; Antachopoulos, C.; Knudsen, T.; Buchanan, W.; Milanovich, J.; Sutton, D.A.; Fothergill, A.; et al. Infections Caused by Scedosporium Spp. Clin. Microbiol. Rev. 2008, 21, 157–197. [Google Scholar] [CrossRef]
- Ramirez-Garcia, A.; Pellon, A.; Rementeria, A.; Buldain, I.; Barreto-Bergter, E.; Rollin-Pinheiro, R.; de Meirelles, J.V.; Xisto, M.I.D.S.; Ranque, S.; Havlicek, V.; et al. Scedosporium and Lomentospora: An Updated Overview of Underrated Opportunists. Med. Mycol. 2018, 56, 102–125. [Google Scholar] [CrossRef]
- Konsoula, A.; Tsioutis, C.; Markaki, I.; Papadakis, M.; Agouridis, A.P.; Spernovasilis, N. Lomentospora Prolificans: An Emerging Opportunistic Fungal Pathogen. Microorganisms 2022, 10, 1317. [Google Scholar] [CrossRef]
- Johnson, L.S.; Shields, R.K.; Clancy, C.J. Epidemiology, Clinical Manifestations, and Outcomes of Scedosporium Infections among Solid Organ Transplant Recipients. Transpl. Infect. Dis. 2014, 16, 578–587. [Google Scholar] [CrossRef]
- Huggins, J.P.; Pease, R.; Stanly, K.; Workman, A.; Reynolds, J.; Alexander, B.D. Safety of Inhaled Amphotericin B Lipid Complex as Antifungal Prophylaxis in Lung Transplant Recipients. Antimicrob. Agents Chemother. 2022, 66, e0028322. [Google Scholar] [CrossRef] [PubMed]
- Borro, J.M.; Solé, A.; de la Torre, M.; Pastor, A.; Fernandez, R.; Saura, A.; Delgado, M.; Monte, E.; Gonzalez, D. Efficiency and Safety of Inhaled Amphotericin B Lipid Complex (Abelcet) in the Prophylaxis of Invasive Fungal Infections Following Lung Transplantation. Transplant. Proc. 2008, 40, 3090–3093. [Google Scholar] [CrossRef] [PubMed]
- Monforte, V.; Roman, A.; Gavalda, J.; Bravo, C.; Tenorio, L.; Ferrer, A.; Maestre, J.; Morell, F. Nebulized Amphotericin B Prophylaxis for Aspergillus Infection in Lung Transplantation: Study of Risk Factors. J. Heart Lung Transplant. 2001, 20, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic Lung Allograft Dysfunction: Definition, Diagnostic Criteria, and Approaches to Treatment-A Consensus Report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019, 38, 493–503. [Google Scholar] [CrossRef]
- Seidel, D.; Meißner, A.; Lackner, M.; Piepenbrock, E.; Salmanton-García, J.; Stecher, M.; Mellinghoff, S.; Hamprecht, A.; Durán Graeff, L.; Köhler, P.; et al. Prognostic Factors in 264 Adults with Invasive Scedosporium Spp. and Lomentospora Prolificans Infection Reported in the Literature and FungiScope®. Crit. Rev. Microbiol. 2019, 45, 1–21. [Google Scholar] [CrossRef]
- Vazirani, J.; Westall, G.P.; Snell, G.I.; Morrissey, C.O. Scedosporium Apiospermum and Lomentospora Prolificans in Lung Transplant Patients—A Single Center Experience over 24 Years. Transpl. Infect. Dis. 2021, 23, e13546. [Google Scholar] [CrossRef]
- Ibáñez-Martínez, E.; Solé, A.; Cañada-Martínez, A.; Muñoz-Núñez, C.F.; Pastor, A.; Montull, B.; Falomir-Salcedo, P.; Valentín, A.; López-Hontangas, J.L.; Pemán, J. Invasive Scedosporiosis in Lung Transplant Recipients: A Nine-Year Retrospective Study in a Tertiary Care Hospital. Rev. Iberoam. Micol. 2021, 38, 184–187. [Google Scholar] [CrossRef]
- Rammaert, B.; Puyade, M.; Cornely, O.A.; Seidel, D.; Grossi, P.; Husain, S.; Picard, C.; Lass-Flörl, C.; Manuel, O.; Le Pavec, J.; et al. Perspectives on Scedosporium Species and Lomentospora Prolificans in Lung Transplantation: Results of an International Practice Survey from ESCMID Fungal Infection Study Group and Study Group for Infections in Compromised Hosts, and European Confederation of Medical Mycology. Transpl. Infect. Dis. 2019, 21, e13141. [Google Scholar] [CrossRef]
- Peghin, M.; Monforte, V.; Martin-Gomez, M.T.; Ruiz-Camps, I.; Berastegui, C.; Saez, B.; Riera, J.; Solé, J.; Gavaldá, J.; Roman, A. Epidemiology of Invasive Respiratory Disease Caused by Emerging Non-Aspergillus Molds in Lung Transplant Recipients. Transpl. Infect. Dis. 2016, 18, 70–78. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Berenguer, J.; Guarro, J.; Kantarcioglu, A.S.; Horre, R.; de Hoog, G.S.; Cuenca-Estrella, M. Epidemiology and Outcome of Scedosporium Prolificans Infection, a Review of 162 Cases. Med. Mycol. 2009, 47, 359–370. [Google Scholar] [CrossRef]
- Konsoula, A.; Agouridis, A.P.; Markaki, L.; Tsioutis, C.; Spernovasilis, N. Lomentospora Prolificans Disseminated Infections: A Systematic Review of Reported Cases. Pathogens 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.F.; Chen, S.C.A.; Crowe, A.; Hamilton, K.; Nguyen, Q.A.; Marriott, D.; Trubiano, J.A.; Spelman, T.; Kong, D.C.M.; Slavin, M.A. Invasive Scedosporium and Lomentospora Prolificans Infections in Australia: A Multicenter Retrospective Cohort Study. Open Forum Infect. Dis. 2023, 10, ofad059. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.-D.; Lackner, M.; et al. ESCMID and ECMM Joint Guidelines on Diagnosis and Management of Hyalohyphomycosis: Fusarium Spp., Scedosporium Spp. and Others. Clin. Microbiol. Infect. 2014, 20 (Suppl. S3), 27–46. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Lackner, M.; Sprute, R.; Al-Hatmi, A.M.S.; Bassetti, M.; et al. Global Guideline for the Diagnosis and Management of Rare Mould Infections: An Initiative of the European Confederation of Medical Mycology in Cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, 21, e246–e257. [Google Scholar] [CrossRef]
- Bupha-Intr, O.; Butters, C.; Reynolds, G.; Kennedy, K.; Meyer, W.; Patil, S.; Bryant, P.; Morrissey, C.O.; Australasian Antifungal Guidelines Steering Committee. Consensus Guidelines for the Diagnosis and Management of Invasive Fungal Disease Due to Moulds Other than Aspergillus in the Haematology/Oncology Setting, 2021. Intern. Med. J. 2021, 51 (Suppl. S7), 177–219. [Google Scholar] [CrossRef]
- Lackner, M.; de Hoog, G.S.; Verweij, P.E.; Najafzadeh, M.J.; Curfs-Breuker, I.; Klaassen, C.H.; Meis, J.F. Species-Specific Antifungal Susceptibility Patterns of Scedosporium and Pseudallescheria Species. Antimicrob. Agents Chemother. 2012, 56, 2635–2642. [Google Scholar] [CrossRef]
- Lackner, M.; Rezusta, A.; Villuendas, M.C.; Palacian, M.P.; Meis, J.F.; Klaassen, C.H. Infection and Colonisation Due to Scedosporium in Northern Spain. An in Vitro Antifungal Susceptibility and Molecular Epidemiology Study of 60 Isolates. Mycoses 2011, 54 (Suppl. S3), 12–21. [Google Scholar] [CrossRef]
- Boutin, C.-A.; Luong, M.-L. Update on Therapeutic Approaches for Invasive Fungal Infections in Adults. Ther. Adv. Infect. Dis. 2024, 11, 20499361231224980. [Google Scholar] [CrossRef]
- Ryder, N.S.; Leitner, I. Synergistic Interaction of Terbinafine with Triazoles or Amphotericin B against Aspergillus Species. Med. Mycol. 2001, 39, 91–95. [Google Scholar] [CrossRef]
- Jenks, J.D.; Seidel, D.; Cornely, O.A.; Chen, S.; van Hal, S.; Kauffman, C.; Miceli, M.H.; Heinemann, M.; Christner, M.; Jover Sáenz, A.; et al. Voriconazole plus Terbinafine Combination Antifungal Therapy for Invasive Lomentospora Prolificans Infections: Analysis of 41 Patients from the FungiScope® Registry 2008-2019. Clin. Microbiol. Infect. 2020, 26, e1–e784. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Johnson, C.; Berman, J.; Coste, A.T.; Cuomo, C.A.; Perlin, D.S.; Bicanic, T.; Harrison, T.S.; Wiederhold, N.; Bromley, M.; et al. The Importance of Antimicrobial Resistance in Medical Mycology. Nat. Commun. 2022, 13, 5352. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In Vitro Activity of Olorofim against Clinical Isolates of Scedosporium Species and Lomentospora Prolificans Using EUCAST and CLSI Methodologies. J. Antimicrob. Chemother. 2020, 75, 3582–3585. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, L.; Dittmer, S.; Buer, J.; Rath, P.-M.; Steinmann, J. In Vitro Activity of Olorofim (F901318) against Fungi of the Genus, Scedosporium and Rasamsonia as Well as against Lomentospora Prolificans, Exophiala Dermatitidis and Azole-Resistant Aspergillus Fumigatus. Int. J. Antimicrob. Agents 2020, 56, 106105. [Google Scholar] [CrossRef] [PubMed]
- Almajid, A.; Bazroon, A.; Al-Awami, H.M.; Albarbari, H.; Alqahtani, I.; Almutairi, R.; Alsuwayj, A.; Alahmadi, F.; Aljawad, J.; Alnimer, R.; et al. Fosmanogepix: The Novel Anti-Fungal Agent’s Comprehensive Review of in Vitro, in Vivo, and Current Insights From Advancing Clinical Trials. Cureus 2024, 16, e59210. [Google Scholar] [CrossRef]
- Georgacopoulos, O.; Nunnally, N.; Law, D.; Birch, M.; Berkow, E.L.; Lockhart, S.R. In Vitro Activity of the Novel Antifungal Olorofim against Scedosporium and Lomentospora Prolificans. Microbiol. Spectr. 2023, 11, e0278922. [Google Scholar] [CrossRef]
- Febles Leyva, S.; Sierra Yuste, M.; Bujaldón Querejeta, N.; De La Pinta Zazo, C.; Belso Candela, A.; Wikman-Jorgensen, P. A Difficult-to-Treat Septic Arthritis by Scedosporium Apiospermum Successfully Treated with Olorofim. J. Antimicrob. Chemother. 2024, 79, 3358–3359. [Google Scholar] [CrossRef]
- Cobo, F.; González-Sierra, P.A.; Ortega-Gavilán, M.C.; Castellano-Sánchez, L.; Navarro-Marí, J.M. Two Cases of Fungemia Due to Lomentospora Prolificans in Haematological Patients with Different Outcome. Diagn. Microbiol. Infect. Dis. 2024, 110, 116527. [Google Scholar] [CrossRef]
- Neoh, C.F.; Chen, S.C.-A.; Lanternier, F.; Tio, S.Y.; Halliday, C.L.; Kidd, S.E.; Kong, D.C.M.; Meyer, W.; Hoenigl, M.; Slavin, M.A. Scedosporiosis and Lomentosporiosis: Modern Perspectives on These Difficult-to-Treat Rare Mold Infections. Clin. Microbiol. Rev. 2024, 37, e0000423. [Google Scholar] [CrossRef]
- Horré, R.; Marklein, G.; Siekmeier, R.; Nidermajer, S.; Reiffert, S.M. Selective Isolation of Pseudallescheria and Scedosporium Species from Respiratory Tract Specimens of Cystic Fibrosis Patients. Respir. Int. Rev. Thorac. Dis. 2009, 77, 320–324. [Google Scholar] [CrossRef]
- Coron, N.; Pihet, M.; Fréalle, E.; Lemeille, Y.; Pinel, C.; Pelloux, H.; Gargala, G.; Favennec, L.; Accoceberry, I.; Durand-Joly, I.; et al. Toward the Standardization of Mycological Examination of Sputum Samples in Cystic Fibrosis: Results from a French Multicenter Prospective Study. Mycopathologia 2018, 183, 101–117. [Google Scholar] [CrossRef]
| Type of Transplant | Age | Gender | Underlying Disease | Species | Time since LTx | Infection/ Colonization | Eradication | Sample (n) | Other Microorganisms | Treatment | Radiological Findings | Outcome | Cause Related to FI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bilateral Retransplantation | 40 | M | Cystic Fibrosis | Scedosporium boydii | 1 y, 5 m | Post-transplant colonization | Yes | BAS (2) | No | No | None | Alive | |
| 2 | Bilateral | 57 | M | COPD | Scedosporium apiospermum | 2 y, 1 m | Localized infection | No | Sputum (8) BAS (7) BAL (3) | No | VOR (IV) | Pulmonary infiltrates | Deceased | No |
| 3 | Bilateral | 67 | M | ILD | Lomentospora prolificans | 1 y, 2 m | Localized infection | Yes | Sputum (1) BAS (5) BAL (3) | Aspergillus Terreus Nocardia farcinica CMV | VOR (PO, INH) TER (PO) | Nodular changes | Deceased | No |
| 4 | Bilateral | 72 | F | ILD | Scedosporium boydii | 6 y, 9 m | Localized infection | Yes | Sputum (2) BAS (3) BAL (2) | No | ISA (IV) AMB (INH) OLO (PO) | Pulmonary infiltrates | Deceased | No |
| 5 | Bilateral | 55 | M | ILD | Lomentospora prolificans | 0 d | Disseminated infection (pre-transplant colonization and post-transplant infection) | No | Sputum (3) BAS (10) BAL (4) Pleural fluid (7) Bronchial biopsy (2) Blood (4) | No | VOR (PO, INH, IP) TER (PO) ANI (IV) AMB (IP) OLO (PO) | Pleural effusion and micronodules | Deceased | Yes |
| 6 | Bilateral | 25 | M | Cystic Fibrosis | Lomentospora prolificans, Scedosporium apiospermum and S. boydii | 0 d | Disseminated infection (pre-transplant colonization and post-transplant infection) | No | Sputum (3) BAS (36) BAL (1) Pleural fluid (14) Pleural biopsy (1) Catheter (1) Wound (2) | Pseudomonas aeruginosa | VOR (PO, IV) POS (PO) ANI (IV) TER (PO) | Pulmonary infiltrates, pleural effusion, mediastinitis, pulmonary thromboembolism | Deceased | Yes |
| 7 | Bilateral | 64 | F | ILD | Scedosporium ellipsoidea | 3 y, 1 m | Post-transplant colonization | Yes | BAL (1) | Staphylococcus aureus | VOR (INH) | None | Alive | |
| 8 | Bilateral | 17 | F | Cystic Fibrosis | Lomentospora prolificans and Scedosporium apiospermum | 7 d | Pre- and post-transplant colonization | Yes | Sputum (7) BAS (2) | CMV | POS (IV) VOR (INH) TER (PO) | None | Alive | |
| 9 | Unilateral | 70 | M | ILD | Scedosporium apiospermum | 3 y, 11 m | Post-transplant colonization | Yes | BAS (1) | No | VOR (INH) TER (PO) | None | Deceased | No |
| 10 | Bilateral | 63 | M | ILD | Scedosporium apiospermum | 9 m | Localized infection | Yes | Sputum (2) Bronchial biopsy (1) BAS (1) | Stenotrophomonas maltophilia, CMV | VOR (IV) | Pulmonary infiltrates | Deceased | No |
| 11 | Bilateral | 64 | M | Bronchiectasis | Scedosporium boydii | 6 y, 4 m | Localized infection | No | Sputum (4) | Aspergillus terreus | POS (IV) TER (PO) MIC (IV) | Tree-in-bud pattern | Deceased | No |
| S/L Isolation | ||||
|---|---|---|---|---|
| No | Yes | Total | ||
| Total (n, %) | 565 (98%) | 11 (2%) | 576 (100%) | |
| Age (mean ± SD) | 56.09 ± 10.31 | 52 ± 16.03 | 56.51 ± 10.43 | p = 0.198 |
| Gender (n, %) | ||||
| Male | 365 (65%) | 8 (73%) | 373 (65%) | |
| Female | 200 (35%) | 3 (27%) | 203 (35%) | |
| Underlying disease (n, %) | ||||
| COPD/emphysema | 227 (40%) | 1 (9%) | 228 (40%) | |
| ILD | 253 (45%) | 6 (55%) | 259 (44%) | |
| Cystic fibrosis | 41 (7%) | 3 (27%) | 44 (8%) | |
| Others | 44 (8%) | 1 (9%) | 45 (8%) | |
| Type of transplant (n, %) | ||||
| Bilateral lung | 487 (86%) | 10 (91%) | 497 (91%) | |
| Unilateral lung | 78 (14%) | 1 (9%) | 79 (9%) | |
| S. apiospermum (mg/L) | S. boydii (mg/L) | L. prolificans (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Tested | Range | MIC50 | No. Tested | Range | MIC50 | No. Tested | Range | MIC50 | |
| Voriconazole | 9 | 0.25–>8 | 0.5 | 6 | 0.5–>8 | 1.5 | 6 | >8 | >8 |
| Isavuconazole | 3 | 8–>8 | 8 | 5 | 2–>8 | >8 | 3 | >8 | >8 |
| Itraconazole | 9 | 8–>8 | >8 | 6 | >8 | >8 | 6 | >8 | >8 |
| Posaconazole | 9 | 0.25–>8 | 1 | 6 | 0.5–>8 | >8 | 6 | >8 | >8 |
| Amphotericin B | 9 | 4–>16 | 4 | 6 | 2–>16 | >16 | 6 | 8–>16 | >16 |
| Terbinafine | 8 | 16–>16 | >16 | 6 | >16 | >16 | 6 | >16 | >16 |
| Anidulafungin | 4 | 0.015–1 | 0.12 | 6 | 0.25–4 | 0,25 | 5 | 0.03–>4 | 0.12 |
| Caspofungin | 9 | 0.5–>16 | 0.5 | 6 | 0.5–8 | 1 | 6 | 0.12–>16 | 1 |
| Micafungin | 4 | 0.03–0.25 | 0.06 | 6 | 0.12–0.5 | 0.25 | 5 | 0.03–>2 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Masedo Fernández, S.; Laporta, R.; Aguilar, M.; García Fadul, C.; Cabrera Pineda, M.; Alastruey-Izquierdo, A.; Royuela, A.; Sánchez Romero, I.; Ussetti Gil, P. Clinical Significance and Therapeutic Challenges of Scedosporium spp. and Lomentospora prolificans Isolates in a Single-Center Cohort of Lung Transplant Recipients. J. Fungi 2025, 11, 291. https://doi.org/10.3390/jof11040291
García-Masedo Fernández S, Laporta R, Aguilar M, García Fadul C, Cabrera Pineda M, Alastruey-Izquierdo A, Royuela A, Sánchez Romero I, Ussetti Gil P. Clinical Significance and Therapeutic Challenges of Scedosporium spp. and Lomentospora prolificans Isolates in a Single-Center Cohort of Lung Transplant Recipients. Journal of Fungi. 2025; 11(4):291. https://doi.org/10.3390/jof11040291
Chicago/Turabian StyleGarcía-Masedo Fernández, Sarela, Rosalía Laporta, Myriam Aguilar, Christian García Fadul, María Cabrera Pineda, Ana Alastruey-Izquierdo, Ana Royuela, Isabel Sánchez Romero, and Piedad Ussetti Gil. 2025. "Clinical Significance and Therapeutic Challenges of Scedosporium spp. and Lomentospora prolificans Isolates in a Single-Center Cohort of Lung Transplant Recipients" Journal of Fungi 11, no. 4: 291. https://doi.org/10.3390/jof11040291
APA StyleGarcía-Masedo Fernández, S., Laporta, R., Aguilar, M., García Fadul, C., Cabrera Pineda, M., Alastruey-Izquierdo, A., Royuela, A., Sánchez Romero, I., & Ussetti Gil, P. (2025). Clinical Significance and Therapeutic Challenges of Scedosporium spp. and Lomentospora prolificans Isolates in a Single-Center Cohort of Lung Transplant Recipients. Journal of Fungi, 11(4), 291. https://doi.org/10.3390/jof11040291







