Exploring Fungicide Sensitivity in Soybean Stem Blight Pathogen Diaporthe longicolla, Emphasizing Genetic Variability Impact on Response to SDHI Fungicides Fluopyram and Pydiflumetofen
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Diaporthe Species Isolates
2.2. DNA Extraction and Identification of Diaporthe Isolates
2.3. Diaporthe Isolates Pathogenicity Testing
2.4. Fungicides
2.5. Sensitivity Assay of Diaporthe Isolates to Eight Fungicides
2.6. Cloning of SdhA, B, C, and D Genes in D. longicolla
2.7. Analysis of SdhB and SdhC in D. longicolla
2.8. Molecular Docking Analysis and Molecular Dynamics (MD) Simulations
2.9. Synergistic Interaction of Mixed Fungicides
2.10. Statistical Analysis
3. Results
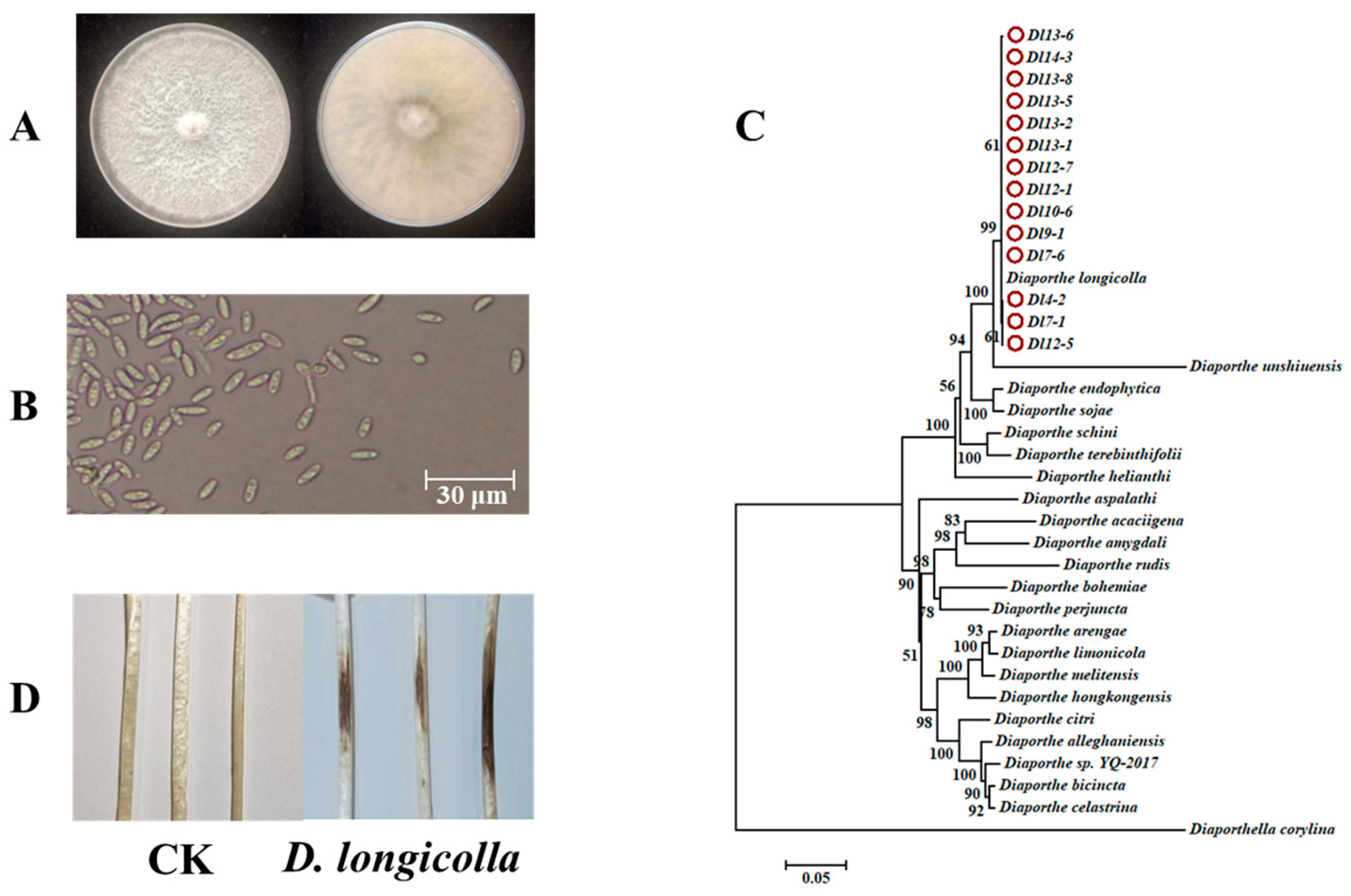
3.1. D. longicolla Was Identified from a Diseased Soybean Stem
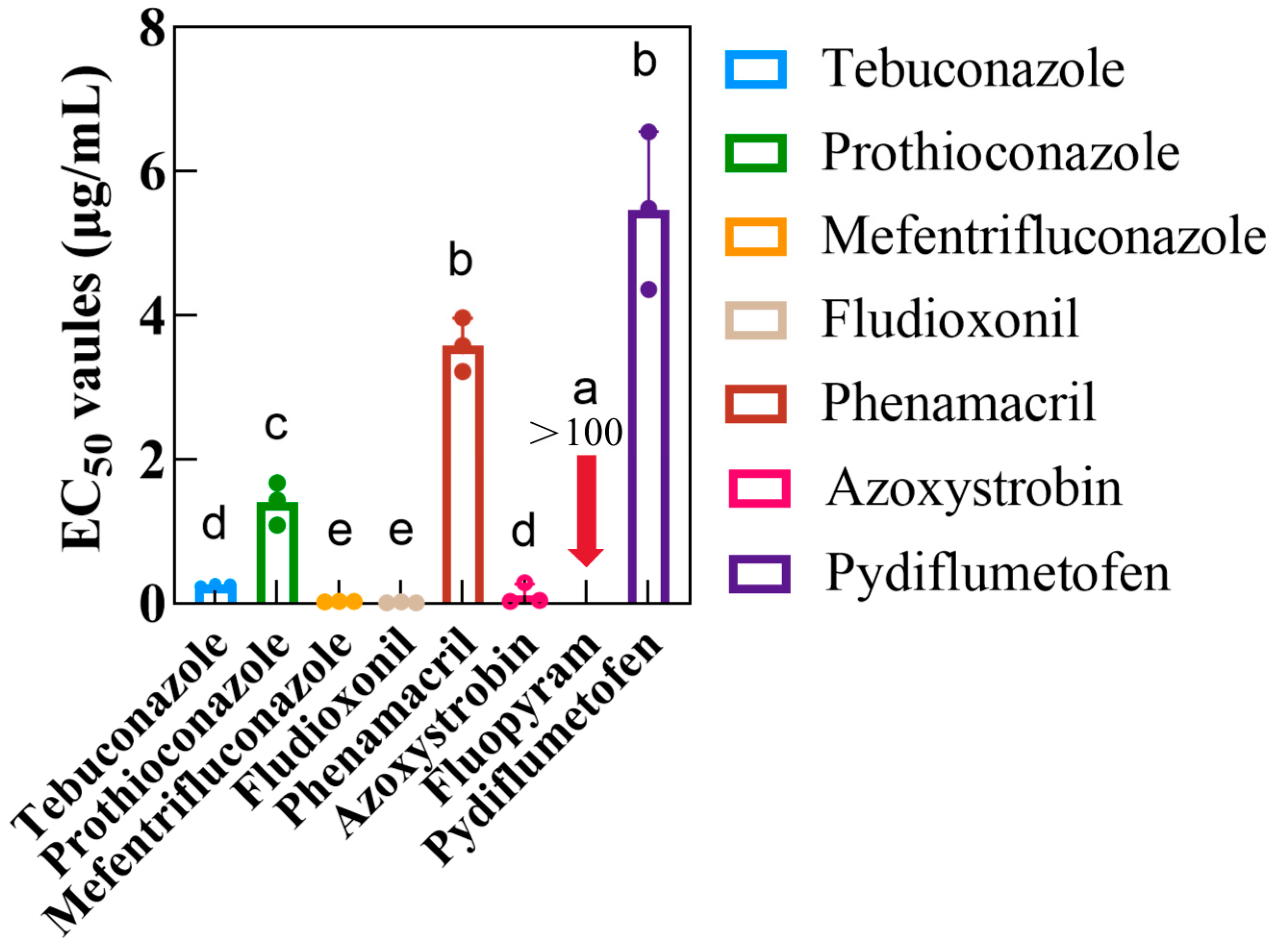
3.2. Determination of EC50 Values of Eight Fungicides and Sensitivity Differentiation in Two SDHIs Against D. longicolla
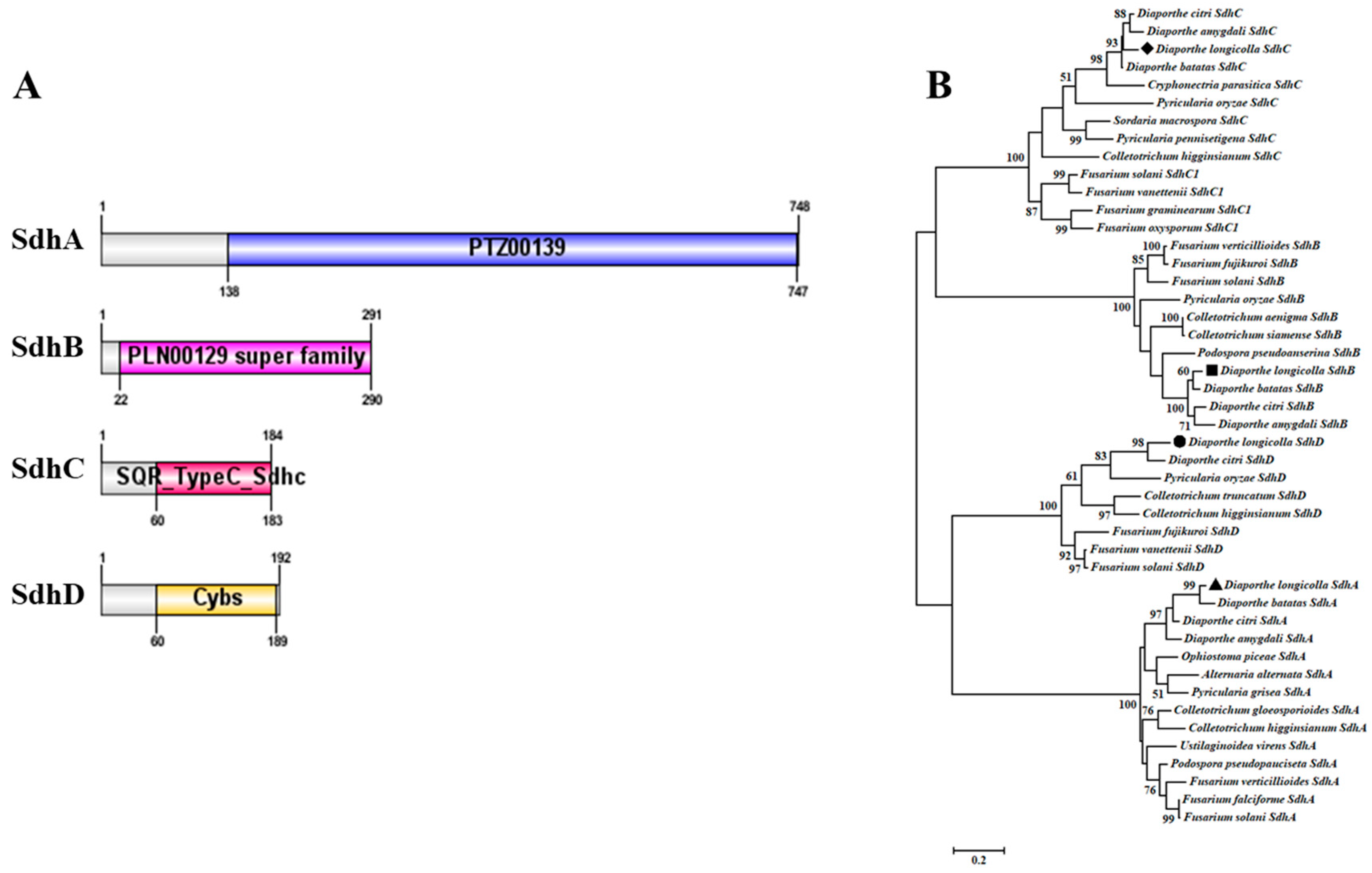
3.3. Characterization of the D. longicolla Sdh (A, B, C, and D) Genes
3.4. Analysis Sdh (B and C) Proteins in D. longicolla
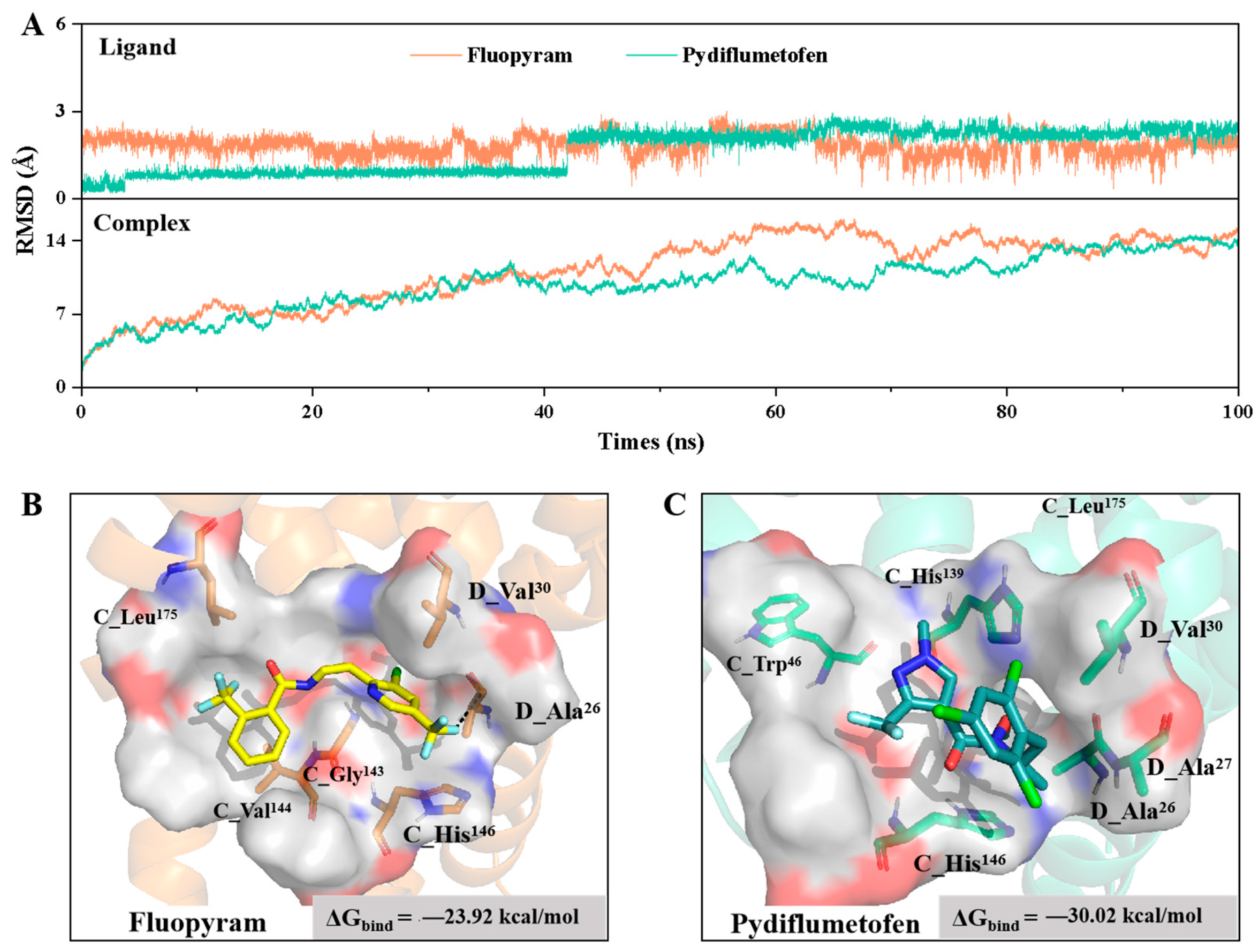
3.5. Molecular Docking and MD Simulation of Two SDHI Fungicides with D. longicolla Sdh Proteins
3.6. Synergy of Fludioxonil, Mefentrifluconazole, and Pydiflumetofen Against D. longicolla
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Borja Reis, A.F.; Tamagno, S.; Moro Rosso, L.H.; Ortez, O.A.; Naeve, S.; Ciampitti, I.A. Historical trend on seed amino acid concentration does not follow protein changes in soybeans. Sci. Rep. 2020, 10, 17707. [Google Scholar] [CrossRef]
- Ramalingam, J.; Alagarasan, G.; Savitha, P.; Lydia, K.; Pothiraj, G.; Vijayakumar, E.; Sudhagar, R.; Singh, A.; Vedna, K.; Vanniarajan, C. Improved host-plant resistance to Phytophthora rot and Powdery mildew in soybean. Sci. Rep. 2020, 10, 13928. [Google Scholar] [CrossRef]
- Yao, H.; Zuo, X.; Zuo, D.; Lin, H.; Huang, X.; Zang, C.F. Study on soybean potential productivity and food security assessment in China under the influence of the COVID-19 outbreak. Georg. Sustain. 2020, 1, 163–171. [Google Scholar] [CrossRef]
- Zhao, J.C.; Wang, Y.X.; Zhao, M.Y.; Wang, K.C.; Li, S.; Gao, Z.Z.; Shi, X.Y.; Chu, Q.Q. Prospects for soybean production increase by closing yield gaps in the Northeast Farming Region, China. Field Crops Res. 2023, 293, 108843. [Google Scholar] [CrossRef]
- Wang, T.; Ma, Y.; Luo, S. Spatiotemporal evolution and influencing factors of soybean production in Heilongjiang Province, China. Land 2023, 12, 2090. [Google Scholar] [CrossRef]
- Uecker, F.A. A World List of Phomopsis Names with Notes on Nomenclature, Morphology and Biology (Mycologia Memoir); J. Cramer: Berlin, Germany, 1988; Volume 13, pp. 1–231. [Google Scholar]
- Santos, J.M.; Phillips, A.J.L. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Divers. 2009, 34, 111–125. [Google Scholar] [CrossRef]
- Petrović, K.; Skaltsas, D.; Castlebury, L.A.; Kontz, B.; Allen, T.W.; Chilvers, M.I.; Gregory, N.; Kelly, H.M.; Koehler, A.M.; Kleczewski, N.M.; et al. Diaporthe seed decay of soybean [Glycine max (L.) Merr.] Is endemic in the United States, but new fungi are involved. Plant Dis. 2021, 105, 1621–1629. [Google Scholar] [CrossRef]
- Rensburg, J.C.J.V.; Lamprecht, S.C.L.; Groenewald, J.Z.; Lisa, A.; Castlebury, L.A.; Crous, P.W. Characterisation of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Stud. Micol. 2006, 55, 65–74. [Google Scholar] [CrossRef]
- Santos, J.M.; Vrandecic, K.; Cosic, J.; Duvnjak, T.; Phillips, A.J.L. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 2011, 27, 9–19. [Google Scholar] [CrossRef]
- Hosseini, B.; ElHasan, A.; Link, T.; Voegele, R.T. Analysis of thespecies spectrum of the Diaporthe/Phomopsis complex in European soybean seeds. Mycol. Prog. 2020, 19, 455–469. [Google Scholar] [CrossRef]
- Abdelmagid, A.; Hafez, M.; Lawley, Y.; Rehal, P.K.; Daayf, F. First report of pod and stem blight and seed decay caused by Diaporthe longicolla on soybean in western Canada. Plant Dis. 2022, 106, 1061. [Google Scholar] [CrossRef]
- Zhao, X.; Li, K.; Zheng, S.; Yang, J.; Chen, C.; Zheng, X.B.; Wang, Y.C.; Ye, W.W. Diaporthe diversity and pathogenicity revealed from a broad survey of soybean stem blight in China. Plant Dis. 2022, 106, 2892–2903. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.L.; Huang, Y.H.; Nelson, R.L.; Noel, G.R. Germplasm evaluation of Glycine max for resistance to Fusarium solani, the causal organism of sudden death syndrome. Plant Dis. 1999, 81, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, S.; Ye, W.; Zheng, X.; Wang, Y. First report of soybean stem blight caused by Diaporthe phaseolorum in Sichuan province, China. Plant Dis. 2021, 10, 3290. [Google Scholar] [CrossRef]
- Mena, E.; Stewart, S.; Montesano, M.; de León, I.P. Current understanding of the Diaporthe/Phomopsis complex causing soybean stem canker: A focus on molecular aspects of the interaction. Plant Pathol. 2024, 73, 31–46. [Google Scholar] [CrossRef]
- Wei, L.L.; Chen, B.; Li, J.W.; Zhang, P.C.; Chen, W.C.; Ye, W.W.; Chen, C.J. Resistance mechanism of Phomopsis longicolla to fludioxonil is associated with modifications in PlOS1, PlOS4 and PlOS5. Pest. Biochem. Physiol. 2024, 201, 105862. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.S.; Duan, Y.B.; Zhou, M.G. Research progress of the resistance to succinate dehydrogenase inhibitors. Chin. J. Pest. Sci. 2022, 24, 937–948. [Google Scholar] [CrossRef]
- Steinhauer, D.; Salat, M.; Frey, R.; Mosbach, A.; Luksch, T.; Balmer, D.; Hansen, R.; Widdison, S.; Logan, G.; Dietrich, R.A.; et al. A dispensable paralog of succinate dehydrogenase subunit C mediates standing resistance towards a subclass of SDHI fungicides in Zymoseptoria tritici. PLoS Pathog. 2019, 15, e1007780. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHl) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, H.; Mao, C.; Wu, J.; Zhang, C. Activity of a SDHI fungicide penflufen and the characterization of natural-resistance in Fusarium fujikuroi. Pestic. Biochem. Physiol. 2021, 179, 104960. [Google Scholar] [CrossRef]
- Chen, W.; Wei, L.; Zhao, W.; Wang, B.; Zheng, H.; Zhang, P.; Lou, T.; Duan, Y.; Hou, Y.; Zhou, M.; et al. Resistance risk assessment for a novel succinate dehydrogenase inhibitor pydiflumetofen in Fusarium asiaticum. Pest. Manag. Sci. 2021, 77, 538–547. [Google Scholar] [CrossRef]
- Shao, W.; Wang, J.; Wang, H.; Wen, Z.; Liu, C.; Zhang, Y.; Zhao, Y.; Ma, Z. Fusarium graminearum FgSdhC1 point mutation A78V confers resistance to the succinate dehydrogenase inhibitor pydiflumetofen. Pest. Manag. Sci. 2022, 78, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. The Diaporthe sojae species complex: Phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol. 2015, 119, 383–407. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Zhu, J.T.; Chen, Y.Y.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Liu, J.K. A re-evaluation of Diaporthe: Refining the boundaries of species and species complexes. Fungal Divers. 2024, 126, 1–125. [Google Scholar] [CrossRef]
- Huang, L.; Lümmen, P.; Berry, E.A. Crystallographic investigation of the ubiquinone binding site of respiratory complex II and its inhibitors. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140679. [Google Scholar] [CrossRef]
- Duan, Y.B.; Yang, Y.; Wang, J.X.; Liu, C.C.; He, L.L.; Zhou, M.G. Development and application of loop-mediated isothermal amplification for detecting the highly benzimidazole-resistant isolates in Sclerotinia sclerotiorum. Sci. Rep. 2015, 5, 17278. [Google Scholar] [CrossRef]
- Kim, W.K.; Mauthe, W.; Hausner, G.; Klassen, G.R. Isolation of high molecular weight DNA and double-stranded RNAs from fungi. Can. J. Bot. 1990, 68, 1898–1902. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Groenewald, J.Z.; Woodhall, J.; Armengol, J.; Cinelli, T.; Eichmeier, A.; Ezra, D.; Fontaine, F.; Gramaje, D.; Gutierrez-Aguirregabiria, A.; et al. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia 2018, 40, 135–153. [Google Scholar] [CrossRef]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for the speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Han, S.; Chen, J.; Zhao, Y.; Cai, H.; Guo, C. Bacillus subtilis HSY21 can reduce soybean root rot and inhibit the expression of genes related to the pathogenicity of Fusarium oxysporum. Pestic. Biochem. Physiol. 2021, 178, 104916. [Google Scholar] [CrossRef]
- Chang, X.; Yan, L.; Naeem, M.; Khaskheli, M.I.; Zhang, H.; Gong, G.; Zhang, M.; Song, C.; Yang, W.; Liu, T.; et al. Maize/Soybean relay strip intercropping reduces the occurrence of Fusarium root rot and changes the diversity of the pathogenic Fusarium species. Pathogens 2020, 9, 211. [Google Scholar] [CrossRef]
- Martin, S.B.; Lucas, L.T.; Campbell, C.L. Comparative sensitivity of Rhizoclonia solani and Rhizoclonia-like fungi to selected fungicides in vitro. Phytopathology 1984, 74, 778–781. [Google Scholar] [CrossRef]
- Gi, S.G.; Kim, W.Y.; Yang, K.Y. Emergence of multiple Diaporthe species causing kiwifruit rot and occurrence of resistance to a methyl benzimidazole carbamate fungicide in South Korea. Crop Prot. 2022, 158, 106016. [Google Scholar] [CrossRef]
- Miao, J.; Dong, X.; Lin, D.; Wang, Q.; Liu, P.; Chen, F.R.; Du, Y.; Liu, X. Activity of the novel fungicide oxathiapiprolin against plant-pathogenic oomycetes. Pest. Manag. Sci. 2016, 72, 1572–1577. [Google Scholar] [CrossRef]
- Miao, J.; Liu, X.; Du, X.; Li, G.; Li, C.; Zhao, D.; Liu, X. Sensitivity of Pythium spp. and Phytopythium spp. and tolerance mechanism of Pythium spp. to oxathiapiprolin. Pest. Manag. Sci. 2020, 76, 3975–3981. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Wang, Y.; Li, X.Y.; Fan, H.J.; Gao, X.H.; Peng, Q.; Li, F.; Lu, L.; Miao, J.; Liu, X. Resistant risk and resistance-related point mutation in SdhC1 of pydiflumetofen in Fusarium pseudograminearum. Pest. Manag. 2023, 79, 4197–4207. [Google Scholar] [CrossRef]
- Aamir, M.; Singh, V.K.; Dubey, M.K.; Meena, M.; Kashyap, S.P.; Katari, S.K.; Upadhyay, R.S.; Umamaheswari, A.; Singh, S. In silico prediction, characterization, molecular docking, and dynamic studies on fungal SDRs as novel targets for searching potential fungicides against fusarium wilt in tomato. Front. Pharmacol. 2018, 9, 1038. [Google Scholar] [CrossRef]
- Tian, C.; Kasavajhala, K.; Belfon, K.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-Acid-Specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. Parallelization of CPPTRAJ enables large scale analysis of molecular dynamics trajectory data. J. Comput. Chem. 2018, 39, 2110–2117. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An efficient program for End-State free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.; Balasubramanian, S.; Rajagopalan, M. Chapter 12—Biomolecular talks—Part 2: Applications and challenges of molecular docking approaches. Methods Mol Biol. 2024, 2780, 203–255. [Google Scholar] [CrossRef]
- Ren, J.J.; Wang, W.H.; Li, H.; Hou, P.X.; Ma, Z.Q.; Feng, J.T.; Wu, H. Efficacy of Ginkgo biloba L. and Derris trifoliata binary extract combinations against aphids (Hemiptera: Aphididae). Crop Prot. 2023, 164, 106144. [Google Scholar] [CrossRef]
- Sun, Y.P.; Johnson, E.R. Synergistic and antagonistic actions of insecticide-synergist combinations and their mode of action. J. Agric. Food Chem. 1960, 8, 261–266. [Google Scholar] [CrossRef]
- Vidić, M.; Petrović, K.; Đorđević, V.; Riccioni, L. Occurrence of Phomopsis longicolla β conidia in naturally infected soybean. J. Phytopathol. 2013, 161, 470–477. [Google Scholar] [CrossRef]
- Wrather, J.A.; Anderson, T.R.; Arsyad, D.M.; Tan, Y.; Ploper, L.D.; Porta-Puglia, A.; Ram, H.H.; Yorinori, J.T. Soybean disease loss estimates for the top ten soybean producing countries in 1998. Can. J. Plant Pathol. 2001, 23, 115–121. [Google Scholar] [CrossRef]
- Li, B.J.; Chen, Q.H.; Lan, Z.C.; Wang, N.N.; Wang, Y.C.; Weng, Q.Y. Identification and pathogenicity test of the pathogens causing soybean root rot in Fu Jian. Fujian J. Agric. Sci. 2011, 26, 798–803. [Google Scholar] [CrossRef]
- Chandra, S.; Choudhary, M.; Bagaria, P.K.; Nataraj, V.; Kumawat, G.; Choudhary, J.R.; Sonah, H.; Gupta, S.; Wani, S.H.; Ratnaparkhe, M.B. Progress and prospectus in genetics and genomics of Phytophthora root and stem rot resistance in soybean (Glycine max L.). Front. Genet. 2022, 13, 939182. [Google Scholar] [CrossRef]
- Carroll, G.C. The biology of endophytism in plants with particular reference to woody perennials. In Microbiology of the Phyllosphere; Cambridge University Press: London, UK, 1986. [Google Scholar]
- Zhao, X.; Zhang, Z.; Zheng, S.; Ye, W.; Zheng, X.; Wang, Y. Genome sequence resource of Phomopsis longicolla YC2-1, a fungal pathogen causing phomopsis stem blight in soybean. Mol. Plant-Microbe Interact. 2021, 34, 842–844. [Google Scholar] [CrossRef]
- Muralli, T.S.; Suryanarayanan, T.S.; Geeta, R. Endophytic Phomopsis species: Host range and implications for diversity estimates. Can. J. Microbiol. 2006, 52, 673–680. [Google Scholar] [CrossRef]
- Garcia-Reyne, A.; López-Medrano, F.; Morales, J.M.; García-Esteban, C.; Martín, I.; Eraña, I.; Meije, Y.; Lalueza, A.; Alastruey-Izquierdo, A.; Rodríguez-Tudela, J.L.; et al. Cutaneous infection by Phomopsis longicolla in a renal transplant recipient from Guinea: First report of human infection by this fungus. Transpl. Infect. Dis. 2010, 13, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Odds, F.C. Resistance of candida species to antifungal agents molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2002, 2, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.C.; Hunte, C. Quinone binding sites of cyt bc complexes analysed by X-ray crystallography and cryogenic electron microscopy. Biochem. Soc. Trans. 2022, 50, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Wang, X.; Cui, K.; Guo, J.; Wang, M.; Hao, Y.; Zhang, F. Exploring the mechanism of Astragali radix for promoting osteogenic differentiation based on network pharmacology, molecular docking, and experimental validation. Chem. Biol. Drug Des. 2023, 102, 1489–1505. [Google Scholar] [CrossRef]
| Fungicide | Concentration Gradient (μg/mL) |
|---|---|
| Tebuconazole | 0, 0.1, 0.4, 0.8, 1, 2.5 |
| Prothioconazole | 0, 1,5, 10, 25, 50, 100 |
| Mefentrifluconazole | 0, 0.005, 0.05, 0.25, 1, 5, 20 |
| Fludioxonil | 0, 0.01, 0.02, 0.05, 0.1, 0.25, 1 |
| Phenamacril | 0, 0.05, 0.25, 0.5, 1.25, 2.5, 10 |
| Pydiflumetofen | 0, 0.2, 0.5, 1, 2.5, 5, 10 |
| Fluopyram | 0, 1, 10, 20, 50, 100 |
| Azoxystrobin | 0, 0.01, 0.02, 0.05, 0.1, 0.25, 1 |
| Primer | Primer Sequence (5′–3′) | Product Length (bp) |
|---|---|---|
| SdhA(F) | TTATCACGGCTGATGTCGGATCG | 2000 |
| SdhA(R) | CCTTCTGGAAAGACAAGGTGTGCTT | |
| SdhB(F) | ATGGCTGCTCTCCGCTCTACCTCCA | 850 |
| SdhB(R) | TACACAGCCATCTCCTTCTTGATCT | |
| SdhC(F) | ATGATTACCTCAAGGGCAGG | 550 |
| SdhC(R) | TTACAGCAGGAACGCAACACCCA | |
| SdhD(F) | ATGGCATCCACTGCTCGTTCGGCT | 600 |
| SdhD(R) | TCATGCTCGCCAAAGCCTCTT |
| Mixture (A:B) | Mixture Ratios (A:B) | EC50 (μg/mL) | Co-Toxicity Index | Interaction Efficiency |
|---|---|---|---|---|
| Mefentrifluconazole and fludioxonil | 1:0 | 0.0663 | - | - |
| 0:1 | 0.0047 | - | - | |
| 1:1 | 0.5070 | 17.3131 | antagonistic | |
| 1:5 | 0.0044 | 126.3899 | synergism | |
| 5:1 | 0.0377 | 55.2262 | antagonistic | |
| Pydiflumetofen and fludioxonil | 1:0 | 6.9921 | - | - |
| 0:1 | 0.0057 | - | - | |
| 10:1 | 0.4220 | 1.4857 | antagonistic | |
| 1:5 | 0.0049 | 695.1258 | synergism | |
| 5:1 | 0.2379 | 2.8747 | antagonistic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, Z.; Chang, Z.; Zheng, Y.; Wang, X.; Li, N.; Huang, Z.; Zhang, C.; Liu, X. Exploring Fungicide Sensitivity in Soybean Stem Blight Pathogen Diaporthe longicolla, Emphasizing Genetic Variability Impact on Response to SDHI Fungicides Fluopyram and Pydiflumetofen. J. Fungi 2025, 11, 292. https://doi.org/10.3390/jof11040292
Chen S, Liu Z, Chang Z, Zheng Y, Wang X, Li N, Huang Z, Zhang C, Liu X. Exploring Fungicide Sensitivity in Soybean Stem Blight Pathogen Diaporthe longicolla, Emphasizing Genetic Variability Impact on Response to SDHI Fungicides Fluopyram and Pydiflumetofen. Journal of Fungi. 2025; 11(4):292. https://doi.org/10.3390/jof11040292
Chicago/Turabian StyleChen, Shanshan, Zhanyun Liu, Zhengjie Chang, Yuxin Zheng, Xueyang Wang, Ningwei Li, Zhongqiao Huang, Can Zhang, and Xili Liu. 2025. "Exploring Fungicide Sensitivity in Soybean Stem Blight Pathogen Diaporthe longicolla, Emphasizing Genetic Variability Impact on Response to SDHI Fungicides Fluopyram and Pydiflumetofen" Journal of Fungi 11, no. 4: 292. https://doi.org/10.3390/jof11040292
APA StyleChen, S., Liu, Z., Chang, Z., Zheng, Y., Wang, X., Li, N., Huang, Z., Zhang, C., & Liu, X. (2025). Exploring Fungicide Sensitivity in Soybean Stem Blight Pathogen Diaporthe longicolla, Emphasizing Genetic Variability Impact on Response to SDHI Fungicides Fluopyram and Pydiflumetofen. Journal of Fungi, 11(4), 292. https://doi.org/10.3390/jof11040292






