Transcriptome Analysis Revealed That Cell Wall Regulatory Pathways Are Involved in the Tolerance of Pleurotus ostreatus Mycelia to Different Heat Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
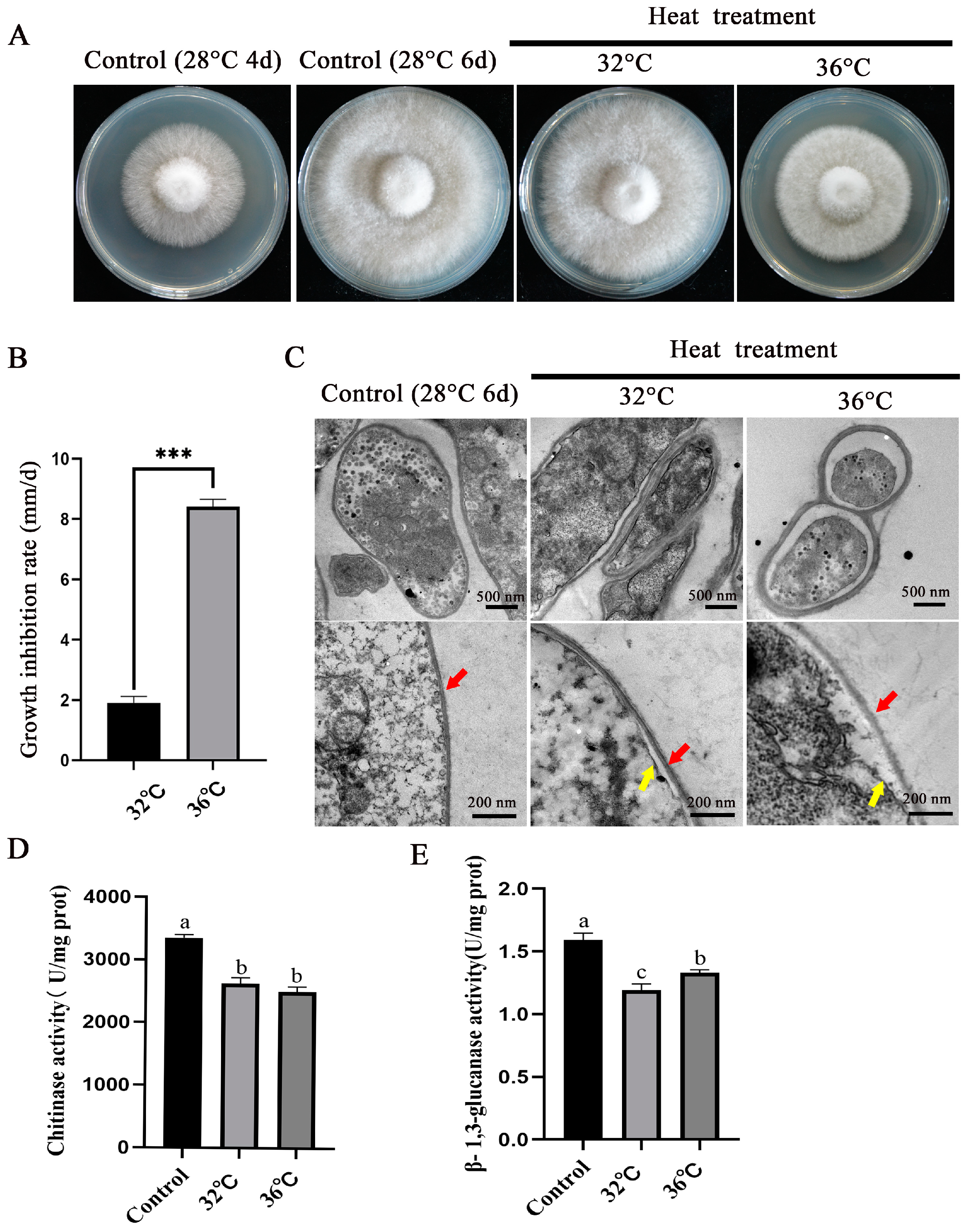
2.2. Electron Microscopy Observation and Physiological Index Determination
2.3. RNA Sequencing (RNA-Seq)
2.4. Differential Expression Analysis and Functional Annotation
2.5. Quantitative Real-Time PCR
3. Results
3.1. Evaluation and Electron Microscopy Observation of the Heat Resistance of P. ostreatus mycelia
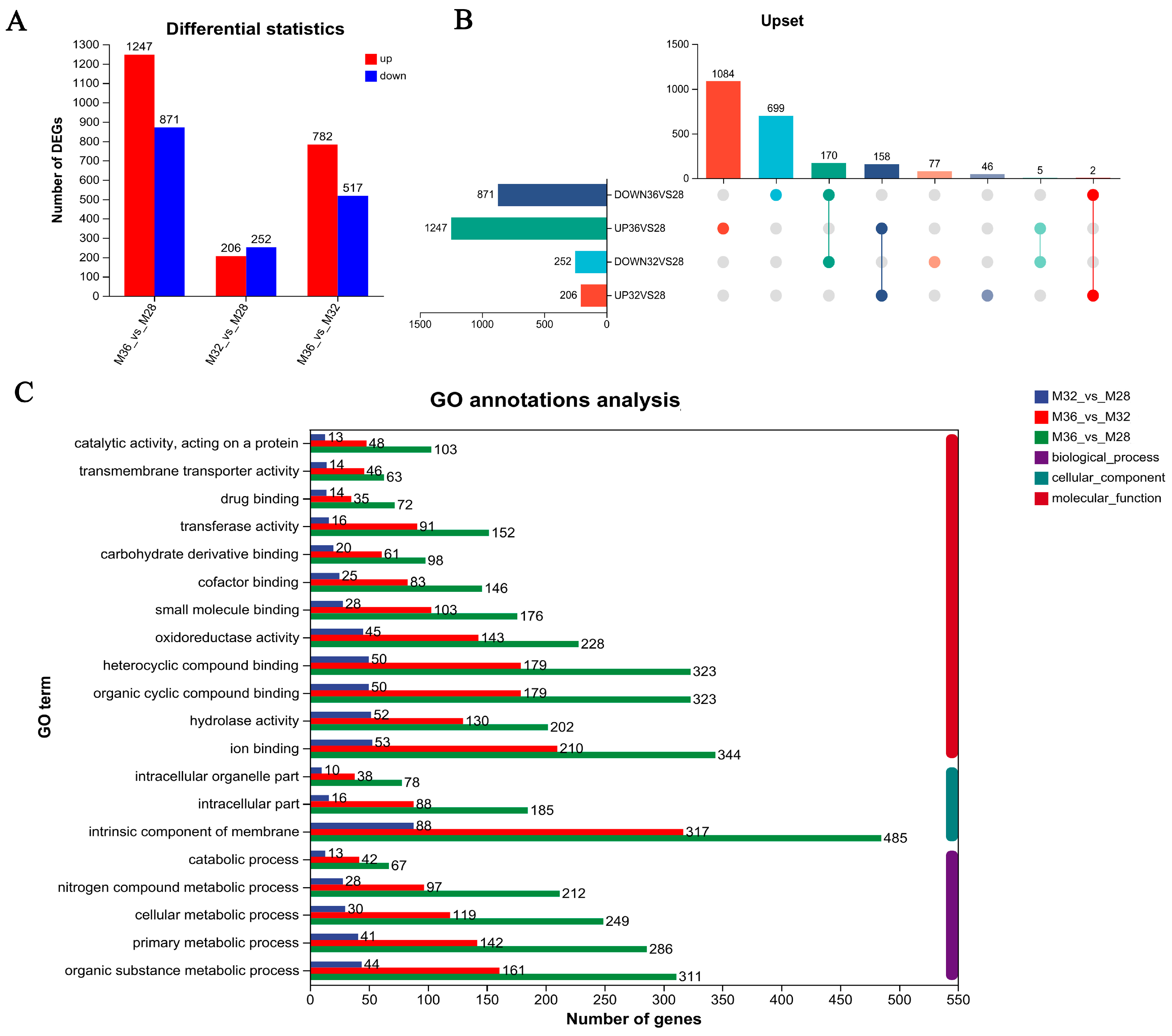
3.2. RNA-Seq Data Analysis
3.3. Identification of Differentially Expressed Genes (DEGs)
3.4. Functional Enrichment of DEGs in Mycelia Under Different Temperature Stresses
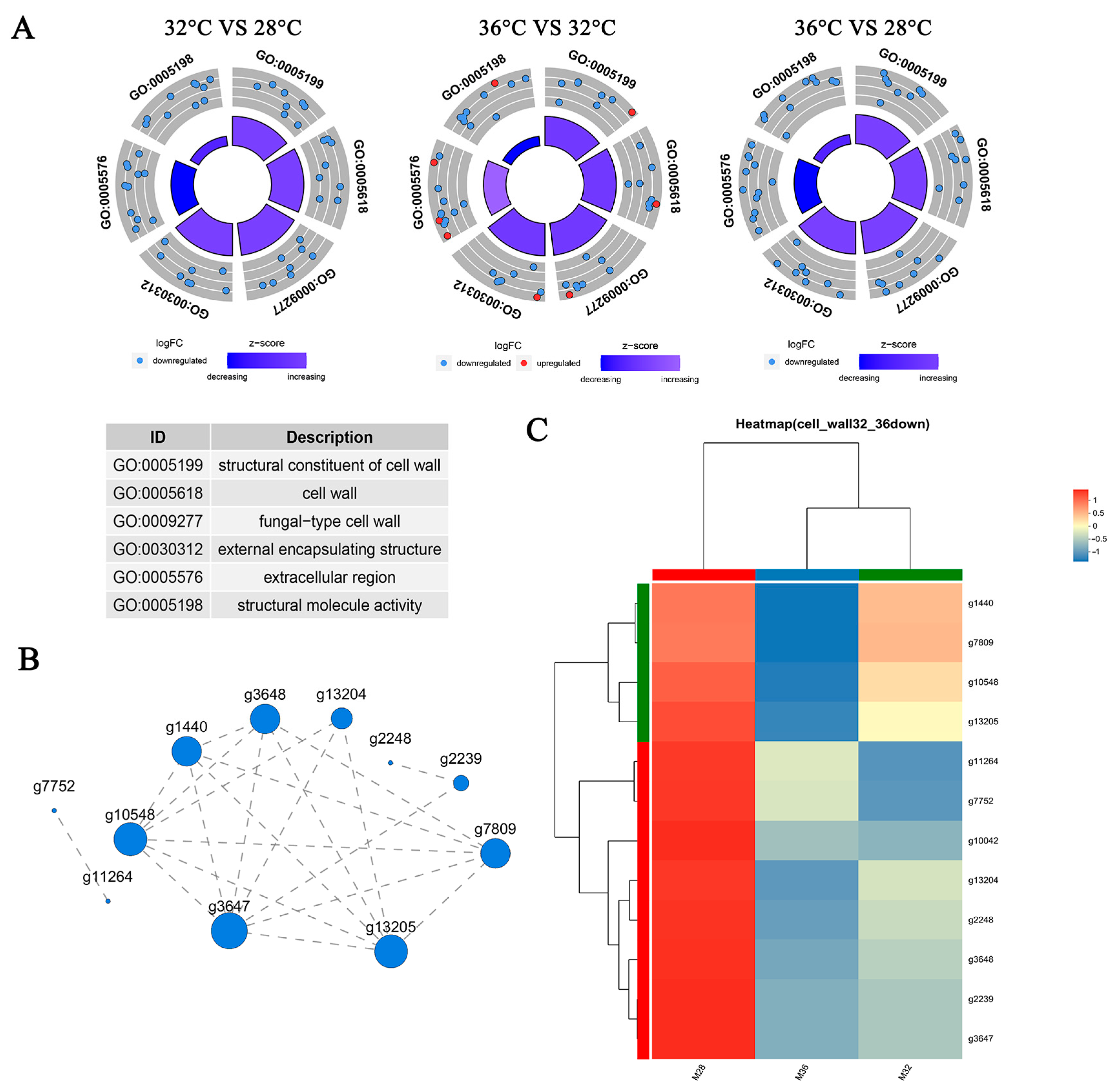
3.5. Functional Enrichment Analysis of Unique DEGs Under Different Heat Stresses
3.6. Functional Enrichment Analysis of Coregulated DEGs Under Different Heat Stresses
3.7. Genes Related to the Cell Wall Respond to Heat Stress
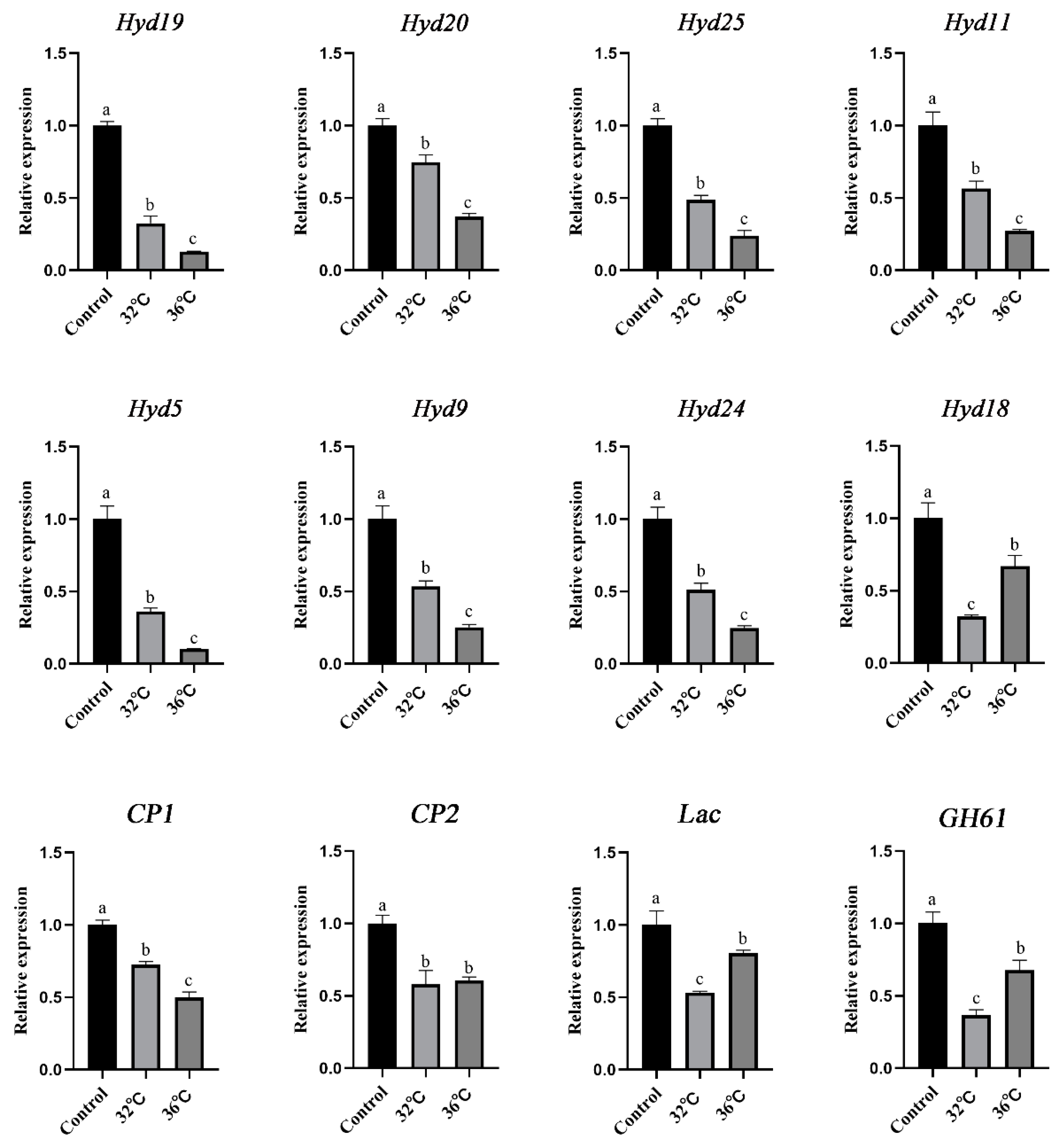
3.8. qRT–PCR Validation of the RNA-Seq Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Tania, M. Nutritional and medicinal importance of Pleurotus mushrooms: An overview. Food Rev. Int. 2012, 28, 313–329. [Google Scholar] [CrossRef]
- Hou, L.D.; Zhao, M.R.; Huang, C.Y.; Wu, X.L.; Zhang, J.X. Nitric oxide improves the tolerance of Pleurotus ostreatus to heat stress by inhibiting mitochondrial aconitase. Appl. Environ. Microbiol. 2020, 86, e02303-19. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.H.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. High temperature induced disruption of the cell wall integrity and structure in Pleurotus ostreatus mycelia. Appl. Microbiol. Biotechnol. 2018, 102, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Y.; Zhao, M.R.; Wu, X.L.; Zhang, J.X. Metabolic response of Pleurotus ostreatus to continuous heat stress. Front. Microbiol. 2019, 10, 3148. [Google Scholar] [CrossRef]
- Pei, J.Q.; Zhao, M.R.; Zhang, L.J.; Wu, X.L. The metacaspase gene PoMCA1 enhances the mycelial heat stress tolerance and regulates the fruiting body development of Pleurotus ostreatus. Horticulturae 2024, 10, 116. [Google Scholar] [CrossRef]
- Yin, C.M.; Zheng, L.S.; Zhu, J.H.; Chen, L.G.; Ma, A. Enhancing stress tolerance by overexpression of a methionine sulfoxide reductase A (MsrA) gene in Pleurotus ostreatus. Appl. Microbiol. Biotechnol. 2015, 99, 3115–3126. [Google Scholar] [CrossRef]
- Hou, L.D.; Wang, L.N.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. Expression patterns of two Pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019, 19, 231. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, P.; Leng, D.D.; Shang, L.; Zhang, C.H.; Wu, Z.W.; Wang, Z.H. salicylic acid treatment alleviates the heat stress response by reducing the intracellular ROS level and increasing the cytosolic trehalose content in Pleurotus ostreatus. Microbiol. Spectr. 2023, 11, e0311322. [Google Scholar] [CrossRef]
- Zhang, B.C.; Gao, Y.H.; Zhang, L.J.; Zhou, Y.H. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Balducci, L.; Deslauriers, A.; Giovannelli, A.; Beaulieu, M.; Delzon, S.; Rossi, S.; Rathgeber, C.B.K. How do drought and warming influence survival and wood traits of Picea mariana saplings? J. Exp. Bot. 2015, 66, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Chen, H.; Li, X.J.; Yang, M.F.; Liu, G.S.; Shen, S.H. A comparative proteomic analysis of rice seedlings under various high-temperature stresses. Biochim. Biophys. Acta BBA-Proteins Proteom. 2009, 1794, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Baldwin, L.; Domon, J.M.; Klimek, J.F.; Fournet, F.; Sellier, H.; Gillet, F.; Pelloux, J.; Lejeune-Hénaut, I.; Carpita, N.C.; Rayon, C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 2014, 104, 37–47. [Google Scholar] [CrossRef]
- Kutsuno, T.; Chowhan, S.; Kotake, T.; Takahashi, D. Temporal cell wall changes during cold acclimation and deacclimation and their potential involvement in freezing tolerance and growth. Physiol. Plant. 2023, 175, e13837. [Google Scholar] [CrossRef]
- Liu, J.; Willick, I.R.; Hiraki, H.; Forand, A.D.; Lawrence, J.R.; Swerhone, G.D.W.; Wei, Y.; Ghosh, S.; Lee, Y.K.; E Olsen, J.; et al. Cold and exogenous calcium alter Allium fistulosum cell wall pectin to depress intracellular freezing temperatures. J. Exp. Bot. 2022, 73, 3807–3822. [Google Scholar] [CrossRef]
- Takahashi, D.; Gorka, M.; Erban, A.; Graf, A.; Kopka, J.; Zuther, E.; Hincha, D.K. Both cold and sub-zero acclimation induce cell wall modification and changes in the extracellular proteome in Arabidopsis thaliana. Sci. Rep. 2019, 9, 2289. [Google Scholar] [CrossRef]
- Ene, I.V.; Walker, L.A.; Schiavone, M.; Lee, K.K.; Martin-Yken, H.; Dague, E.; Gow, N.A.R.; Munro, C.A.; Brown, A.J.P. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. mBio 2015, 6, e00986. [Google Scholar] [CrossRef]
- Laforet, L.; Moreno, I.; Sánchez-Fresneda, R.; Martínez-Esparza, M.; Martínez, J.P.; Argüelles, J.-C.; de Groot, P.W.J.; Valentín-Gomez, E. Pga26 Mediates filamentation and biofilm formation and is required for virulence in Candida albicans. FEMS Yeast Res. 2011, 11, 389–397. [Google Scholar] [CrossRef]
- Delgado, M.L.; Gil, M.L.; Gozalbo, D. Starvation and temperature upshift cause an increase in the enzymatically active cell wall-associated glyceraldehyde-3-phosphate dehydrogenase protein in yeast. FEMS Yeast Res. 2003, 4, 297–303. [Google Scholar] [CrossRef]
- Qu, J.B.; Zhao, M.R.; Hsiang, T.; Feng, X.; Zhang, J.X.; Huang, C.Y. Identification and characterization of small noncoding rnas in genome sequences of the edible fungus Pleurotus ostreatus. BioMed Res. Int. 2016, 2016, 2503023. [Google Scholar] [CrossRef] [PubMed]
- Tizro, P.; Choi, C.; Khanlou, N. Sample preparation for transmission electron microscopy. In Biobanking; Humana Press: New York, NY, USA, 2019; pp. 417–424. ISBN 978-1-4939-8935-5. [Google Scholar]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. Goatools: A python library for gene ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed]
- Castanera, R.; López-Varas, L.; Pisabarro, A.G.; Ramírez, L. Validation of reference genes for transcriptional analyses in Pleurotus ostreatus by using reverse transcription-quantitative PCR. Appl. Environ. Microbiol. 2015, 81, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.J.; Liu, H.; Xue, P.; Hong, M.T.; Guo, X.Y.; Xing, Z.Z.; Zhao, M.W.; Zhu, J. Function of a hydrophobin in growth and development, nitrogen regulation, and abiotic stress resistance of Ganoderma lucidum. FEMS Microbiol. Lett. 2023, 370, fnad051. [Google Scholar] [CrossRef]
- Yang, G.G.; Tang, L.G.; Gong, Y.D.; Xie, J.T.; Fu, Y.P.; Jiang, D.H.; Li, G.Q.; Collinge, D.B.; Chen, W.D.; Cheng, J.S. A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum. New Phytol. 2018, 217, 739–755. [Google Scholar] [CrossRef]
- Bai, Y.S.; Ali, S.; Liu, S.; Zhou, J.J.; Tang, Y.L. Characterization of plant laccase genes and their functions. Gene 2023, 852, 147060. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, A.; Li, M.J.; Cao, P.F.; Chen, T.X.; Zhang, G.; Shi, L.; Jiang, A.L.; Zhao, M.W. Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis, and hyphal branching of Ganoderma lucidum via cytosolic Ca2+. Appl. Environ. Microbiol. 2016, 82, 4112–4125. [Google Scholar] [CrossRef]
- Higgins, S.M.; Lilly, W.W. Multiple responses to heat stress by the basidiomycete Schizophyllum commune. Curr. Microbiol. 1993, 26, 123–127. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Aguilar-Uscanga, B.; Francois, J.M. A study of the yeast cell wall composition and structure in response to growthconditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Huang, B.F.; Chen, T.; Huang, J.; Qiu, L.L.; Lin, W.X. Study on the optimization of theautolytic conditions of Pleurotus tuber-regium (Fr.) Sing Mycelium. Chin. Agric. Sci. Bull. 2010, 26, 254–256. (In Chinese) [Google Scholar]
- Suwa, R.; Hakata, H.; Hara, H.; El-Shemy, H.A.; Adu-Gyamfi, J.J.; Nguyen, N.T.; Kanai, S.; Lightfoot, D.A.; Mohapatra, P.K.; Fujita, K. High Temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea Mays L.) genotypes. Plant Physiol. Biochem. 2010, 48, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.B.; dos Santos, T.B.; Vieira, L.G.E.; Ferrarese, M.d.L.L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; Petkowicz, C.L.d.O. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee Leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, S.S.; Wu, J.Y.; Sun, X.Y.; Ma, A. Heat stress in macrofungi: Effects and response mechanisms. Appl. Microbiol. Biot. 2021, 105, 7567–7576. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads Jr, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Guo, L.F.; Li, T.L.; Zhang, B.S.; Yan, K.X.; Meng, J.L.; Chang, M.C.; Hou, L.D. Family identification and functional study of copper transporter genes in Pleurotus ostreatus. Int. J. Mol. Sci. 2024, 25, 12154. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhang, T.J.; Lu, X.X.; Ma, B.L.; Ren, A.; Shi, L.; Jiang, A.L.; Yu, H.S.; Zhao, M.W. Membrane fluidity is involved in the regulation of heat stress induced secondary metabolism in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 1653–1668. [Google Scholar] [CrossRef]
- Schiphof, K.; Kawauchi, M.; Tsuji, K.; Yoshimi, A.; Tanaka, C.; Nakazawa, T.; Honda, Y. Functional analysis of basidiomycete specific chitin synthase genes in the agaricomycete fungus Pleurotus ostreatus. Fungal Genet. Biol. 2024, 172, 103893. [Google Scholar] [CrossRef]
- Lei, M.; Wu, X.L.; Huang, C.Y.; Qiu, Z.H.; Wang, L.N.; Zhang, R.Y.; Zhang, J.X. Trehalose induced by reactive oxygen species relieved the radial growth defects of Pleurotus ostreatus under heat stress. Appl. Microbiol. Biotechnol. 2019, 103, 5379–5390. [Google Scholar] [CrossRef]
- Hou, L.D.; Zhao, M.R.; Huang, C.Y.; He, Q.; Zhang, L.J.; Zhang, J.X. Alternative oxidase gene induced by nitric oxide is involved in the regulation of ROS and enhances the resistance of Pleurotus ostreatus to heat stress. Microb. Cell Fact. 2021, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.D.; Liu, J.; Zhang, S.X.; Zhou, Y.H.; Wang, J.; Du, M.; Lin, Y.; Chang, H. Calcium chloride treatment delays the quality deterioration of king oyster mushroom (Pleurotus eryngii) through positive regulation of antioxidants by PeCaMK1. Food Biosci. 2024, 61, 104778. [Google Scholar] [CrossRef]
- Cai, M.J.; Wu, X.X.; Liang, X.W.; Hu, H.P.; Liu, Y.C.; Yong, T.Q.; Li, X.M.; Xiao, C.; Gao, X.; Chen, S.D.; et al. Comparative proteomic analysis of two divergent strains provides insights into thermotolerance mechanisms of Ganoderma lingzhi. Fungal Genet. Biol. 2023, 167, 103796. [Google Scholar] [CrossRef]
- Hao, H.B.; Huang, J.C.; Wang, Q.; Juan, J.X.; Xiao, T.T.; Song, X.X.; Chen, H.; Zhang, J.J. Effects of heat stress on the differential expression of antioxidant enzymes and heat shock protein genes of Agaricus bisporus. Mycosystema 2021, 40, 616–625. [Google Scholar] [CrossRef]
- Wang, G.Z.; Ma, C.J.; Luo, Y.; Zhou, S.S.; Zhou, Y.; Ma, X.L.; Cai, Y.L.; Yu, J.J.; Bian, Y.B.; Gong, Y.H. Proteome and transcriptome reveal involvement of heat shock proteins and indoleacetic acid metabolism process in Lentinula Edodes thermotolerance. Cell Physiol. Biochem. 2018, 50, 1617–1637. [Google Scholar] [CrossRef]
- Yang, K.A.; Lim, C.J.; Hong, J.K.; Park, C.Y.; Cheong, Y.H.; Chung, W.S.; Lee, K.O.; Lee, S.Y.; Cho, M.J.; Lim, C.O. Identification of cell wall genes modified by a permissive high temperature in Chinese cabbage. Plant Sci. 2006, 171, 175–182. [Google Scholar] [CrossRef]
- Yu, E.; Fan, C.; Yang, Q.; Li, X.; Wan, B.; Dong, Y.; Wang, X.; Zhou, Y. Identification of heat responsive genes in Brassica napus siliques at the seed-filling stage through transcriptional profiling. PLoS ONE 2014, 9, e101914. [Google Scholar] [CrossRef]
- Song, Y.P.; Ci, D.; Tian, M.; Zhang, D.Q. Comparison of the physiological effects and transcriptome responses of Populus Simonii under different abiotic stresses. Plant Mol. Biol. 2014, 86, 139–156. [Google Scholar] [CrossRef]
- Neil, A.R.G.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 3. [Google Scholar] [CrossRef]



| Sample | Raw Reads | Clean Reads | Total Mapped | Multiple Mapped | Uniquely Mapped |
|---|---|---|---|---|---|
| M28_1 | 48052110 | 47638650 | 37745127 (79.23%) | 326372 (0.69%) | 37418755 (78.55%) |
| M28_2 | 44714218 | 44328286 | 35661000 (80.45%) | 297190 (0.67%) | 35363810 (79.78%) |
| M28_3 | 43469900 | 43070352 | 34767913 (80.72%) | 276462 (0.64%) | 34491451 (80.08%) |
| M32_1 | 48827640 | 48430718 | 38597088 (79.70%) | 322669 (0.67%) | 38274419 (79.03%) |
| M32_2 | 52328856 | 51949474 | 41105356 (79.13%) | 349492 (0.67%) | 40755864 (78.45%) |
| M32_3 | 54948006 | 54518710 | 43782677 (80.31%) | 355754 (0.65%) | 43426923 (79.66%) |
| M36_1 | 79999062 | 79401364 | 62828943 (79.13%) | 533955 (0.67%) | 62294988 (78.46%) |
| M36_2 | 47631544 | 47215264 | 37457481 (79.33%) | 320792 (0.68%) | 37136689 (78.65%) |
| M36_3 | 43746956 | 43421102 | 33967284 (78.23%) | 293893 (0.68%) | 33673391 (77.55%) |
| Gene ID | Name | Gene Function |
|---|---|---|
| g1440 | Hyd5 | hydrophobin modified 4 |
| g7809 | Hyd11 | putative class I hydrophobin superfamily |
| g10548 | Hyd25 | class I hydrophobin superfamily |
| g13205 | Hyd19 | hydrophobin |
| g11264 | GH61 | glycoside hydrolase family 61 protein |
| g7752 | Hyd18 | fungal hydrophobin |
| g10042 | Lac | laccase |
| g13204 | Hyd20 | class I hydrophobin superfamily |
| g2248 | CP1 | cerato-platanin-related secreted protein1 |
| g3648 | Hyd9 | class I hydrophobin superfamily |
| g2239 | CP2 | cerato-platanin-related secreted protein2 |
| g3647 | Hyd24 | class I hydrophobin superfamily |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Wang, J.; Li, T.; Zhang, B.; Yan, K.; Zhang, Z.; Geng, X.; Chang, M.; Meng, J. Transcriptome Analysis Revealed That Cell Wall Regulatory Pathways Are Involved in the Tolerance of Pleurotus ostreatus Mycelia to Different Heat Stresses. J. Fungi 2025, 11, 266. https://doi.org/10.3390/jof11040266
Hou L, Wang J, Li T, Zhang B, Yan K, Zhang Z, Geng X, Chang M, Meng J. Transcriptome Analysis Revealed That Cell Wall Regulatory Pathways Are Involved in the Tolerance of Pleurotus ostreatus Mycelia to Different Heat Stresses. Journal of Fungi. 2025; 11(4):266. https://doi.org/10.3390/jof11040266
Chicago/Turabian StyleHou, Ludan, Jingyi Wang, Tonglou Li, Baosheng Zhang, Kexing Yan, Zehua Zhang, Xueran Geng, Mingchang Chang, and Junlong Meng. 2025. "Transcriptome Analysis Revealed That Cell Wall Regulatory Pathways Are Involved in the Tolerance of Pleurotus ostreatus Mycelia to Different Heat Stresses" Journal of Fungi 11, no. 4: 266. https://doi.org/10.3390/jof11040266
APA StyleHou, L., Wang, J., Li, T., Zhang, B., Yan, K., Zhang, Z., Geng, X., Chang, M., & Meng, J. (2025). Transcriptome Analysis Revealed That Cell Wall Regulatory Pathways Are Involved in the Tolerance of Pleurotus ostreatus Mycelia to Different Heat Stresses. Journal of Fungi, 11(4), 266. https://doi.org/10.3390/jof11040266





