Harnessing Nanoparticles and Nanosuspensions to Combat Powdery Mildew: A Frontier in Vegetable and Fruit Protection
Abstract
1. Introduction
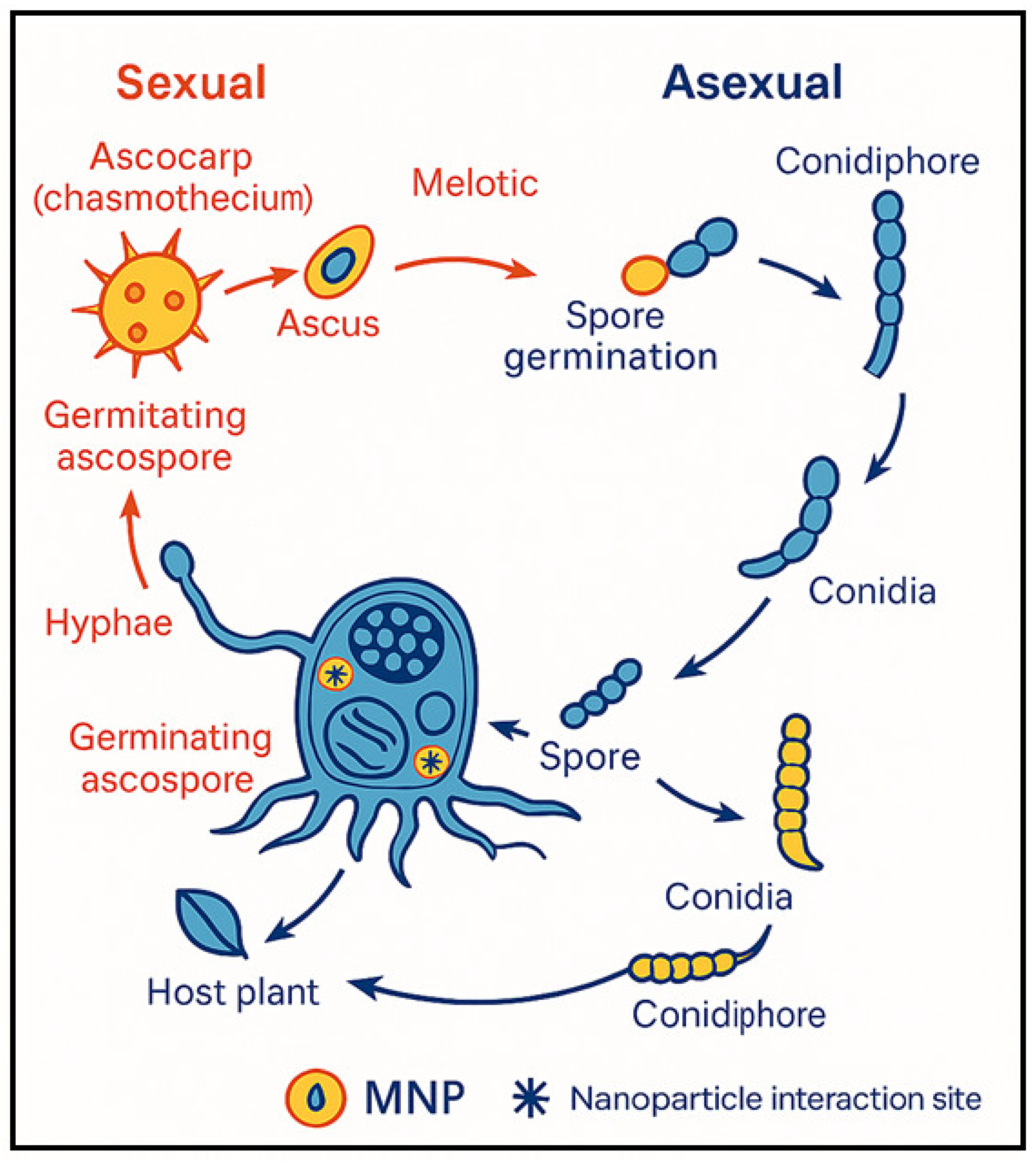
2. Powdery Mildew: Biology and Impact
3. Nanoparticles and Nanosuspensions Applications on Powdery Mildew
3.1. Metallic Nanoparticles (MNPs) Effect on Powdery Mildew
3.1.1. Synthesis Methods of Nanoparticles Affecting Antifungal Activity
3.1.2. Properties of Nanoparticles Affecting Antifungal Activity
3.2. Non-Metallic Nanoparticles (NMNPs) Effect on Powdery Mildew
3.3. Nano-Encapsulated Fungicides and Essential Oils
| Nanoparticle | Crop Name | Size (nm) | Concentration/Dose | Synthesis Method | Application Method | Effectiveness/Efficiency | Additional Benefits | References |
|---|---|---|---|---|---|---|---|---|
| Chitosan + CSEO | Cucumber | 300 | 1–3 mg·mL−1 | CS | Foliar | Significant reduction in powdery mildew severity | Increased chlorophyll, phenolics, flavonoids, defense enzyme activity, and gene expression | [16] |
| Nutragreen® nanoscale carrier | Hop | NS | 30% v/v | CS | Foliar | ~70–90% reduction in powdery mildew severity | Reduced pesticide use; improved cone yield and α-acid content; enhanced leaf and cone protection | [36] |
| Sulfur | Okra | 50–90 | 1000 ppm | CS | Foliar | 100% inhibition of conidial germination | Disrupted cleistothecia, reduced phytotoxicity | [64] |
| Sulfur | Apple | 50–90 | 1000 ppm | CS | Foliar | Effective at lower doses | Safer than conventional sulfur for fruit crops | [64] |
| Sulfur | Mango | 85 | 100 ppm | CS | Foliar | 14.6% reduction in powdery mildew | 342% increase in productivity; improved fruit quality; enhanced antioxidant enzyme activity | [64] |
| Sulfur | Cucumber | 12.2–23.5 | 500 mg·L−1 | CS | Foliar | 60.9% reduction in powdery mildew | Matched azoxystrobin (74%) and diniconazole (68.8%) in efficacy; highest fruit yield and quality | [65] |
| Chitosan oligomers + Streptomyces metabolites/hydrolyzed gluten | Grapevine | <2 kDa | ~40 mL | Enzymatic hydrolysis/fermentation | Foliar & root | Comparable to conventional fungicides | Effective against Erysiphe necator; biostimulant effects; reduced overwintering inoculum | [68] |
| Chitosan NPs + SSEO | Cucumber | ~116.2 | 400 µg·mL−1 | CS | Foliar | Significant reduction severity | High encapsulation efficiency; spherical morphology; elevated phenolics, flavonoids, and antioxidant enzymes activity | [69] |
| Thyme oil nanoemulsion | Lettuce | ~83 | 10% (v/v) | Ultrasonic emulsification | Foliar | ~75% disease reduction | Maintains beneficial microbes; stable for >3 months; effective even when diluted | [70] |
| Chitosan | Cucumber | 150–250 | 0.1–0.2% (w/v) | CS | Foliar | ~70% disease reduction | Enhances chlorophyll and defense enzymes | [82] |
| Chitosan | Tomato | 20–100 | 0.1–1% (w/v) | CS | Foliar | Effective against powdery mildew compared with tebuconazole at early stage | Induces systemic resistance, enhances growth | [83] |
| Chitosan | Cucumber | 20–100 | 0.1–1% (w/v) | CS | Foliar | Effective against powdery mildew compared with tebuconazole | Improved resistance, growth promotion | [83] |
| SiO2- | Grape | 50–80 | 50–100 mg L−1 | CS | Foliar | 85–90% reduction | Strengthens epidermis; Si-mediated resistance | [84] |
| SiO2 | Cucumber | 40–60 | 50 mg L−1 | CS | Foliar | 80–90% mildew reduction | Reinforces cuticle; nontoxic | [84] |
| SiO2 | Watermelon | Mesoporous SNPs | NS | CS | Root dip | 40% reduction in disease severity | Downregulation of stress genes | [85] |
| SiO2 | Cucumber | 10–100 | NS | CS/GS | Foliar | High efficacy | Improved photosynthesis, enzyme activity, stomatal conductance | [85] |
| SiO2 | Cucumber | 10–100 | 1.7–56 mM | CS | Foliar | Up to 87% reduction in powdery mildew | Improved resistance, structural strength | [86] |
| Silica–alginate nanocomposite | Pumpkin | 70–150 | 25–75 mg L−1 | CS | Foliar | 80% mildew control | Reinforces epidermis; water balance | [87] |
| Silica–chitosan | Spinach | 80–150 | 0.1% (w/v) | CS | Foliar | 65% infection reduction | Improves leaf turgidity; safe | [87] |
| Silica–pectin | Apple | 40–90 | 50 mg L−1 | CS | Foliar | 75% reduction | Biodegradable; strengthens cuticle | [88] |
| Silica–pectin | Peach | 40–100 | 50 mg L−1 | CS | Foliar | 78% mildew suppression | Strengthens fruit epidermis | [88] |
| Carbon nanotubes | Tomato | 10–40 | 10–25 mg L−1 | CS | Foliar | ~55% mildew reduction | Boosts antioxidants | [89] |
| Graphene oxide nanosheets | Cucumber | 30–200 | 25–50 mg L−1 | CS | Foliar | ~60% reduction | Activates enzymes; nutrient uptake | [90] |
| Nano-encapsulated lemongrass EO (Alginate) | Strawberry | 150–300 | 2–4 mg mL−1 | CS | Foliar | ~85% infection reduction | Antioxidant; flavor-safe | [91] |
| Nanobubble water | Papaya | 70–130 | 5 × 108 to 5 × 109 bubbles/mL | CS | Foliar | Effective against powdery mildew | Non-toxic, enhances root zone health | [92] |
4. Mechanisms of Action Against Powdery Mildew
4.1. Direct Antifungal Effects of Metallic Nanoparticles
4.2. Indirect Effects: Induced Resistance and Gene Regulation
4.3. Synergy with Conventional Fungicides
5. Challenges and Limitations
6. Future Perspectives and Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghalehgolabbehbahani, A.; Zinati, G.; Hamido, S.; Bhusal, N.; Dhakal, M.; Afshar, R.K.; Contina, J.B.; Caetani, R.; Smith, A.; Panday, D. Integrated control of powdery mildew using UV light exposure and OMRI-certified fungicide for greenhouse organic lettuce production. Horticulturae 2025, 11, 246. [Google Scholar] [CrossRef]
- Rehman, A.U.; Davik, J.; Karisto, P.; Astakhova, T.; Sønsteby, A. A major QTL region associated with powdery mildew resistance in leaves and fruits of the reconstructed garden strawberry. Theor. Appl. Genet. 2025, 138, 93. [Google Scholar] [CrossRef]
- Amsalem, L.; Freeman, S.; Rav-David, D.; Nitzani, Y.; Sztejnberg, A.; Pertot, I.; Elad, Y. Effect of climatic factors on powdery mildew caused by Sphaerotheca macularis f. sp. fragariae on strawberry. Eur. J. Plant Pathol. 2006, 114, 283–292. [Google Scholar] [CrossRef]
- McGrath, M.T.; Staniszewska, H.; Shishko, N. Fungicide Sensitivity of Sphaerotheca fuliginea populations in the United States. Plant Dis. 1996, 80, 697–703. [Google Scholar] [CrossRef]
- Saharan, G.S.; Mehta, N.; Saharan, M.S. Powdery Mildew Disease of Crucifers: Biology, Ecology and Disease Management; Chapter 3, The Pathogen; Springer Nature: Singapore, 2019. [Google Scholar] [CrossRef]
- Nie, J.; Yuan, Q.; Zhang, W.; Pan, J. Genetics, resistance mechanism, and breeding of powdery mildew resistance in cucumbers (Cucumis sativus L.). Hortic. Plant J. 2023, 9, 603–615. [Google Scholar] [CrossRef]
- Qi, Q.; Fan, C.; Wu, H.; Sun, L.; Cao, C. Preparation of Trichoderma asperellum microcapsules and biocontrol of cucumber powdery mildew. Microbiol. Spectr. 2023, 11, e05084-22. [Google Scholar] [CrossRef]
- Elagamey, E.; Abdellatef, M.A.; Haridy, M.S.; Abd El-aziz, E.S.A. Evaluation of natural products and chemical compounds to improve the control strategy against cucumber powdery mildew. Eur. J. Plant Pathol. 2023, 165, 385–400. [Google Scholar] [CrossRef]
- Magyarosy, A.C.; Malkin, R. Effect of powdery mildew infection of sugar beet on the content of electron carriers in chloroplasts. Physiol. Plant Pathol. 1978, 13, 183–188. [Google Scholar]
- Jaenisch, J.; Xue, H.; Schläpfer, J.; McGarrigle, E.R.; Louie, K.; Northen, T.R.; Wildermuth, M.C. Powdery mildew infection induces a non-canonical route to storage lipid formation at the expense of host thylakoid lipids to fuel its spore production. bioRxiv 2023. [Google Scholar] [CrossRef]
- Percival, G.C.; Fraser, G.A. The influence of powdery mildew infection on photosynthesis, chlorophyll fluorescence, leaf chlorophyll and carotenoid content of three woody species. Arboric. J. 2002, 26, 333–347. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef]
- Lin, Z.; Pu, X.; Du, W. The effect of powdery mildew on the cell structure and cell wall composition of red clover. Eur. J. Plant Pathol. 2024, 171, 603–614. [Google Scholar] [CrossRef]
- Lu, C.; Du, J.; Chen, H.; Gong, S.; Jin, Y.; Meng, X.; Zhang, T.; Fu, B.; Molnár, I.; Holušová, K.; et al. Wheat Pm55 alleles exhibit distinct interactions with an inhibitor to cause different powdery mildew resistance. Nat. Commun. 2024, 15, 503. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Yan, Y.; Wang, W.; Gebretsadik, K.; Qi, X.; Xu, Q.; Chen, X. Comparative proteomic analysis of cucumber powdery mildew resistance between a single-segment substitution line and its recurrent parent. Hortic. Res. 2019, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Zarei, A. Chitosan nanoparticle encapsulation of celery seed essential oil: Antifungal activity and defense mechanisms against cucumber powdery mildew. Carbohydr. Polym. Technol. Appl. 2024, 8, 100531. [Google Scholar] [CrossRef]
- Németh, M.Z.; Seress, D.; Nonomura, T. Fungi Parasitizing Powdery Mildew Fungi: Ampelomyces Strains as Biocontrol Agents against Powdery Mildews. Agronomy 2023, 13, 1991. [Google Scholar] [CrossRef]
- Gilardi, G.; Gullino, M.L.; Garibaldi, A. Efficacy of biological and chemical products against powdery mildew on zucchini. J. Plant Dis. Prot. 2008, 115, 241–246. [Google Scholar]
- MSU Extension. Integrated Pest Management (IPM) for Powdery Mildew in Horticultural Crops; Michigan State University (MSU) Extension: East Lansing, MI, USA, 2023. Available online: https://www.canr.msu.edu/ipm/diseases/powdery_mildew (accessed on 5 September 2025).
- Lebeda, A.; Mieslerová, B. Taxonomy, distribution and biology of lettuce powdery mildew (Golovinomyces cichoracearum sensu stricto). Plant Pathol. 2011, 60, 400–415. [Google Scholar] [CrossRef]
- Aldeeb, M.M.E.; Wilar, G.; Suhandi, C.; Elamin, K.M.; Wathoni, N. Nanosuspension-Based Drug Delivery Systems for Topical Applications. Int. J. Nanomed. 2024, 19, 825–844. [Google Scholar] [CrossRef]
- Parada, J.; Tortella, G.; Seabra, A.B.; Fincheira, P.; Rubilar, O. Potential antifungal effect of copper oxide nanoparticles combined with fungicides against Botrytis cinerea and Fusarium oxysporum. Antibiotics 2024, 13, 215. [Google Scholar] [CrossRef]
- Sangeeta, M.K.; Gunagambhire, V.M.T.; Bhat, M.P.; Nagaraja, S.K.; Gunagambhire, P.V.; Kumar, R.S.; Mahalingam, S.M. In-vitro evaluation of Talaromyces islandicus mediated zinc oxide nanoparticles for antibacterial, anti-inflammatory, bio-pesticidal and seed growth promoting activities. Waste Biomass Valorization 2024, 15, 1901–1915. [Google Scholar] [CrossRef]
- Bruno, A.; Tripodi, F.; Armanni, A.; Barbieri, L.; Colombo, A.; Fumagalli, S.; Moukham, H.; Tomaino, G.; Kukushkina, E.; Lorenzi, R.; et al. Advancements in nanosensors for detecting pathogens in healthcare environments. Environ. Sci. Nano 2024, 11, 4449–4474. [Google Scholar] [CrossRef]
- Ly, P.-D.; Ly, K.-N.; Phan, H.-L.; Nguyen, H.H.T.; Duong, V.-A.; Nguyen, H.V. Recent advances in surface decoration of nanoparticles in drug delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Xu, Y.; Du, S.; Li, M.; Tan, H.; Sohail, X.; Liu, X.; Qi, X.; Yang, X.; Chen, A. Genome-wide association study reveals molecular mechanism underlying powdery mildew resistance in cucumber. Genome Biol. 2024, 25, 252. [Google Scholar] [CrossRef]
- Eichmann, R.; Hückelhoven, R. Accommodation of powdery mildew fungi in intact plant cells. J. Plant Physiol. 2008, 165, 5–18. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2002, 39, 385–417. [Google Scholar] [CrossRef]
- Heffer, V.; Johnson, K.B.; Powelson, M.L.; Shishkoff, N. Identification of powdery mildew fungi. Plant Health Instr. 2006, 6. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Pearson, R.C.; Gadoury, D.M. Cleistothecia, the source of primary inoculum for grape powdery mildew in New York. Phytopathology 1987, 77, 1509–1514. [Google Scholar] [CrossRef]
- Pirondi, A.; Vela-Corcía, D.; Dondini, L.; Brunelli, A.; Pérez-García, A.; Collina, M. Genetic diversity analysis of the cucurbit powdery mildew fungus Podosphaera xanthii suggests a clonal population structure. Fungal Biol. 2015, 119, 791–801. [Google Scholar] [CrossRef]
- Polonio, Á.; Pérez-García, A.; Martínez-Cruz, J.; Fernández-Ortuño, D.; de Vicente, A. The haustorium of phytopathogenic fungi: A short overview of a specialized cell of obligate biotrophic plant parasites. In Progress in Botany; Springer: Cham, Switzerland, 2020; Volume 82, pp. 337–355. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.; Liu, R.; Jiang, Y. Effect of powdery mildew on interleaf microbial communities and leaf antioxidant enzyme systems. For. Res. 2023, 34, 1535–1547. [Google Scholar] [CrossRef]
- Lamsal, K.; Kim, S.; Jung Kim, Y.S.; Kim, K.S.; Lee, Y.S. Inhibition Effects of Silver Nanoparticles against Powdery Mildews on Cucumber and Pumpkin. Mycobiology 2011, 39, 26–32. [Google Scholar] [CrossRef]
- Porteous-Álvarez, A.J.; Maldonado-González, M.M.; Mayo-Prieto, S.; Lorenzana, A.; Paniagua-García, A.I.; Casquero, P.A. Green Strategies of Powdery Mildew Control in Hop: From Organic Products to Nanoscale Carriers. J. Fungi 2021, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Almusallam, A.H.; Ismail, A.M.; Elshewy, E.S.; El-Gawad, M.E.A. Green synthesized zinc oxide nanoparticles: Antifungal activity against powdery mildew disease of pepper (Capsicum annuum L.) and genotoxicity potentials. Biopestic. Int. 2024, 20, 193–206. [Google Scholar] [CrossRef]
- Zhu, Z.; Shao, C.; Guo, Y.; Feng, J.; Chen, C.; Yang, H. Facile pathway to generate agrochemical nanosuspensions integrating super-high load, eco-friendly excipients, intensified preparation process, and enhanced potency. Nano Res. 2021, 14, 2179–2187. [Google Scholar] [CrossRef]
- Rossier, B.; Jordan, O.; Allémann, E.; Roderguez-Nogales, C. Nanocrystals and nanosuspensions: An exploration from classic formulations to advanced drug delivery systems. Drug Deliv. Transl. Res. 2024, 14, 3438–3451. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Shahbaz, M.; Khalid, F.; Rasheed, Y.; Asif, K.; Naz, N.; Zulfiqar, U.; Zulfiqar, F.; Moosa, A.; Alamer, K.H.; et al. Biogenic nanoparticles application in agriculture for ROS mitigation and abiotic stress tolerance: A review. Plant Stress. 2023, 10, 100281. [Google Scholar] [CrossRef]
- Mussin, J.; Giusiano, G. Biogenic silver nanoparticles as antifungal agents. Front. Chem. 2022, 10, 1023542. [Google Scholar] [CrossRef]
- Tada, D.B.; Baptista, M.S. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 33. [Google Scholar] [CrossRef]
- Wu, Q.; Cen, F.; Xie, Y.; Ning, X.; Wang, J.; Lin, Z.; Huang, J. Nanoparticle-based antifungal therapies: Innovations, mechanisms, and future prospects. PeerJ 2025, 13, e19199. [Google Scholar] [CrossRef]
- Vizitiu, D.E.; Sardarescu, D.I.; Fierascu, I.; Fierascu, R.C.; Soare, L.C.; Ungureanu, C.; Buciumeanu, E.C.; Guta, I.C.; Pandelea, L.M. Grapevine plants management using natural extracts and phytosynthesized silver nanoparticles. Materials 2022, 15, 8188. [Google Scholar] [CrossRef]
- Shandila, P.; Mahatmanto, T.; Hsu, J.-L. Metal-Based nanoparticles as nanopesticides: Opportunities and challenges for sustainable. Crop Protection. Process. 2025, 13, 1278. [Google Scholar] [CrossRef]
- Ruiz-Jiménez, L.; Polonio, Á.; Vielba-Fernández, A.; Pérez-García, A.; Fernández-Ortuño, D. Gene mining for conserved, non-annotated proteins of Podosphaera xanthii identifies novel target candidates for controlling powdery mildews by spray-induced gene silencing. J. Fungi 2021, 7, 735. [Google Scholar] [CrossRef] [PubMed]
- Indermaur, E.J.; Day, C.T.C.; Dunn-Silver, A.R.; Smar, C.D. Biorational Fungicides to manage cucurbit powdery mildew on winter squash in New York. Plant Health Prog. 2024, 25, 249–254. [Google Scholar] [CrossRef]
- Włodarczyk, K.; Smoli´nska, B.; Majak, I. How Nano-ZnO Affect Tomato Fruits (Solanum lycopersicum L.)? Analysis of Selected Fruit Parameters. Int. J. Mol. Sci. 2024, 25, 8522. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Abd El-Gawed, M.E. Antifungal activity of MgO and ZnO nanoparticles against powdery mildew of pepper under greenhouse condition. Egypt. J. Agric. Res. 2021, 4, 421–434. [Google Scholar]
- Masoud, S.A.; Morsy, S.M.; El-Dein, M.M.Z. Controlling powdery mildew using Fe3O4 NPs, yeast, and Bio-Arc and their effects on the performance of lettuce Lactuca sativa L. Egypt. J. Chem. 2023, 66, 393–401. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.; Jia, Y.; Chen, Z.; Kang, D.; Zhang, L.; Pan, C. Nano-selenium enhances melon resistance to Podosphaera xanthii by enhancing the antioxidant capacity and promoting alterations in the polyamine, phenylpropanoid and hormone signaling pathways. J. Nanobiotechnol 2023, 21, 377. [Google Scholar] [CrossRef]
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Sci. Rep. 2017, 7, 2737. [Google Scholar] [CrossRef]
- Haq, I.U.; Cai, X.; Ali, H.; Akhtar, M.R.; Ghafar, M.A.; Hyder, M.; Hou, Y. Interactions between nanoparticles and tomato plants: Influencing host physiology and the tomato leaf miner’s molecular response. Nanomaterials 2024, 14, 1788. [Google Scholar] [CrossRef]
- Al-Goul, S.T. The impact of eco-friendly nanoparticles on the management of phytopathogenic fungi: A comprehensive review. J. Plant Pathol. 2024, 107, 265–290. [Google Scholar] [CrossRef]
- Amin, H.H.; Elsayed, A.B.; Maswada, H.F.; Elsheery, N.I. Enhancing sugar beet plant health with zinc nanoparticles: A sustainable solution for disease management. J. Soil Plant Environ. 2023, 2, 1–20. [Google Scholar] [CrossRef]
- Jan, H.; Khan, M.A.; Shah, M.R.; Ahmed, I.; Hussain, S.; Khan, M.M. Plant-based synthesis of zinc oxide nanoparticles (ZnO-NPs) using aqueous leaf extract of Aquilegia pubiflora: Characterization and bioactivity. Evid. Based Complement. Altern. Med. 2021, 2021, 4786227. [Google Scholar] [CrossRef]
- Wagh, S.S.; Kadam, V.S.; Jagtap, C.V.; Salunkhe, D.B.; Patil, R.S.; Pathan, H.M.; Patole, S.P. Comparative studies on synthesis, characterization and photocatalytic activity of Ag-doped ZnO nanoparticles. ACS Omega 2023, 8, 7779–7790. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Oshiro-Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Farhat, M.; Thabet, M.; Haggag, W.; Mosa, A. Efficacy of silicon and titanium nanoparticles biosynthesis by some antagonistic fungi and bacteria for controlling powdery mildew disease of wheat plants. Int. J. Agric. Technol. 2018, 14, 661–674. [Google Scholar]
- Elsharkawy, M.M.; El-Kot, G.A.N.; Hegazi, M. Management of rose powdery mildew by nanosilica, diatomite and bentocide. Egypt. J. Biol. Pest Control. 2015, 25, 545–553. [Google Scholar]
- Rashad, Y.M.; El-Sharkawy, H.H.A.; Belal, B.E.A.; Abdel Razik, E.S.; Galilah, D.A. Silica nanoparticles as a probable anti-oomycete compound against downy mildew, and yield and quality enhancer in grapevines: Field Evaluation, Molecular, Physiological, Ultrastructural, and Toxicity Investigations. Front. Plant Sci. 2021, 12, 763365. [Google Scholar] [CrossRef] [PubMed]
- Gogol, R.; Singh, P.K.; Kumar, R.; Nair, K.K.; Alam, I.; Sirvasava, C.; Yadav, S.; Gopal, M.; Choudhury, S.R.; Goswaml, A. Suitability of nano-sulphur for biorational Management of Powdery mildew of Okra (Abelmoschus esculentus Moench) caused by Erysiphe cichoracearum. J. Plant Pathol. Microbiol. 2013, 4, 171. [Google Scholar] [CrossRef]
- Abdel-Rahman, H.R.; Alkolaly, A.M.A. Comparative studies on nanosulfur and certain fungicides to control cucumber powdery mildew disease and their residues in treated fruits. Sci. Exch. J. 2020, 41, 54–70. [Google Scholar] [CrossRef]
- Chen, H.; Yada, R.Y. Nanotechnologies in agriculture: New tools for sustainable development. Trends Food Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Rathore, A.; Hasan, W.; Ramesha, N.M.; Pujar, K.; Singh, R.; Panotra, N.; Satapathy, S.N. Nanotech for Crop Protection: Utilizing Nanoparticles for Targeted Pesticide Delivery. Uttar Pradesh J. Zool. 2024, 45, 46–71. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Sánchez-Hernández, E.; Baquero-Foz, R.; Martín-Ramos, P.; Martín-Gil, J.; Torres-Sánchez, S.; Casanova-Gascón, J. Chitosan-based bioactive formulations for the control of powdery mildew in viticulture. Agronomy 2022, 12, 495. [Google Scholar] [CrossRef]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Ghanadian, M. Characterization, biochemical defense mechanisms, and antifungal activities of chitosan nanoparticle-encapsulated spinach seed essential oil. J. Agric. Food Res. 2025, 22, 102016. [Google Scholar] [CrossRef]
- Patel, A.; Ghosh, V. Thyme (Thymus vulgaris) Essential Oil–Based Antimicrobial Nanoemulsion Formulation for Fruit Juice Preservation. In Biotechnological Applications in Human Health; Sadhukhan, P., Premi, S., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Assessment of nanoencapsulated Cananga odorata essential oil in chitosan nanopolymer as a green approach to boost the antifungal, antioxidant and in situ efficacy. Int. J. Biol. Macromol. 2021, 171, 480–490. [Google Scholar] [CrossRef]
- Sreelatha, S.; Kumar, N.; Yin, T.S.; Rajani, S. Evaluating the antibacterial activity and mode of action of thymol-loaded chitosan nanoparticles against plant bacterial pathogen Xanthomonas campestris pv. campestris. Front. Microbiol. 2022, 12, 792737. [Google Scholar] [CrossRef]
- Desouky, M.M.; Abou-Saleh, R.H.; Moussa, T.A.A.; Fahmy, H.M. Nano-chitosan-coated, green-synthesized selenium nanoparticles as a novel antifungal agent against Sclerotinia sclerotiorum: In vitro study. Sci. Rep. 2025, 15, 1004. [Google Scholar] [CrossRef] [PubMed]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and thyme essential oils encapsulated in chitosan nanoparticles as effective antimicrobial agents against foodborne pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; Hiremani, V.D.; Chougale, R.B.; Masti, S.P.; Vootla, S.K.; Mudigoudra, B.S. Chitosan/pullulan-based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohydr. Polym. 2022, 277, 118866. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, M.; Gao, Y.; Su, P.; Jia, H.; Tang, C.; Meng, H.; Wu, L. Nano protective membrane coated wheat to resist powdery mildew. Front. Plant Sci. 2024, 15, 1369330. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kar, K.; Mazumder, R. Exploration of different strategies of nanoencapsulation of bioactive compounds and their ensuing approaches. Future J. Pharm. Sci. 2024, 10, 644. [Google Scholar] [CrossRef]
- Adnan, I.; Waleed, M. Evaluation of the effectiveness of biologically synthesized copper and zinc nanoparticles in controlling powdery mildew on Rose hybrida. Nat. Eng. Sci. 2025, 10, 552–562. [Google Scholar] [CrossRef]
- Jiménez-Pérez, O.; GallegosMorales, G.; Espinoza-Ahumada, C.A.; Delgado-Luna, C.; Rangel, P.; Espinosa-Palomeque, B. Potential of chitosan for the control of powdery mildew (Leveillula taurica (Lév.) Arnaud) in a Jalapeño Pepper (Capsicum annuum L.) cultivar. Plants 2024, 13, 915. [Google Scholar] [CrossRef]
- Nandhini, R.; Rajeswari, E.; Harish, S.; Sivakumar, V.; Selvi, R.G.; Sundrasharmila, D.J. Role of chitosan nanoparticles in sustainable plant disease management. J. Nanoparticle Res. 2025, 27, 13. [Google Scholar] [CrossRef]
- Ahmed, F.; Adnan, F.; Bazai, M.J.; Fareed, S.R.; Tareen, J.K.; Uddin, M.; Kakar, H. Powdery mildew: A disease of grapes and the fungicides’ mode of action—A review. Biosci. Res. 2022, 3, 78. [Google Scholar] [CrossRef]
- Naaz, H.; Rawat, K.; Saffeullah, P.; Umar, S. Silica nanoparticles synthesis and applications in agriculture for plant fertilization and protection: A review. Environ. Chem. Lett. 2023, 21, 539–559. [Google Scholar] [CrossRef]
- Laane, H.-M. The effects of foliar sprays with different silicon compounds. Plants 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Krishnani, K.K.; Boddu, V.M.; Chadha, N.K.; Saffeullah, P.; Umar, S. Metallic and non-metallic nanoparticles from plant, animal, and fisheries wastes: Potential and valorization for application in agriculture. Environ. Sci. Pollut. Res. 2022, 29, 81130–81165. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized silica nanoparticles: Classification, synthetic approaches and recent advances in adsorption applications. Nanoscale 2021, 13, 16200–16230. [Google Scholar] [CrossRef]
- González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Cabrera, R.I.; Juárez-Maldonado, A. Carbon Nanotubes Decrease the Negative Impact of Alternaria solani in Tomato Crop. Nanomaterials 2021, 11, 1080. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.; Wang, J.; Li, F.; Zhao, J. Graphene Oxide Exhibits Antifungal Activity against Bipolaris sorokiniana In Vitro and In Vivo. Microorganisms 2022, 10, 1994. [Google Scholar] [CrossRef]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef]
- Tanaka, H. Method of Controlling Powdery Mildew Using Nanobubble Water. WO2019230789A1, 29 May 2019. [Google Scholar]
- Venugopal, E.; Krishna, S.B.N.; Golla, N.; Patil, S.J. Nanofungicides: A Promising Solution for Climate-Resilient Plant Disease Management. In Plant Quarantine Challenges under Climate Change Anxiety; Abd-Elsalam, K.A., Abdel-Momen, S.M., Eds.; Springer: Cham, Switzerland, 2024; pp. 513–532. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Hanwate, R.M.; Babu, R.H.; Wadhave, A.A.; Mishra, V. Advancements in Nanosuspension Technology for Drug Delivery. Biomed. Mater. Devices 2025. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 10. [Google Scholar] [CrossRef]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional nanoparticles and nanopesticides in agricultural application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological environments. Chem. Soc. Rev. 2018, 47, 3862–3871. [Google Scholar] [CrossRef]
- Wohlmuth, J.; Tekielska, D.; Čechová, J.; Baránek, M. Interaction of the Nanoparticles and Plants in selective growth stages—Usual effects and resulting impact on usage perspectives. Plants 2022, 11, 2405. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lu, H. Protein PEPylation: A New Paradigm of Protein–Polymer Conjugation. Bioconjugate Chem. 2019, 30, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Lanke, N.P.; Chandak, M.B. Recent trends in deep learning and hyperspectral imaging for fruit quality analysis: An overview. J. Opt. 2025, 15, 1–24. [Google Scholar] [CrossRef]
- Singh, J.; Kashyap, R.; Bansal, K.; Das, R.; Sandhu, K.; Singh, G.; Saini, D.K. Transition from conventional to AI-based methods for detection of foliar disease symptoms in vegetable crops: A comprehensive review. J. Plant Pathol. 2025, 107, 1791–1814. [Google Scholar] [CrossRef]
- Vu Thanh, C.; Gooding, J.J.; Kah, M. Learning lessons from nanomedicine to improve the design and performances of nano-agrochemicals. Nat. Commun. 2025, 16, 2306. [Google Scholar] [CrossRef]
| Nanoparticle Type | Crop | Size (nm) | Concentration | Synthesis Method | Application Method | Effectiveness/Efficiency | Additional Benefits | Reference |
|---|---|---|---|---|---|---|---|---|
| Ag | Eggplant | 7–25 | 10–100 ppm | CS | Foliar | Effective in reducing powdery mildew | Mycelial and spore deformation | [35] |
| Ag | Beans | ~25 | 10–100 ppm | CS and GS | Foliar | Effective against powdery mildew and Botrytis cinerea | ↓ Disease incidence, ↑ yield potential | [35] |
| Ag | Melons | 7–25 | 10–100 ppm | CS | Foliar | 100 ppm: 20% disease incidence | Spore deformation, ↑ efficacy pre-infection | [35] |
| Ag | Radish | 7–25 | 10–100 ppm | CS | Foliar | Effective in reducing powdery mildew (extrapolated) | Spore deformation, safe for leafy crops | [35] |
| Ag | cucumber & pumpkin | ~10–50 | 100 ppm | GS | Foliar | Highest inhibition rate | Damages fungal hyphae and conidia | [35] |
| Ag | Grapevine | ~20–23 | crude | GS | Foliar | Improved control of E. necator | Superior leaf adhesion, enhanced uptake, prolonged protection vs. copper formulations | [39] |
| Ag | Grapevine | ~17 | crude | GS | Foliar | Protective effect against Uncinula necator | enhanced sugar, starch, water content; increased shoot length and grape yield | [44] |
| Cu | Squash | ~40–60 | ~300 mg·L−1 | CS | Foliar | Highest among tested | Outperformed biological and botanical alternatives; consistent disease suppression | [47] |
| ZnO | Tomato, Pepper | ~40 | 50–250 mg·L−1 | CS | Foliar & Soil | Significant reduction in powdery mildew | Enhanced chlorophyll, lycopene, β-carotene, sugar content; reduced oxidative stress | [48] |
| ZnO | Pepper | 79.5 | 100, 150, 200 mg·L−1 | CS | Foliar | Significant reduction in disease severity | Increased chlorophyll; no substantial cytotoxicity (mitotic index unaffected); alternative to penconazole | [49] |
| MgO | Pepper | 53 | 100, 150, 200 mg·L−1 | CS | Foliar | Significant reduction in disease severity | Increased chlorophyll; no substantial cytotoxicity (mitotic index unaffected); alternative to penconazole | [49] |
| Fe3O4 | Lettuce | ~20–50 | ~200 mg·L−1 | CS | Foliar | Significant reduction in disease severity | Increased chlorophyll, carotene, phenolics, protein; elevated antioxidant enzymes activity | [50] |
| Se | Melons | ~50–100 | 25–75 mg·L−1 | CS | Foliar | ~21–45% reduction | Enhances antioxidant enzymes; alters polyamine, phenylpropanoid, and hormone pathways | [51] |
| Se | Various crops | ~50–100 | 25–100 mg·L−1 | GS | Foliar | High antifungal activity; effective against resistant strains | Antioxidant, biocompatible, low toxicity; safe fungicide alternative | [52] |
| Ag, ZnO, TiO2 | Tomato | 10–100 | Varies | GS | Foliar | Effective against fungal & insect pests | ↑ Photosynthesis | [53] |
| CuO and ZnO | Mustard | ~50–80 | 100–300 mg·L−1 | GS | Foliar | Promising antifungal activity | Eco-friendly alternative to fungicides | [54] |
| TiO2 | Spinach | ~20 | 50–100 mg·L−1 | Sol-gel/GS | Foliar | ↑ Photosynthesis, ↓ fungal stress | ↑ Biomass, ↑ Chlorophyll content | [54] |
| CuO | Lettuce | 230–400 | 100 mg·L−1 | GS | Foliar | ↓ Fungal colonization | ↑ Leaf health, ↓ oil evaporation | [54] |
| Ag and ZnO | Grapes | 10–50 | 50–200 ppm | GS | Foliar | Effective against Erysiphe necator | ↑ Fruit quality, ↓ chemical residues | [54] |
| ZnO and CuO | Oranges | 20–80 | 100–300 ppm | GS | Foliar | Antifungal & antibacterial | ↑ Shelf life, ↑ Disease resistance | [54] |
| ZnO | Beetroot (Sugar beet) | — | 10, 50, 100 ppm | Engineered | Foliar | ↓ Disease severity, ↑ chlorophyll, | Induced resistance via ROS and phenolics | [55] |
| Ag | Cucurbits | ~10–50 | 25–100 mg·L−1 | CS/GS | Foliar | Up to 90% reduction | Minimal phytotoxicity; eco-friendly; strong antifungal activity | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geremew, A.; Shembo, A.; Carson, L. Harnessing Nanoparticles and Nanosuspensions to Combat Powdery Mildew: A Frontier in Vegetable and Fruit Protection. J. Fungi 2025, 11, 896. https://doi.org/10.3390/jof11120896
Geremew A, Shembo A, Carson L. Harnessing Nanoparticles and Nanosuspensions to Combat Powdery Mildew: A Frontier in Vegetable and Fruit Protection. Journal of Fungi. 2025; 11(12):896. https://doi.org/10.3390/jof11120896
Chicago/Turabian StyleGeremew, Addisie, Alemayehu Shembo, and Laura Carson. 2025. "Harnessing Nanoparticles and Nanosuspensions to Combat Powdery Mildew: A Frontier in Vegetable and Fruit Protection" Journal of Fungi 11, no. 12: 896. https://doi.org/10.3390/jof11120896
APA StyleGeremew, A., Shembo, A., & Carson, L. (2025). Harnessing Nanoparticles and Nanosuspensions to Combat Powdery Mildew: A Frontier in Vegetable and Fruit Protection. Journal of Fungi, 11(12), 896. https://doi.org/10.3390/jof11120896






