Effects of Thermal and Antibiotic Treatments on the Viral Accumulation of FcMV1 in Fusarium circinatum Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Mycoviruses
2.2. Mycovirus-Curing Treatments
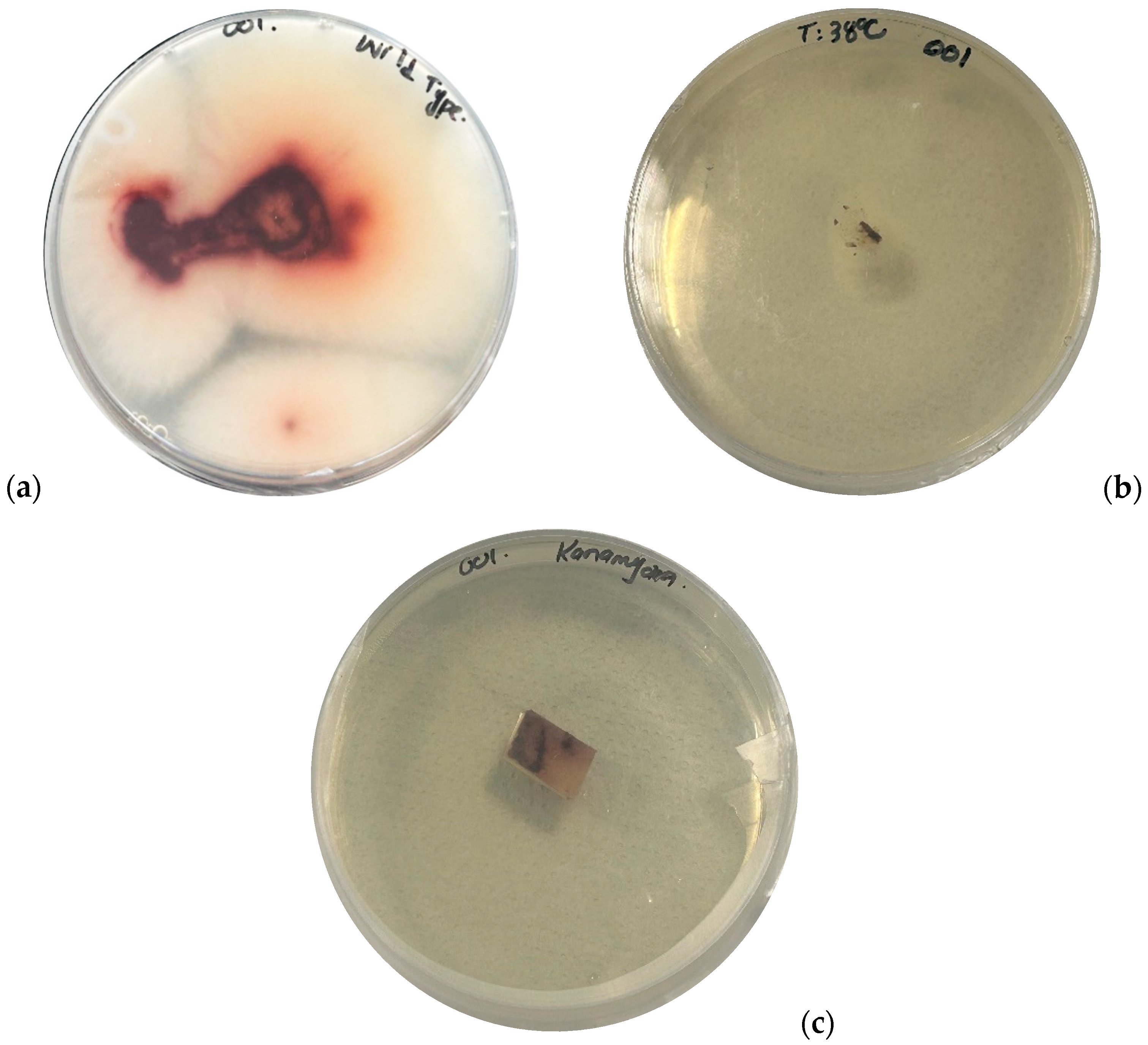
2.2.1. Thermal Treatment
2.2.2. Antibiotic Treatment
2.2.3. Effect of Mycoviruses on Fungal Growth
2.3. RNA Extraction and cDNA Synthesis
2.4. Mycovirus Detection Through qPCR
2.5. Statistical Analysis
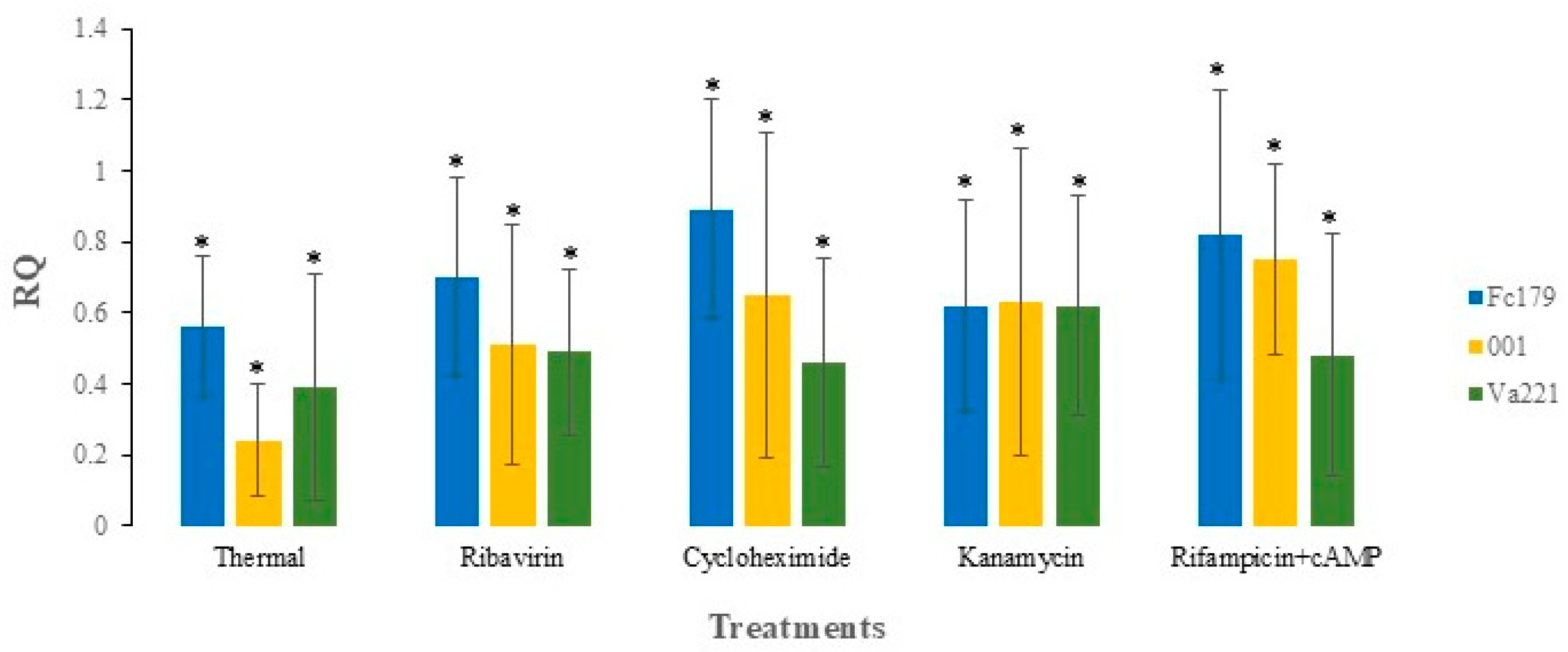
3. Results
3.1. Confirmation of FcMV1 Infection Before Treatments
3.2. Stability of Mitovirus FcMV1 After Thermal Treatment
3.3. Stability of Mitovirus FcMV1 After Antibiotic Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | |
| qPCR | Quantitative polymerase chain reaction |
| RQ | Relative quantification |
| CT | Cycle threshold |
| W1 | Week 1 |
| W5 | Week 5 |
| PDA | Potato dextrose agar |
| uL | Microliter |
| ug | Microgram |
| min | Minute |
| mg | Milligram |
| L | Liters |
References
- Ghabrial, S.A.; Suzuki, N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-Plus Years of Fungal Viruses. Virology 2015, 479, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Botella, L.; Jung, M.H.; Rost, M.; Jung, T. Natural Populations from the Phytophthora palustris Complex Show a High Diversity and Abundance of ssRNA and dsRNA Viruses. J. Fungi 2022, 8, 1118. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses (ICTV). Report on Virus Classification and Taxon Nomenclature. Available online: https://ictv.global/report (accessed on 18 May 2023).
- Hillman, B.I.; Suzuki, N. Viruses of the Chestnut Blight Fungus, Cryphonectria parasitica. Adv. Virus Res. 2004, 63, 423–472. [Google Scholar] [CrossRef] [PubMed]
- Hillman, B.I.; Cai, G. The Family Narnaviridae: Simplest of RNA Viruses. Adv. Virus Res. 2013, 86, 149–176. [Google Scholar] [CrossRef]
- Ong, J.W.; Li, H.; Sivasithamparam, K.; Dixon, K.W.; Jones, M.G.K.; Wylie, S.J. The challenges of using high-throughput sequencing to track multiple bipartite mycoviruses of wild orchid-fungus partnerships over consecutive years. Virology 2017, 510, 297–304. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Yi, X.; Jiang, D. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, L.; Li, G.; Jiang, D.; Ghabrial, S.A. Genome characterization of a debilitation-associated mitovirus infecting the phytopathogenic fungus Botrytis cinerea. Virology 2010, 406, 117–126. [Google Scholar] [CrossRef]
- Heitman, J.; Sun, L.; Suzuki, N.; Jiang, D.; Turina, M.; Xie, J. Frontiers in Fungal Virus Research. Front. Cell. Infect. Microbiol. 2020, 9, 456. [Google Scholar] [CrossRef]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal Viruses Unveiled: A Comprehensive Review of Mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef]
- Tran, T.T.; Li, H.; Nguyen, D.Q.; Jones, M.G.K.; Wylie, S.J. Co-Infection with Three Mycoviruses Stimulates Growth of Monilinia fructicola Isolate on Nutrient Medium but Does Not Induce Hypervirulence in a Natural Host. Viruses 2019, 11, 89. [Google Scholar] [CrossRef]
- Hong, Y.; Dover, S.L.; Cole, T.E.; Brasier, C.M.; Buck, K.W. Multiple Mitochondrial Viruses in an Isolate of the Dutch Elm Disease Fungus Ophiostoma novo-ulmi. Virology 1999, 258, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.M.; Jeon, J.J.; Yea, S.J.; Kim, Y.H.; Yun, S.H.; Lee, Y.W.; Kim, K.H. Double-stranded RNA mycovirus from Fusarium graminearum. Appl. Environ. Microbiol. 2002, 68, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Kramer, K.; Valdivia, L.; Ortiz, S.; Castillo, A. A double-stranded RNA mycovirus confers hypovirulence-associated traits to Botrytis cinerea. FEMS Microbiol. Lett. 2003, 228, 87–91. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef]
- Nuss, D.L. Biological Control of Chestnut Blight: An example of Virus-Mediated Attenuation of Fungal Pathogenesis. Microbiol. Rev. 1992, 56, 561–576. [Google Scholar] [CrossRef]
- Myteberi, I.F.; Lushaj, A.B.; Keča, N.; Lushaj, A.B.; Lushaj, B.M. Diversity of Cryphonectria parasitica, hypovirulence, and possibilities for biocontrol of chestnut canker in Albania. Int. J. Microbiol. Res. 2013, 1, 11–21. [Google Scholar]
- Howitt, R.L.J.; Beever, R.E.; Pearson, M.N.; Forster, R.L.S. Genome characterization of a flexuous rod-shaped mycovirus, Botrytis virus X, reveals high amino acid identity to genes from plant “potex-like” viruses. Arch. Biol. Vir. 2006, 151, 563–579. [Google Scholar] [CrossRef]
- Xie, J.; Wei, D.; Jiang, D.; Fu, Y.; Li, G.; Ghabrial, S.; Peng, Y. Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J. Gen. Virol. 2006, 87, 241–249. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lim, W.S.; Park, S.H.; Park, M.R.; Kim, K.H. Molecular Characterization of a dsRNA Mycovirus, Fusarium graminearum Virus-DK21, which Is Phylogenetically Related to Hypoviruses but Has a Genome Organization and Gene Expression Strategy Resembling Those of Plant Potex-like Viruses. Mol. Cells 2007, 23, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Kanematsu, S.; Onoue, M.; Oikawa, Y.; Nakamura, H.; Yoshida, K. Artificial infection of Rosellinia necatrix with purified viral particles of a member of the genus Mycoreovirus reveals its uneven distribution in single colonies. Phytopathology 2007, 97, 278–286. [Google Scholar] [CrossRef]
- Li, P.; Bhattacharjee, P.; Wang, S.; Zhang, L.; Ahmed, I.; Guo, L. Mycoviruses in Fusarium species: An update. Front. Cell. Infect. Microbiol. 2019, 9, 257. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Pearson, M.N. Characterisation of a novel hypovirus from Sclerotinia sclerotiorum potentially representing a new genus within the Hypoviridae. Virology 2014, 464–465, 441–449. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, S.; Liu, L.; Cheng, J.; Fu, Y.; Jiang, D.; Xie, J. A mitovirus related to plant mitochondrial gene confers hypovirulence on the phytopathogenic fungus Sclerotinia sclerotiorum. Virus Res. 2015, 197, 127–136. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Pando, V.; Diez, J.J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant Pathol. 2014, 63, 1086–1094. [Google Scholar] [CrossRef]
- Walmsley, J.D.; Godbold, D.L. Stump harvesting for bioenergy—A review of the environmental impacts. Forestry 2010, 83, 17–38. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Pérez-Sierra, A.; Armengol, J.; García-Jiménez, J.; Berbegal, M. Efficacy of hot water treatment to reduce the incidence of Fusarium circinatum on Pinus radiata seeds. Forestry 2012, 85, 629–635. [Google Scholar] [CrossRef]
- Zamora-Ballesteros, C.; Diez, J.J.; Martín-García, J.; Witzell, J.; Solla, A.; Ahumada, R.; Capretti, P.; Cleary, M.; Drenkhan, R.; Dvořák, M.; et al. Pine Pitch Canker (PPC): Pathways of Pathogen Spread and Preventive Measures. Forests 2019, 10, 1158. [Google Scholar] [CrossRef]
- Vainio, E.J.; Jurvansuu, J.; Streng, J.; Rajamä, M.-L.; Hantula, J.; Valkonen, J.P.T. Diagnosis and discovery of fungal viruses using deep sequencing of small RNAs. J. Gen. Virol. 2015, 96, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Adalia, E.J.; Fernández, M.M.; Diez, J.J. The use of mycoviruses in the control of forest diseases. Biocontrol. Sci. Technol. 2016, 26, 577–604. [Google Scholar] [CrossRef]
- Flores-Pacheco, J.A.; Muñoz-Adalia, E.J.; Martínez-Álvarez, P.; Pando, V.; Diez-Casero, J.J.; Martín-García, J. Effect of mycoviruses on growth, spore germination and pathogenicity of the fungus Fusarium circinatum. For. Syst. 2017, 26. [Google Scholar] [CrossRef]
- Cao, C.; Li, H.; Jones, M.G.K.; Wylie, S.J. Challenges to elucidating how endornaviruses influence fungal hosts: Creating mycovirus-free isogenic fungal lines and testing them. J. Virol. Methods 2019, 274, 113745. [Google Scholar] [CrossRef]
- Herrero, N.; Zabalgogeazcoa, I. Mycoviruses infecting the endophytic and entomopathogenic fungus Tolypocladium cylindrosporum. Virus Res. 2011, 160, 409–413. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Chiba, S.; Kondo, H.; Kanematsu, S.; Suzuki, N. A novel single-stranded RNA virus isolated from a phytopathogenic filamentous fungus, Rosellinia necatrix, with similarity to hypo-like viruses. Front. Microbiol. 2014, 5, 360. [Google Scholar] [CrossRef]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- Fink, G.R.; Styles, C.A. Curing of a killer factor in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1972, 69, 2846–2849. [Google Scholar] [CrossRef]
- Varga, J.; Kevei, F.; Vágvölgyi, C.; Vriesema, A.; Croft, J.H. Double-stranded RNA mycoviruses in section Nigri of the Aspergillus genus. Can. J. Microbiol. 1994, 40, 325–329. [Google Scholar] [CrossRef]
- Bhatti, M.F.; Jamal, A.; Petrou, M.A.; Cairns, T.C.; Bignell, E.M.; Coutts, R.H.A. The effects of dsRNA mycoviruses on growth and murine virulence of Aspergillus fumigatus. Fungal Genet. Biol. 2011, 48, 1071–1075. [Google Scholar] [CrossRef]
- Kim, J.M.; Song, H.Y.; Choi, H.J.; Yun, S.H.; So, K.K.; Ko, H.K.; Kim, D.H. Changes in the mycovirus (LeV) titer and viral effect on the vegetative growth of the edible mushroom Lentinula edodes. Virus Res. 2015, 197, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Thapa, V.; Turner, G.G.; Hafenstein, S.; Overton, B.E.; Vanderwolf, K.J.; Roossinck, M.J. Using a Novel Partitivirus in Pseudogymnoascus destructans to Understand the Epidemiology of white-nose syndrome. PLoS Pathog. 2016, 12, e1006076. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Diez, J.J.; Fernández, M.M.; Hantula, J.; Vainio, E.J. Characterization of small RNAs originating from mitoviruses infecting the conifer pathogen Fusarium circinatum. Arch. Virol. 2018, 163, 1009–1018. [Google Scholar] [CrossRef]
- Vainio, E.J.; Martínez-Álvarez, P.; Bezos, D.; Hantula, J.; Diez, J.J. Fusarium circinatum isolates from northern Spain are commonly infected by three distinct mitoviruses. Arch. Virol. 2015, 160, 2093–2098. [Google Scholar] [CrossRef]
- Romeralo, C.; Bezos, D.; Martínez-Álvarez, P.; Diez, J.J. Vertical transmission of Fusarium circinatum mitoviruses FcMV1 and FcMV2-2 via microconidia. Forests 2018, 9, 356. [Google Scholar] [CrossRef]
- Kwon, B.S.; Kim, M.-N.; Sung, H.; Koh, Y.; Kim, W.-S.; Song, J.-W.; Oh, Y.-M.; Lee, S.-D.; Lee, S.W.; Lee, J.-S.; et al. In vitro MIC values of rifampin and ethambutol and treatment outcome in Mycobacterium avium complex lung disease. J. Antimicrob. Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kassawara Martins, M.; Cristina, M.; Daniel, F.; Sosa-Gomez, R.; Rodrigues, M.; Maria, F.; Fungaro, H.P. Double-stranded RNA in the entomopathogenic fungus Metarhizium flavoviride. Curr. Genet. 1999, 36, 94–97. [Google Scholar] [CrossRef]
- Romo, M.; Leuchtmann, A.; García, B.; Zabalgogeazcoa, I. A totivirus infecting the mutualistic fungal endophyte Epichloë festucae. Virus Res. 2007, 124, 38–43. [Google Scholar] [CrossRef]
- Carroll, K.; Wickner, R.B. Translation and M1 Double-Stranded RNA Propagation: MAK18 RPL41B and Cycloheximide Curing. J. Bacteriol. 1995, 177, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ballesteros, C.; Wingfield, B.D.; Wingfield, M.J.; Martín-García, J.; Diez, J.J. Residual effects caused by a past mycovirus infection in Fusarium circinatum. Forests 2021, 12, 11. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharyya, S. Effect of temperature on viral infection and its control: A mathematical approach. J. Theor. Biol. 2007, 247, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Parsell, D.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–497. [Google Scholar] [CrossRef] [PubMed]
- Applen Clancey, S.; Ruchti, F.; LeibundGut-Landmann, S.; Heitman, J.; Ianiri, G. A novel mycovirus evokes transcriptional rewiring in the fungus Malassezia and stimulates beta interferon production in macrophages. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Wang, L.; He, H.; Wang, S.; Chen, X.; Qiu, D.; Kondo, H.; Guo, L. Evidence for a novel negative-stranded RNA mycovirus isolated from the plant pathogenic fungus Fusarium graminearum. Virology 2018, 518, 232–240. [Google Scholar] [CrossRef]
- Romon-Ochoa, P.; Smith, O.; Lewis, A.; Kupper, Q.; Shamsi, W.; Rigling, D.; Pérez-Sierra, A.; Ward, L. Temperature Effects on the Cryphonectria hypovirus 1 Accumulation and Recovery within Its Fungal Host, the Chestnut Blight Pathogen Cryphonectria parasitica. Viruses 2023, 15, 1260. [Google Scholar] [CrossRef]
- Vainio, E.J.; Korhonen, K.; Tuomivirta, T.T.; Hantula, J. A novel putative partitivirus of the saprotrophic fungus Heterobasidion ecrustosum infects pathogenic species of the Heterobasidion annosum complex. Fungal Biol. 2010, 114, 955–965. [Google Scholar] [CrossRef]
- da Silva Xavier, A.; de Barros, A.P.O.; Godinho, M.T.; Zerbini, F.M.; de Oliveira Souza, F.; Bruckner, F.P.; Alfenas-Zerbini, P. A novel mycovirus associated to Alternaria alternata comprises a distinct lineage in Partitiviridae. Virus Res. 2018, 244, 21–26. [Google Scholar] [CrossRef]
- Xie, J.; Ghabrial, S.A. Molecular characterizations of two mitoviruses co-infecting a hyovirulent isolate of the plant pathogenic fungus Sclerotinia sclerotiorum. Virology 2012, 428, 77–85. [Google Scholar] [CrossRef]
- Khalili, J.S.; Zhu, H.; Mak, N.S.A.; Yan, Y.; Zhu, Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J. Med.Virol. 2020, 92, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Ghany, M.G.; Liang, T.J. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir. Chem. Chemother. 2012, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Herrero Asensio, N.; Sánchez Márquez, S.; Zabalgogeazcoa, I. Mycovirus effect on the endophytic establishment of the entomopathogenic fungus Tolypocladium cylindrosporum in tomato and bean plants. BioControl 2013, 58, 225–232. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Redda, T.; Mei, J.; Zhang, J.; Wu, B.; Jiang, X. A novel double-stranded RNA mycovirus isolated from Trichoderma harzianum. Virol. J. 2019, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, M.; Wang, J.; Bian, Y.; Xu, Z. Curing two predominant viruses occurring in Lentinula edodes by chemotherapy and mycelial fragmentation methods. J. Virol. Methods 2022, 300, 114370. [Google Scholar] [CrossRef]
- Parker, W.B. Metabolism and antiviral activity of ribavirin. Virus Res. 2005, 107, 165–171. [Google Scholar] [CrossRef]
- Eski, A.; Biryol, S.; Acici, O.; Demir, İ. Biocontrol of the western conifer seed bug, Leptoglossuss occidentalis Heidemann (Heteroptera: Coreidae) using indigenous entomopathogenic fungi. Egypt. J. Biol. Pest Control 2022, 32, 140. [Google Scholar] [CrossRef]
- Elias, K.S.; Cotty, P.J. Incidence and stability of infection by double-stranded RNA genetic elements in Aspergillus section flavi and effects on aflatoxigenicity. Can. J. Bot. 1996, 74, 716–725. [Google Scholar] [CrossRef]
- Khankhum, S.; Valverde, R.A.; Pastor-Corrales, M.A.; Osorno, J.M.; Sabanadzovic, S. Two endornaviruses show differential infection patterns between gene pools of Phaseolus vulgaris. Arch. Virol. 2015, 160, 1131–1137. [Google Scholar] [CrossRef]
- Thekke Veetil, T.; Ho, T.; Moyer, C.; Whitaker, V.M.; Tzanetakis, I.E. Detection of Strawberry necrotic shock virus using conventional and TaqMan® quantitative RT-PCR. J. Virol. Methods 2016, 235, 176–181. [Google Scholar] [CrossRef]
- van Diepeningen, A.D.; Debets, A.J.; Hoekstra, R.F. Dynamics of dsRNA mycoviruses in black Aspergillus populations. Fungal Genet. Biol. 2006, 43, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.; Mascarin, G.M.; Lopes, M.d.S.; Alves, M.C.D.F.; Rezende, J.M.; Gatti, M.S.V.; Dunlap, C.A.; Júnior, Í.D. Identification of double-stranded RNA viruses in Brazilian strains of Metarhizium anisopliae and their effects on fungal biology and virulence. Plant Gene. 2017, 11, 49–58. [Google Scholar] [CrossRef]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural Mechanism for Rifampicin Inhibition of Bacterial RNA Polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Kwon, Y.C.; Jeong, D.W.; Gim, S.I.; Ro, H.S.; Lee, H.S. Curing viruses in Pleurotus ostreatus by growth on a limited nutrient medium containing cAMP and rifamycin. J. Virol. Methods 2012, 185, 156–159. [Google Scholar] [CrossRef]
- Darissa, O.; Adam, G.; Schäfer, W. A dsRNA mycovirus causes hypovirulence of Fusarium graminearum to wheat and maize. Eur. J. Plant Pathol. 2012, 134, 181–189. [Google Scholar] [CrossRef]
- Lemus-Minor, C.G.; Canizares, M.C.; Garciá-Pedrajas, M.D.; Pérez-Artés, E. Fusarium oxysporum f. sp. Dianthi virus 1 accumulation is correlated with changes in virulence and other phenotypic traits of its fungal host. Phytopathology 2018, 108, 957–963. [Google Scholar] [CrossRef]
| Isolates | Mean Ct Values |
|---|---|
| Fc179 | 17.7 |
| 001 | 18.2 |
| Va221 | 19.6 |
| Single-Factor ANOVA | ||||||
|---|---|---|---|---|---|---|
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| Fc179.T | 3 | 89.031 | 29.677 | 2.375652 | ||
| 001.T | 3 | 79.994 | 26.66467 | 2.57666 | ||
| Va221.T | 3 | 87.631 | 29.21033 | 2.928101 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between-Groups | 15.77235 | 2 | 7.886174 | 3.002193 | 0.124863 | 5.143253 |
| Within-Groups | 15.76083 | 6 | 2.626805 | |||
| Total | 31.53318 | 8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, H.; Zamora-Ballesteros, C.; Diez-Casero, J.J. Effects of Thermal and Antibiotic Treatments on the Viral Accumulation of FcMV1 in Fusarium circinatum Isolates. J. Fungi 2025, 11, 267. https://doi.org/10.3390/jof11040267
Amin H, Zamora-Ballesteros C, Diez-Casero JJ. Effects of Thermal and Antibiotic Treatments on the Viral Accumulation of FcMV1 in Fusarium circinatum Isolates. Journal of Fungi. 2025; 11(4):267. https://doi.org/10.3390/jof11040267
Chicago/Turabian StyleAmin, Huma, Cristina Zamora-Ballesteros, and Julio Javier Diez-Casero. 2025. "Effects of Thermal and Antibiotic Treatments on the Viral Accumulation of FcMV1 in Fusarium circinatum Isolates" Journal of Fungi 11, no. 4: 267. https://doi.org/10.3390/jof11040267
APA StyleAmin, H., Zamora-Ballesteros, C., & Diez-Casero, J. J. (2025). Effects of Thermal and Antibiotic Treatments on the Viral Accumulation of FcMV1 in Fusarium circinatum Isolates. Journal of Fungi, 11(4), 267. https://doi.org/10.3390/jof11040267








