New Species of Byssosphaeria (Melanommataceae, Pleosporales) from the Mexican Tropical Montane Cloud Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphology
2.2. Extraction, Amplification, and Sequencing of DNA
2.3. Phylogenetic Analysis
| Fungal Species | Strain/Voucher | ITS | LSU | SSU | tef1-α | Reference |
|---|---|---|---|---|---|---|
| Byssosphaeria bautistae | T. Raymundo 6308 ENCB Holotype | PQ778308 | PQ773902 | PQ779856 | PV009316 | In this study |
| B. bautistae | R. Valenzuela 16932 ENCB | PQ778309 | PQ773903 | PQ779857 | PV009317 | In this study |
| B. chrysostoma | T. Raymundo 6221 ENCB Holotype | PQ778310 | PQ773904 | PQ779858 | PV009318 | In this study |
| B. chrysostoma | A. Cobos-Villagrán 352 ENCB | PQ778311 | PQ773905 | PQ779859 | PV009319 | In this study |
| B. guangdongense | ZHKUCC 22-0335 | OQ449320 | OQ449288 | OQ449337 | – | [32] |
| B. guangdongense | ZHKUCC 22-0336 | OQ449321 | OQ449289 | OQ449338 | – | |
| B. jamaicana | SMH 1403 | – | GU385152 | – | – | [1] |
| B. jamaicana | SMH 3464 | – | GU385153 | – | – | |
| B. jamaicana | SMH 3085 | – | GU385154 | – | – | |
| B. macarangae | MFLUCC 17 2655 | MH389782 | MH389778 | MH389780 | MH389784 | [45] |
| B. musae | MFLUCC 11 0146 | NR185364 | NG228735 | NG228698 | MH581149 | [46] |
| B. musae | MFLUCC 11 0182 | KP744435 | KP744477 | – | – | |
| B. neorhodomphala | E. Escudero-Leyva 190 ENCB Holotype | PQ778314 | PQ773908 | PQ779860 | PV009320 | In this study |
| B. neorhodomphala | T. Raymundo 4481 ENCB | PQ778315 | PQ773909 | PQ779861 | PV009321 | In this study |
| B. neoschiedermayriana | R. Valenzuela 16092 ENCB Holotype | PQ778312 | PQ773906 | PQ779862 | PV009322 | In this study |
| B. neoschiedermayriana | T. Raymundo 4199 ENCB | PQ778313 | PQ773907 | PQ779863 | PV009323 | In this study |
| B. poaceicola | HFJAU10337 | PP460781 | PP460774 | PP460766 | PP475455 | [47] |
| B. poaceicola | HFJAU10338 | PP460782 | PP460775 | PP460767 | PP475456 | |
| B. phoenicis | ZHKUCC 21-0122 | ON180685 | ON180683 | ON180691 | ON243583 | [48] |
| B. phoenicis | ZHKUCC 21-0123 | ON180686 | ON180684 | ON180692 | ON243584 | |
| B. rhodomphala | SMH3086 | – | GU385155 | – | – | [1] |
| B. rhodomphala | SMH 4363 | – | GU385156 | – | – | |
| B. rhodomphala | GKM L153N | – | GU385157 | – | GU327747 | |
| B. rhodomphala | ANM 942 | – | GU385160 | – | – | |
| B. rhodomphala | SMH 3402 | – | GU385170 | – | – | |
| B. rhodomphala | HA 400 | – | KT313008 | – | – | |
| B. rhodomphala | HA 200 | – | – | – | KT313006 | [49] |
| B. salebrosa | SMH 2387 | – | GU385162 | – | GU327748 | [1] |
| B. schiedermayeriana | MFLUCC 10-0100 | – | KT289894 | KT289896 | – | [7] |
| B. schiedermayeriana | SMH 1269 | – | GU385158 | – | – | [1] |
| B. schiedermayeriana | SMH 1816 | – | GU385159 | – | – | |
| B. schiedermayeriana | SMH 3157 | – | GU385163 | – | GU327745 | |
| B. schiedermayeriana | GKM 152N | – | GU385168 | – | GU327749 | |
| B. siamensis | MFLUCC 10 0099 | – | KT289895 | KT289897 | KT962059 | [7] |
| B. siamensis | MFLUCC 17 1800 | MG543923 | MG543914 | MG543917 | – | |
| B. siamensis | MFLU 18 0032 | MH388334 | MH376706 | MH388303 | MH388370 | [50] |
| B. siamensis | HFJAU10336 | PP460780 | PP460773 | PP460765 | PP475454 | [47] |
| B. taiwanense | MFLUCC 17 2643 | MH389783 | MH389779 | MH389781 | MH389785 | [45] |
| B. villosa | GKM 204N | – | GU385151 | – | GU327751 | [1] |
| Fusiconidium aquaticum | KUMCC 15-0300 | – | KX641894 | KX641895 | KX641896 | [51] |
| F. mackenziei | MFLU 14-0434 | – | KX611112 | KX611114 | KX611118 |
3. Results
3.1. Molecular Analysis
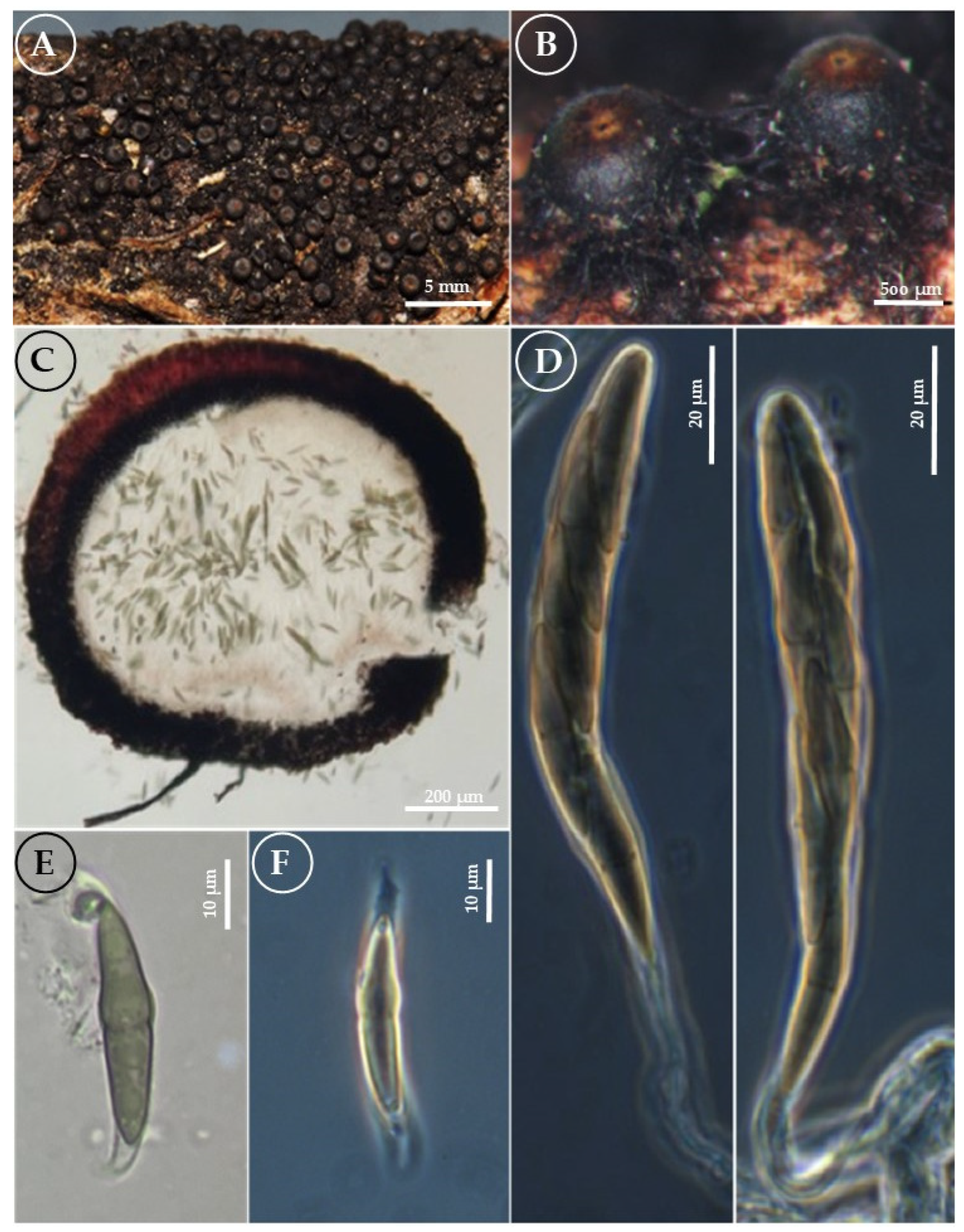
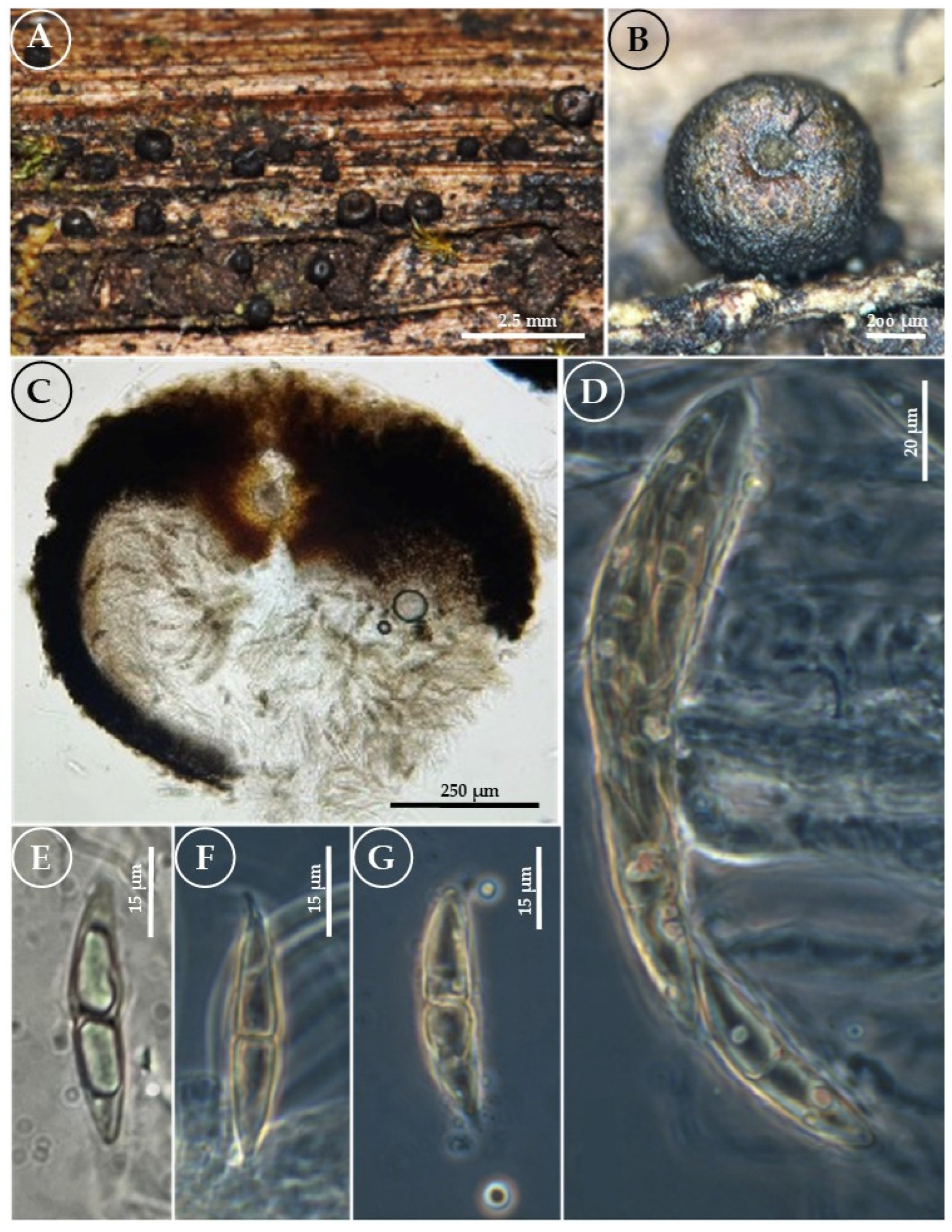
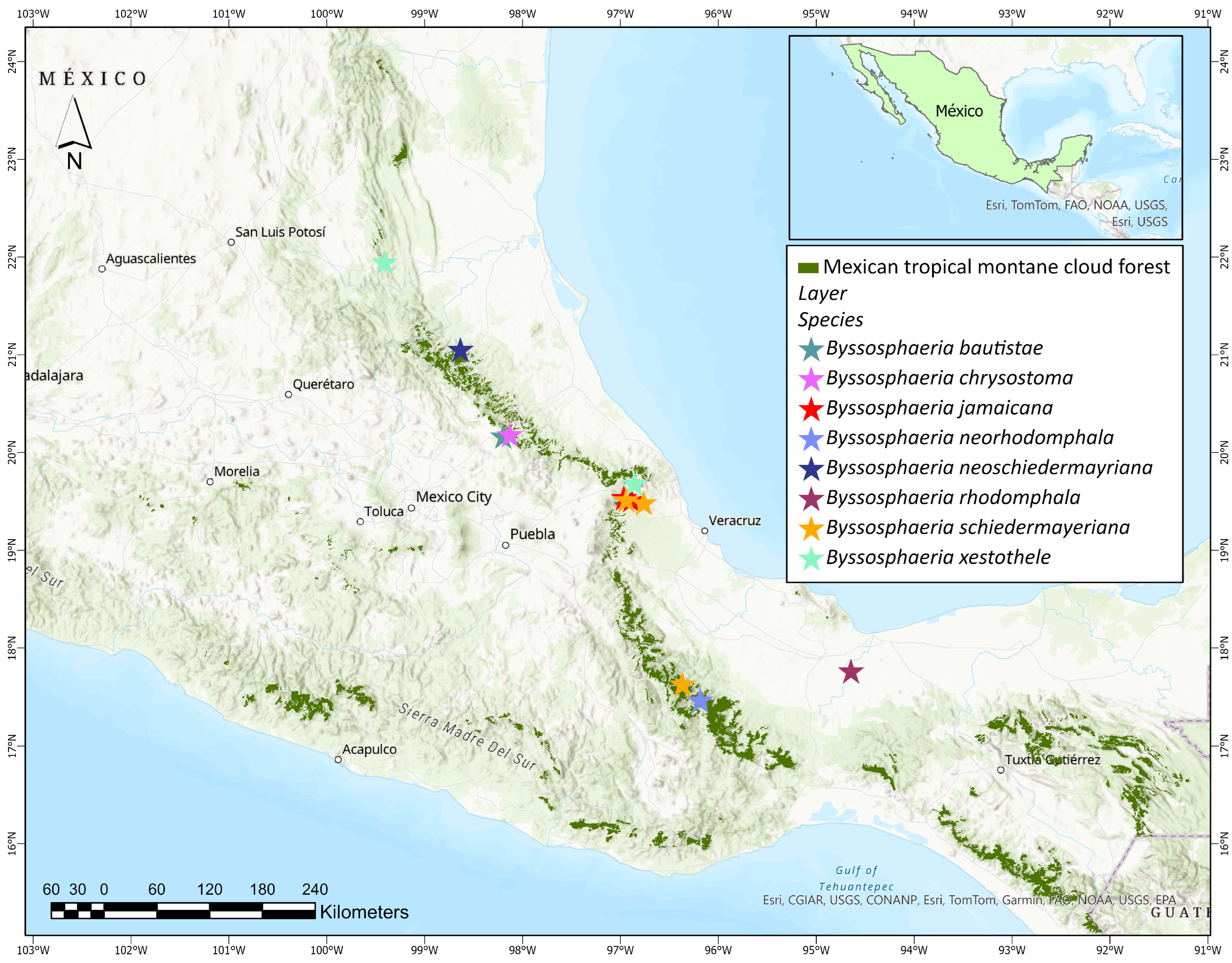
3.2. Taxonomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mugambi, G.K.; Huhndorf, S.M. Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae recircumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Stud. Mycol. 2009, 64, 103–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Crous, P.W.; Schoch, C.L.; Hyde, K.D. Pleosporales. Fungal Divers. 2012, 53, 1–221. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.K.; Ariyawansa, H.A.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Cooke, M.C.; Plowright, C.B. British Sphaeriacei. Grevillea 1879, 7, 77–89. [Google Scholar]
- Barr, M.E. Herpotrichia and its segregates. Mycotaxon 1984, 20, 1–38. [Google Scholar]
- Hyde, K.D.; Goh, T.K.; Taylor, J.E.; Fröhlich, J. Byssosphaeria, Chaetosphaeria, Niesslia and Ornatispora gen. nov., from palms. Mycol. Res. 1999, 103, 1423–1439. [Google Scholar] [CrossRef]
- Tian, Q.; Liu, J.K.; Hyde, K.D.; Wanasinghe, D.N.; Boonmee, S.; Jayasiri, S.C.; Luo, Z.L.; Taylor, J.E.; Phillips, A.J.L.; Bhat, D.J.; et al. Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales). Fungal Divers. 2015, 74, 267–324. [Google Scholar] [CrossRef]
- Dong, W.; Wang, B.; Hyde, K.D.; McKenzie, E.H.C.; Raja, H.A.; Tanaka, K.; Abdel-Wahab, M.A.; Abdel-Aziz1, F.A.; Doilom, M.; Phookamsak, R.; et al. Freshwater Dothideomycetes. Fungal Divers. 2020, 105, 319–575. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Kirk, P.M.; Sutton, B.C.; Pegler, D.N. Ainsworth & Bisby’s Dictionary of the Fungi, 8th ed.; CABI: Wallingford, UK, 1995; p. 616. [Google Scholar]
- Samuels, G.J.; Müller, E. Life-history studies of Brazilian ascomycetes 4. Three species of Herpotrichia and their Pyrenochaeta-like anamorphs. Sydowia 1978, 31, 157–168. [Google Scholar]
- Hyde, K.D.; McKenzie, E.H.C.; Koko, T.W. Towards incorporating anamorphic fungi in a natural classification—Checklist and notes for 2010. Mycosphere 2011, 2, 1–88. [Google Scholar]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Kirk, P.M. Index Fungorum. 2024. Available online: http://www.indexfungorum.org/names/names.asp (accessed on 21 October 2024).
- Medel, R. First records of the genus Byssosphaeria (Pleosporales) in Mexico and Venezuela. Mycotaxon 2007, 100, 247–250. [Google Scholar]
- Chacón-Zapata, S.; Tapia-Padilla, F. Algunas especies del género Byssosphaeria (Melanommataceae, Pleosporales) de Veracruz, México. Rev. Mex. Biodivers. 2013, 84, 739–745. [Google Scholar] [CrossRef]
- Raymundo, T.; Soto-Agudelo, R.; Bautista-Hernández, S.; Morales-Campos, A.; Valenzuela, R. Catálogo de los ascomicetos del bosque mesófilo de montaña de Tlanchinol, Hidalgo (México). Bol. Soc. Micol. Madrid 2016, 40, 79–104. [Google Scholar]
- Valenzuela, R.; Raymundo, T.; Reyes, P.; Guzmán-Guillermo, J.; Acosta, S.; Ramírez-Martínez, C.R.; Luna-Vega, I. Ascomycetes from the relic forest of Oreomunnea mexicana, Oaxaca, Mexico. Phytotaxa 2021, 528, 19–44. [Google Scholar] [CrossRef]
- Raymundo, T.; Valenzuela, R.; García-Martínez, Y.; Bravo-Álvarez, M.A.; Ramírez-Martínez, J.C.; Bautista-Hernández, S.; Palacios-Pacheco, M.; Luna-Vega, I. Ascomycetes (Fungi) from the relic forest of Fagus grandifolia subsp. mexicana in eastern Mexico. Phytotaxa 2019, 418, 1–41. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia, G.; Mena-Portales, J. Adiciones al conocimiento de la diversidad de los hongos anamorfos del bosque mesófilo de montaña del estado de Veracruz III. Acta Bot. Mex. 2010, 90, 19–42. [Google Scholar] [CrossRef]
- Medel-Ortiz, R.; Lorea-Hernández, F.G.; Baeza Guzmán, Y.; Palestina-Villa, E.N.; Belingheri-Lagunes, M.E. Ascomicetos asociados a angiospermas en el bosque mesófilo de montaña del centro de Veracruz, México. Acta Bot. Mex. 2019, 126, e1542. [Google Scholar] [CrossRef]
- Raymundo, T.; Valenzuela, R.; Ramírez-Martínez, C.R.; Martínez-Pineda, M.; Cobos-Villagrán, A.; Trejo-Arana, A.; Sánchez-Flores, M.; Gay-González, A.D.; Luna-Vega, I. New records of Ascomycetes from the Tropical Montane Cloud Forests of eastern Mexico. Phytotaxa 2020, 454, 161–185. [Google Scholar] [CrossRef]
- Gual-Díaz, M. Sistema de Información del Bosque Mesófilo de Montaña en México: Recopilación y sistematización de datos e información. In Bosques Mesófilos de Montaña de México. Diversidad, Ecología y Manejo, 1st ed.; Gual-Díaz, M., Rendón-Correa, A., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2014; pp. 90–92. [Google Scholar]
- INEGI 2010. Compendio de Información Geográfica Municipal de los Estados Unidos Mexicanos. Acaxochitlán y Tlanchinol Hidalgo; Naupan, Puebla; Santiago Camotlán, Oaxaca. Available online: https://www.inegi.org.mx/app/areasgeograficas/#collapse-Resumen (accessed on 21 October 2024).
- Chen, C.Y.; Hsieh, W.H. Byssosphaeria and Herpotrichia from Taiwan, with notes on the taxonomic relationship between hese two genera. Sydowia 2004, 56, 24–38. [Google Scholar]
- Martínez-González, C.R.; Ramírez-Mendoza, R.; Jiménez-Ramírez, J.; Gallegos-Vázquez, C.; Luna-Vega, I. Improved method for genomic DNA extraction for Opuntia Mill. (Cactaceae). Plant Methods 2017, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 135–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A Method for Designing Primer Sets for Speciation Studies in Filamentous Ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Xiong, Y.; Manawasinghe, I.S.; Wanasinghe, D.N.; Hongsanan, S.; Hyde, K.D.; Biao, X.; Dong, Z. Two new species and a new host record of Pleosporales (Dothideomycetes) from palm (Arecaceae) in Guangdong Province, China. New Zeal. J. Bot. 2023, 62, 165–191. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Müller, J.; Müller, K.; Neinhuis, C.; Quandt, D.; PhyDE®-Phylogenetic Data Editor. Program distributed by the author, v.0.9971. 2010. Available online: http://www.phyde.de (accessed on 7 October 2024).
- Maddison, W.; Maddison, D. Mesquite: A Modular System for Evolutionary Analysis. Version 3.31. 2023. Available online: http://mesquiteproject.org (accessed on 10 October 2024).
- Peng, J.; Swofford, D.L.; Kubatko, L. PAUP* Phylogenetics Estimation of speciation times under the multispecies coalescent. Bioinformatics 2022, 38, 5182–5190. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Kainer, D.; Mayer, C.; Stamatakis, A. Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evol. Biol. 2014, 14, 82. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, P.B.; Calcott, B.; Mayer, C.; Lanfear, R. Automatic selection of partitioning schemes for phylogenetic analyses using iterative k-means clustering of site rates. BMC Evol. Biol. 2015, 15, 13. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partition Finder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v.1.4.4. A Graphical Viewer of Phylogenetic Trees. 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 October 2024).
- Tennakoon, D.S.; Jeewon, R.; Kuo, C.H.; Hyde, K.D. Phylogenetic and morphological characterization of Byssosphaeria macarangae sp. nov., and B. taiwanense sp. nov. from Macaranga tanarius. Phytothaxa 2018, 364, 211–226. [Google Scholar] [CrossRef]
- Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Ariyawansa, H.A.; Bhat, D.J.; Boonmee, S.; Maharachchikumbura, S.S.N.; McKenzi, E.H.C.; Phookamsak, R.; Phukhamsakda, C.; et al. Fungal diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015, 72, 1–197. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Thambugala, K.M.; de Silva, N.I.; Song, H.Y.; Suwannarach, N.; Chen, F.S.; Hu, D.M. An overview of Melanommataceae (Pleosporales, Dothideomycetes): Current insight into the host associations and geographical distribution with some interesting novel additions from plant litter. MycoKeys 2024, 106, 43–96. [Google Scholar] [CrossRef] [PubMed]
- Kularathnage, N.D.; Wanasinghe, D.N.; Senanayake, I.C.; Yang, Y.; Manawasinghe, I.S.; Phillips, A.J.L.; Hyde, K.D.; Dong, W.; Song, J. Microfungi associated with ornamental palms: Byssosphaeria phoenicis sp. nov. (Melanommataceae) and Pseudocoleophoma rhapidis sp. nov. (Dictyosporiaceae) from south China. Phytotaxa 2022, 568, 149–169. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Thambugala, K.M.; Manamgoda, D.S.; Jayawardena, R.; Camporesi, R.; Boonmee, S.; Wanasinghe, D.N.; Phookamsak, R.; Hongsanan, S.; Singtripop, C.; et al. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers. 2015, 71, 85–139. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; McKenzie, E.H.C.; Bhat, D.J.; Phillips, A.J.L.; Wanasinghe, D.N.; Samarakoon, M.C.; Jayawardena, R.S.; Dissanayake, A.J.; Tennakoon, D.S.; et al. Fungal diversity notes 840–928: Micro-fungi associated with Pandanaceae. Fungal Divers. 2018, 93, 1–160. [Google Scholar] [CrossRef]
- Li, J.; Jeewon, R.; Luo, Z.; Phookamsak, R.; Bhat, D.J.; Mapook, A.; Phukhamsakda, C.; Camporesi, E.; Lumyong, S.; Hyde, K.D. Morphological characterization and DNA based taxonomy of Fusiconidium gen. nov. with two novel taxa within Melanommataceae (Pleosporales). Phytotaxa 2017, 308, 206–218. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhuang, W.Y. Notes on the genus Byssosphaeria (Melanommataceae) from China. Mycosystema 2008, 27, 48–53. [Google Scholar]
- Cooke, M.C. Synopsis Pyrenomycetum. In A Quarterly Record of Cryptogamic Botany and Its Literature; Cooke, M.C., Ed.; Grevillea: London, UK, 1887; pp. 80–82. [Google Scholar]
- Wang, Y.Z.; Aptroot, A.; Hyde, K.D. Revision of the Ascomycete Genus Amphisphaeria, 1st ed.; Fungal Diversity Press: Hong Kong, 2004; p. 168. [Google Scholar]
- Barr, M.E. Melanommatales (Loculoascomycetes). North Am. Flora II 1990, 13, 11–29. [Google Scholar]
- Liu, J.K.; Hyde, K.D.; Jeewon, R.; Phillips, A.; Maharachchikumbura, S.S.N.; Ryberg, M.; Liu, Z.Y.; Zhao, Q. Ranking higertaxa using divergence times: A case study in Dothideomycetes. Fungal Divers. 2017, 84, 75–99. [Google Scholar] [CrossRef]



| Loci/Segment | Primer | Sequence 5′-3′ | T(°C) | Reference |
|---|---|---|---|---|
| ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | 58 | [28] |
| ITS4 | TCCTCCGCTTATTGATATGC | 58 | ||
| SSU | NS1 | GTAGTCATATGCTTGTCTC | 56 | |
| NS2 | GGCTGCTGGCACCAGACTTGC | 56 | ||
| LSU | LROR | ACCCGCTGAACTTAAGC | 48 | [29] |
| LR3 | GGTCCGTGTTTCAAGAC | 48 | ||
| tef1-α | EF1-983F | GCYCCYGGHCAYCGTGAYTTYAT | 56 | [30] |
| EF1-2218R | ATGACACCRACRGCRACRGTYTG | 56 | [31] |
| Species | Ascomata Diam × High (µm) | Peridium | Asci (µm) | Ascospores | Habitat | Type | Distribution | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wide (µm) | Color Around the Ostiole | Color in Contact with KOH | Size Large × Wide (µm) | Number of Septa | Color | |||||||
| B. alnea | 220–460 | 40–50 | Shining black, pallid | – | 105–140 × 7.5–12 | 19.5–24 × 4–5 | 1–3 | Light brown | On branches of Alnus | USA (NY) | USA, Nova Scotia, China | [5] |
| B. andurnensis | – | – | – | – | – | – | 0 | Brown | – | – | – | [13] |
| B. bautistae | 900–1100 × 700–1000 | 55–90 | Pale yellowish-to-orange zone | Orange pigment that turns vinaceous | 105–125 × 11–12 | (31–) 33–33 (–36) × 5–6 | 1 | Olive-brown | On decaying wood | Mexico (Hgo.) | Mexico | In this study |
| B. byssiseda | – | – | – | – | – | – | On Salix alba | – | – | [13] | ||
| B. chrysostoma | 700–800 × 850–950 | 75–150 | Light brown-to-slightly golden | Red-to-light pink-orange | 122–152 × 10–13 | (37–)40–47(–50) × 6–8 | 1 | Grayish-brown | On fallen twigs | Mexico (Pue.) | Mexico | In this study |
| B. epileuca | – | – | – | – | – | – | – | Sri Lanka | Sri Lanka | [53] | ||
| B. erumpens | 420–520 × 450–550 | 20–30 to 70 | Pallid | – | 105–130 × 12–15 | 20–25 × 5–6 | 1 | Dark brown | On dead stems of Litsea sp. | Taiwan | Taiwan, China | [24] |
| B. erythrinae | – | – | – | – | – | – | – | – | On dead branches of Erythrina indica | – | New Caledonia | [13] |
| B. guangdongense | 480–640 × 310–500 | 40–70 | Orange-to-light brown | – | 110–160 × 10–25 | 30–40 × 5–10 | 1 | Hyaline-to-brown | Saprobic on rotting branches on Phoenix roebelenii (Arecaceae) | China | China | [32] |
| B. hainanensis | 182–291 × 165–280 | 33–58 | – | – | 115–130 × 7–11 | 10.5–13.9 × 3.5–5.2 | 1 | Light clear brown | On decaying wood | China | China | [52] |
| B. holophaea | 500 | – | – | – | – | 22–24 × 8 | 2 | – | On branches | USA (PA) | USA | [53] |
| B. imposita | – | – | – | – | – | 25 × 6 | 0 | Brown | On dead branches | USA (PA) | USA | |
| B. jamaicana | 340–500 | 50–60 | Dark reddish brown | – | 80–120 × 12–15 | 25–35 × 7–8 | 1–3 | Light-to-clear brown | On decorticated rooting wood | Jamaica | Jamaica, Puerto Rico, Trinidad & Tobago, China, Mexico | [5] |
| B. juniperi | – | – | – | – | 100–120 × 20 | 30–35 × 10–12 | – | – | On bark of Juniperus monosperma | USA (CO) | USA | [54] |
| B. macarangae | 60–120 × 80–150 | 20–30 | Black | – | 100 × 7–10 | 20–25 × 4–5 | 1 with 2 euseptate | Hyaline | On decaying wood of Macaranga tanarius | Taiwan | Taiwan | [45] |
| B. musae | 450–630 × 430–540 | 35–80 | Orange-to-yellow | – | (120–) 125–135 (–145) × (11.5–) 12–14 (–17) | 30–33 (–36) × (4–) 5–6 | 1 (–3) | Hyaline-to-light brown | On leaf sheath of Musa sp. (Musaceae) | Thailand | Thailand | [46] |
| B. neorhodomphala | 300–500 × 400–600 | 25–65 | Reddish-orange-to-intense red | Reddish to a wine color to pink | 90–95 × 12–13 | (18–) 20–23 (–24) × 5.5–6 (–7) | 1 | Brown-olive-to-dark brown | On decaying wood | Mexico (Oax.) | Mexico | In this study |
| B. neoschiedermayriana | 400–770 | 60–90 | Reddish orange | Pink-fuchsia | 142–158 (–170) × 13–14 (–15) | 36–46 (–50) × 6–7 | 1 | Pale brown | On decaying wood | Mexico (Hgo.) | Mexico | In this study |
| B. oviformis | 1000–1500 | 100–130 | Dull black | – | 120–130 × 7–9 | 25–30 × (2.5–) 3–3.5 | 1 | Hyalinae | On blackened decorticated wood | Jamaica | Jamaica, Hong Kong, China | [5] |
| B. pardalios | – | – | – | – | – | 8.8 long | 0 | Brown | – | – | – | [13] |
| B. phoenicis | 580–625 × 600–650 | 90–110 | Reddish orange | – | 100–160 × 10–15 | 25–30 × 5–7 | 1 (–3) | Pale brown-to-pale olivaceous | On dead petioles of Phoenix roebelenii (Arecaceae) | China | China | [48] |
| B. poaceicola | 550–650 × 600–800 | 30–45 | Orange-to-yellow | – | 165–180 × 12–15 | 32–40 × 7–8 | 1 | Hyaline-to-pale brown | On dead stem of Arundo pliniana (Poaceae) | China | China | [47] |
| B. picta | – | – | – | – | – | 20–22 × 8–10 | 0 | Brown | On decorticated wood | India | India | [53] |
| B. purpureofusca | – | – | – | – | – | – | 0 | Brown | On branches of Quercus | USA (PA) | USA | |
| B. rhodomelaena | – | – | – | – | – | 10 × 6 | 0 | Brown | On rotten wood. | USA (Carolina & PA) | USA | |
| B. rhodomphala | 220–500 | 20–60 | Red, orange, or yellow pulverulence | Pigment leaching | (50–) 85–120 × 10–13 | (16–) 18–23 (–25) × (5–) 6–7.5 (–9) | 1(–3) | Light brown | On wood and periderm of various trees | USA (OH) | Cosmopolitan | [55] |
| B. rubiginosa | – | – | – | – | – | 20–24 × 4–6 | 2 | Hyaline-to-pale brown | – | USA (NY) | USA | [53] |
| B. salebrosa | 440–800 | 30–35 to 55–100 (in base) | Yellowish | Non-leaching pigment | 120–150 × 13–16.5 | (30–) 40–50 × (6–) 7– 9 | 1–3 (–5) | Hyaline-to-light brown | On woody substrates, on dead roots of Acer spicatum (Sapindaceae), and on branches of Vaccinium or Andromeda | USA (NY) | USA, Canada | [5] |
| B. schiedermayeriana | 500–825 | 50–100 | Red or orange | – | (80–) 100–150 × 12–15 | (25–) 32–42 × 5–8 (–9) | 1–3 (–5) | Light brown | On varied substrates, rotting logs and branches, endocarps of coconut, culms, and petioles on wood or cord in greenhouses. On rotten branches of Sambucus nigra | Austria | Cosmopolitan | |
| B. semen | 400–600 × 330–550 | 40–84 | Pallid | – | 80–110 × 9–12 | 20–30 × 3.5–4.5 (–6) | 1 (–3) | Hyaline-to-light brown | On decaying petioles of Sorbus sp., rotting hardwood | USA (NY) | USA | |
| B. siamensis | 501–692 × 561–720 | 39–42 | Orange-to-yellow | – | 112–148 × 10–16 | 40.5–50 × 7–11 | 1 (–3) | Hyaline-to-pale yellow | On decaying wood and unidentified host | Thailand | Thailand | [7] |
| B. taiwanense | 450–500 × 460–540 | 35–50 to 75–85 (in base) to 75–95 (wide near ostiole) | Orange-to-yellow | – | (120–) 125–150 (–155) × (11.6–) 12–14 (–14.8) | 30–35 × 7–8 | 1 | Hyaline-to-light brown | On decaying wood of Macaranga tanarius | Taiwan | Taiwan | [45] |
| B. xestothele | 330–440 × 330–550 | 30–52 | Reddish | – | 70–100 × 9–12 | 20–26 × 4.5–6 | 1–3 | – | On fallen branches of Cornus florida or old leathery leaves | USA (SC) | USA, Mexico | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobos-Villagrán, A.; Pérez-Valdespino, A.; Valenzuela, R.; Martínez-González, C.R.; Luna-Vega, I.; Villa-Tanaca, L.; Rodríguez-Tovar, A.V.; Raymundo, T. New Species of Byssosphaeria (Melanommataceae, Pleosporales) from the Mexican Tropical Montane Cloud Forest. J. Fungi 2025, 11, 89. https://doi.org/10.3390/jof11020089
Cobos-Villagrán A, Pérez-Valdespino A, Valenzuela R, Martínez-González CR, Luna-Vega I, Villa-Tanaca L, Rodríguez-Tovar AV, Raymundo T. New Species of Byssosphaeria (Melanommataceae, Pleosporales) from the Mexican Tropical Montane Cloud Forest. Journal of Fungi. 2025; 11(2):89. https://doi.org/10.3390/jof11020089
Chicago/Turabian StyleCobos-Villagrán, Aurora, Abigail Pérez-Valdespino, Ricardo Valenzuela, César Ramiro Martínez-González, Isolda Luna-Vega, Lourdes Villa-Tanaca, Aída Verónica Rodríguez-Tovar, and Tania Raymundo. 2025. "New Species of Byssosphaeria (Melanommataceae, Pleosporales) from the Mexican Tropical Montane Cloud Forest" Journal of Fungi 11, no. 2: 89. https://doi.org/10.3390/jof11020089
APA StyleCobos-Villagrán, A., Pérez-Valdespino, A., Valenzuela, R., Martínez-González, C. R., Luna-Vega, I., Villa-Tanaca, L., Rodríguez-Tovar, A. V., & Raymundo, T. (2025). New Species of Byssosphaeria (Melanommataceae, Pleosporales) from the Mexican Tropical Montane Cloud Forest. Journal of Fungi, 11(2), 89. https://doi.org/10.3390/jof11020089









