Effects of Insect Cuticular Compounds on Appressorium Formation and Metabolic Activity in Beauveria bassiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Strains
2.2. Reagents
2.3. Determination of Conidial Germination Rates and Adhesive Spore Formation Rates of B. bassianan in Analogues of Different Insect Cuticular Compounds
2.4. Metabolic Effects of Insect Cuticle Compound Analogues on B. bassiaan Appressorium Formation
2.4.1. Sample Preparation
2.4.2. LC-MS Analysis
2.5. Data Processing
3. Result
3.1. Effects of Different Insect Cuticular Compound Analogues on Spore Germination and Appressorium Formation in B. bassiana
3.2. Effects of the Insect Cuticle Compound Analogue Carnitine C3:0 on the Metabolism of B. bassiana Adhesive Spores
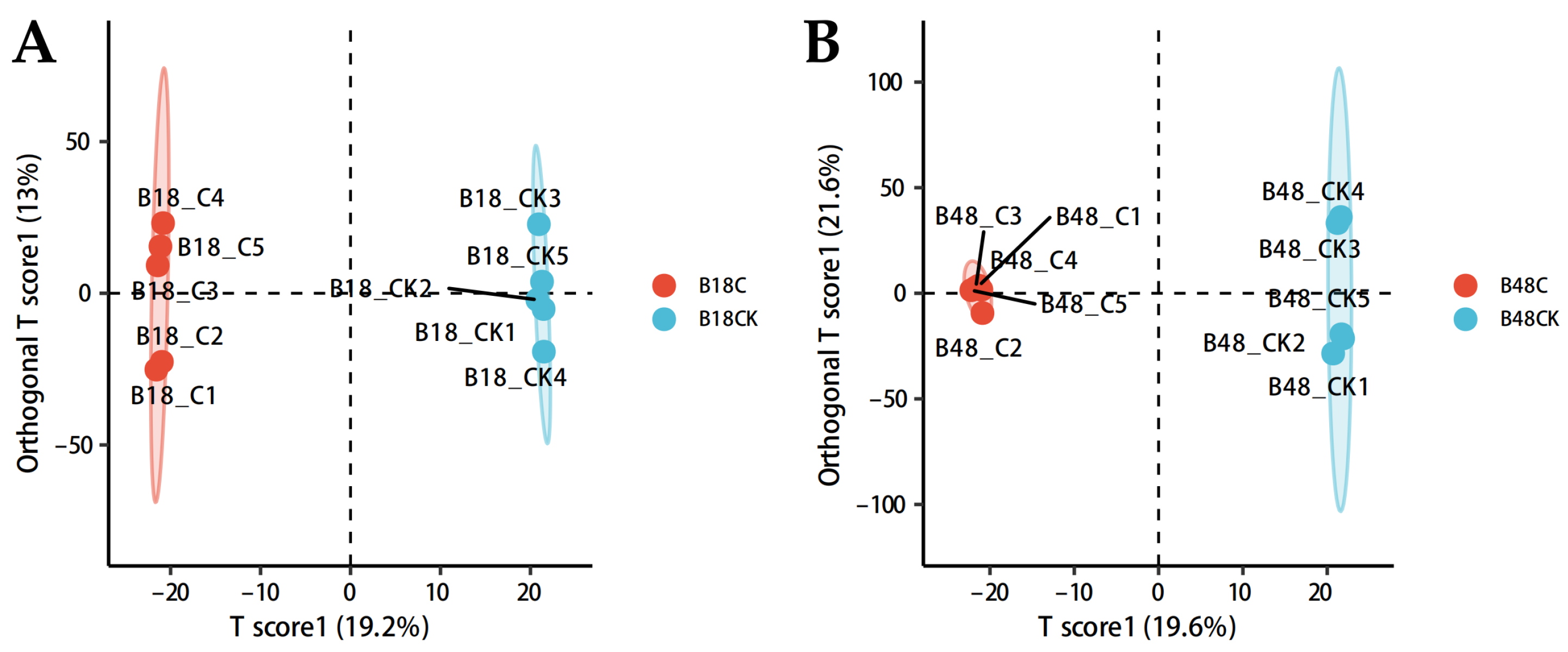
3.2.1. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
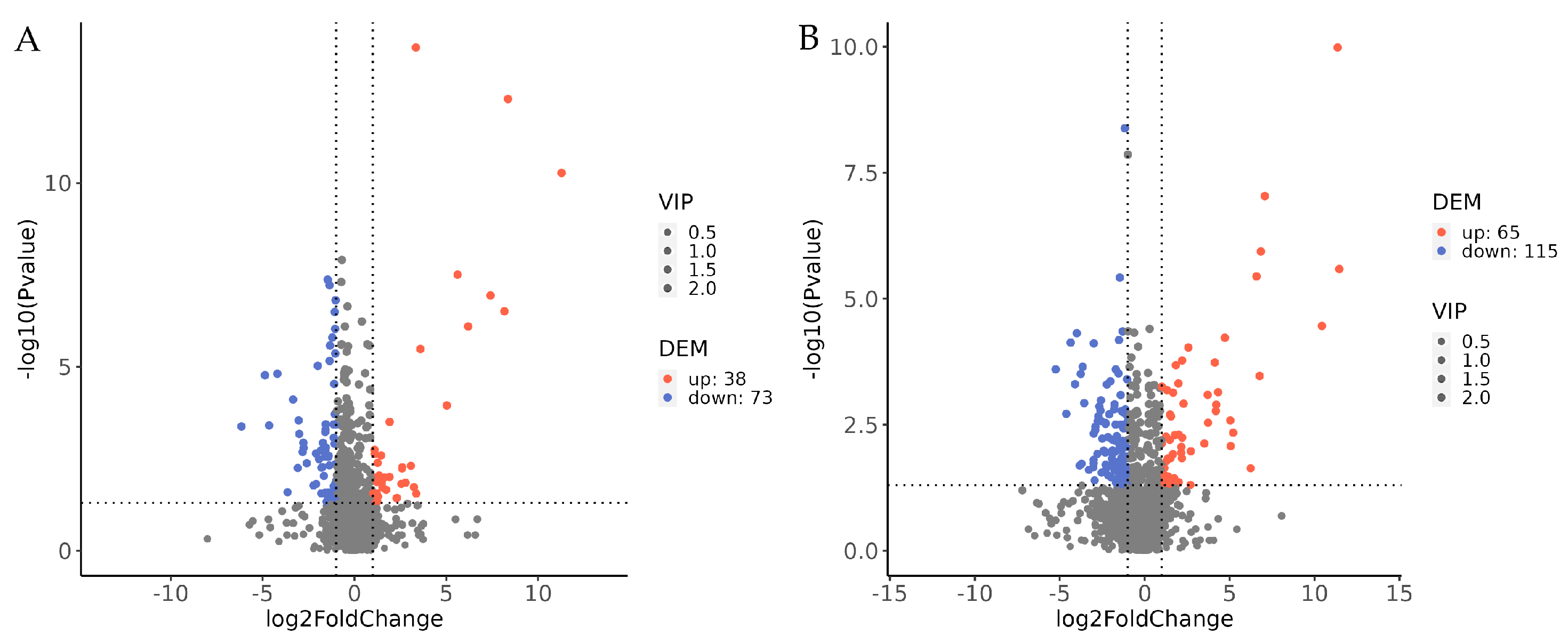
3.2.2. Volcano Analysis
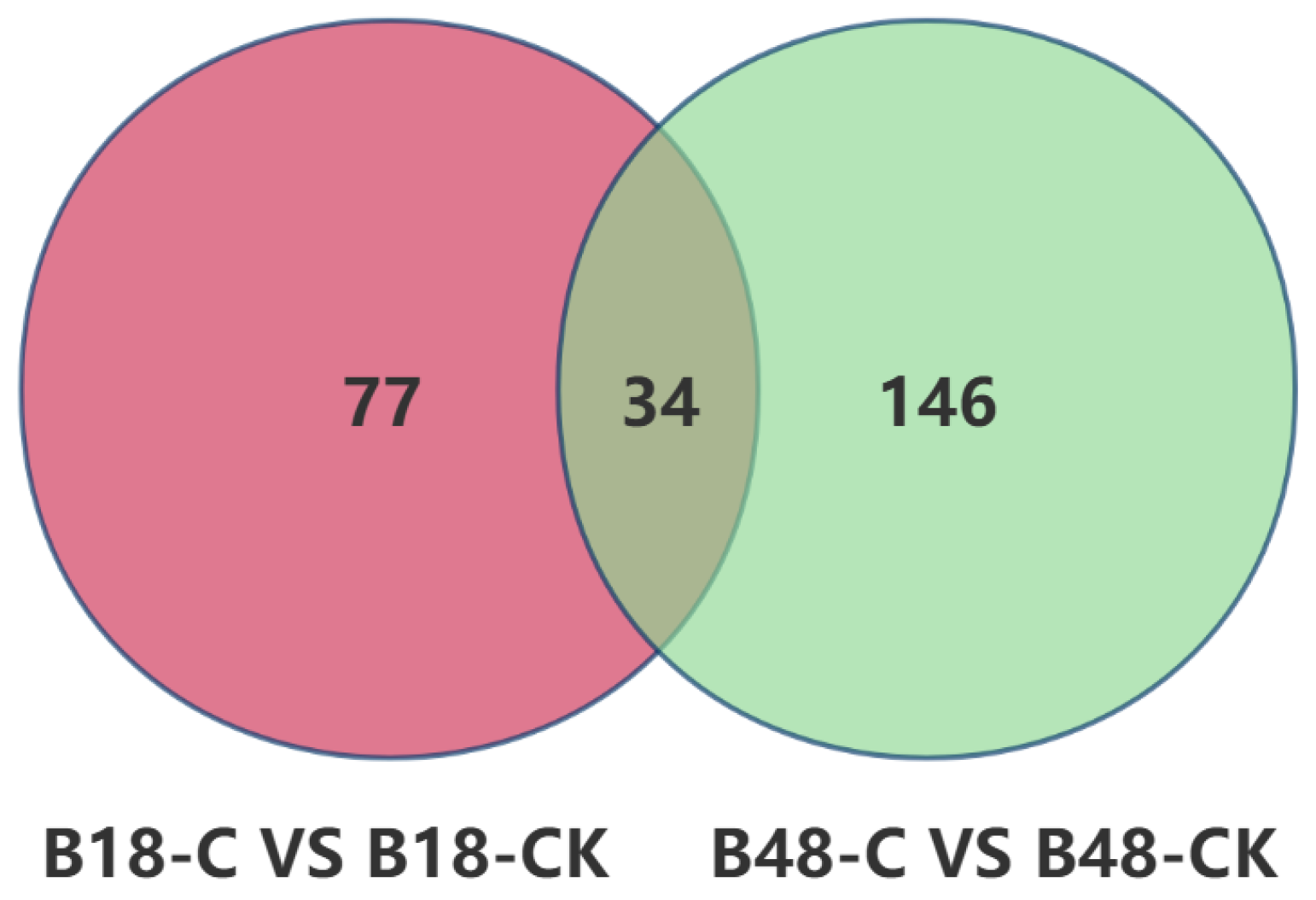
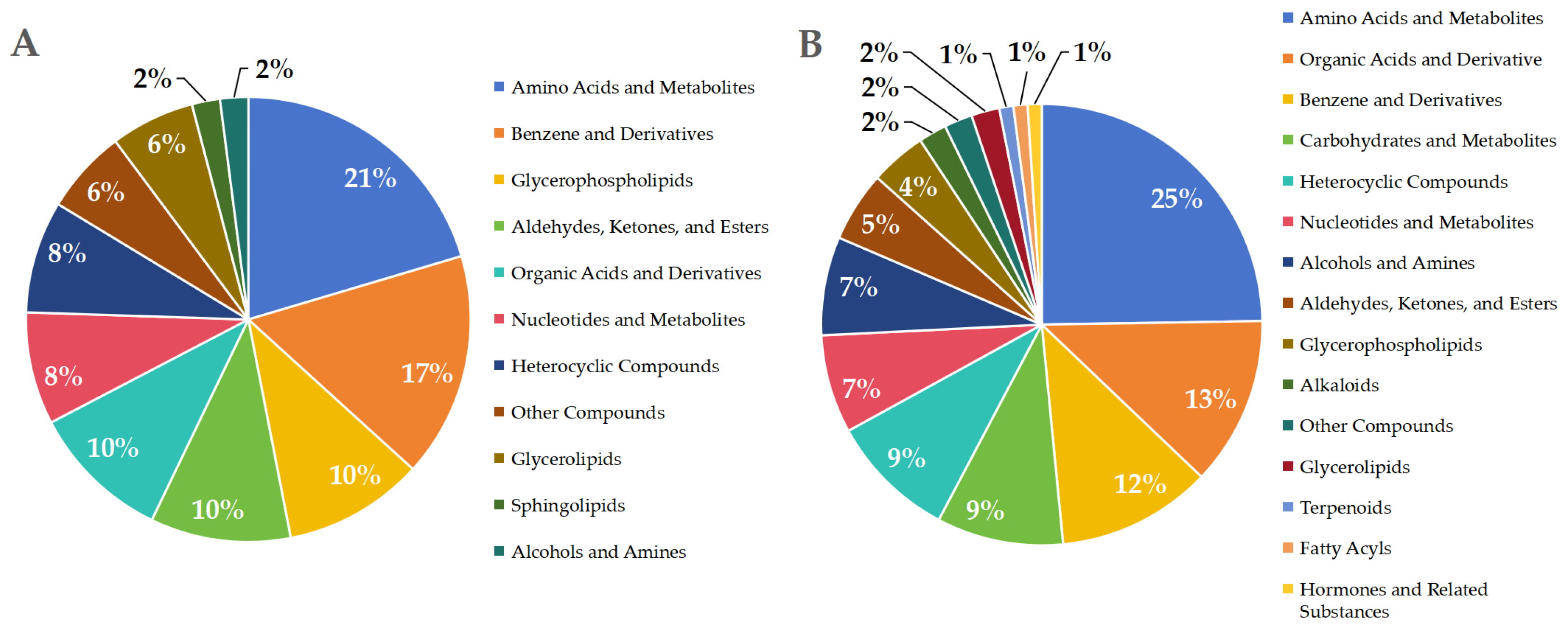
3.2.3. Differential Metabolites Following B. bassiana Spore Formation in the Carnitine C3:0-Treated Group
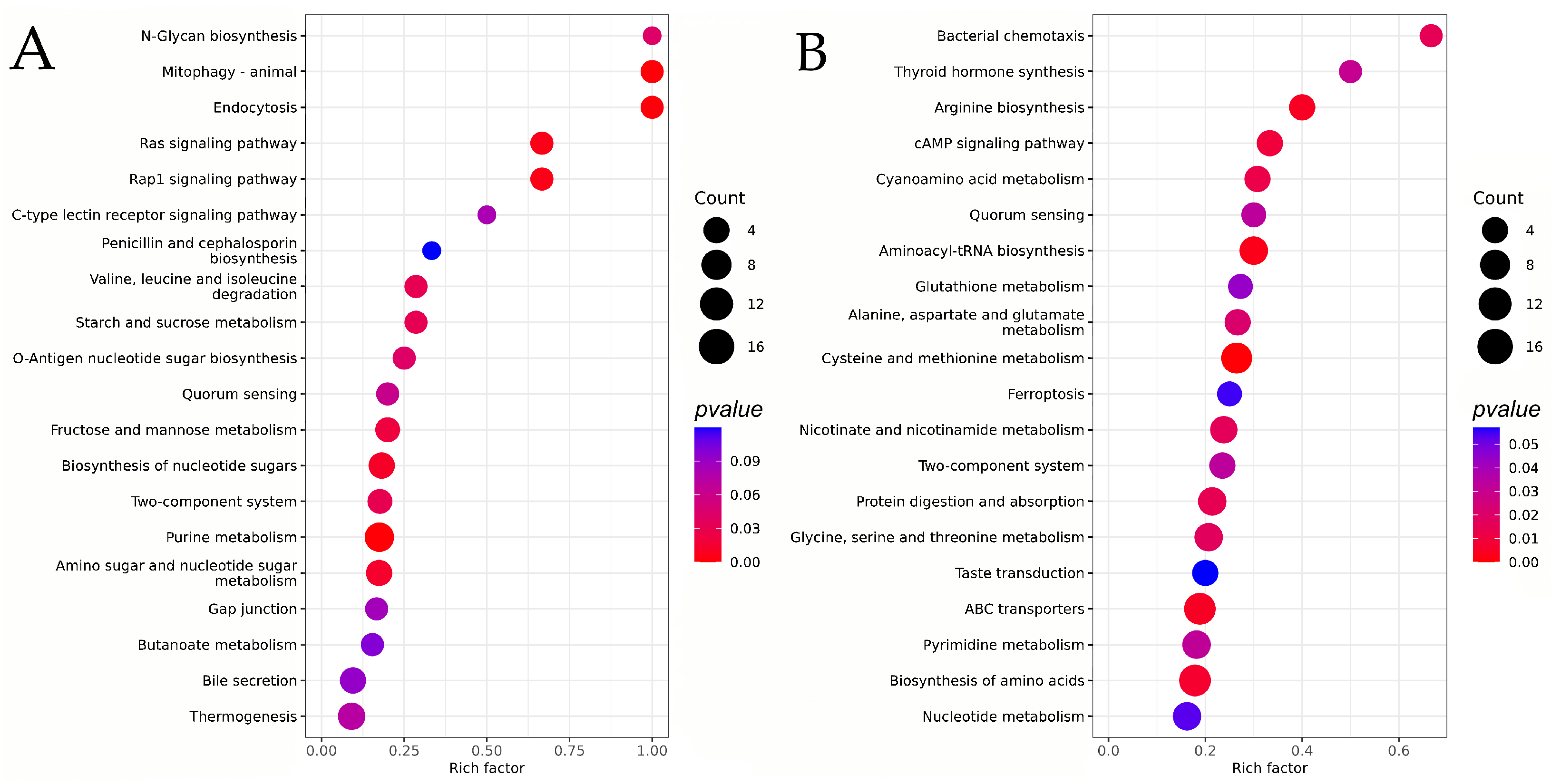
3.2.4. KEGG Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pu, Z. Insect Mycology; Anhui Science and Technology Press: Hefei, China, 1996. [Google Scholar]
- Wang, Y.X.; Wang, X.R.; Chen, J.Y.; Huang, J.B.; Zheng, L. Screening for highly virulent Beauveria bassiana Walker strains against Dendrolimus punctatus. J. South. Agric. 2016, 47, 662–666. [Google Scholar]
- Zhang, L.W.; Kang, K.; Liu, Y.J.; Zhang, J.; Sun, L. Evaluation of Beauveria bassiana isolates as potential agents for control of Hyphantria cunea (Lepidoptera: Arctiidae). Acta Entomol. Sin. 2016, 59, 111–118. [Google Scholar]
- He, R.; Cui, X.; Ying, Y.; Qu, L.J.; Wang, R.Z. Screening and Identification of Beauveria bassiana Strains for Biocontrol of Monochamus alternatus Adults (Coleoptera: Cerambycidae). Sci. Silvae Sin. 2020, 56, 129–134. [Google Scholar]
- He, X.Y.; Cai, S.P.; Tong, Y.H.; Xiong, Y.; Huang, Y.; Xie, J.D.; Chen, S.L. Pathogenicity evaluation of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against adults of Basilepta melanopus (Coleoptera: Eumolpidae). Acta Entomol. Sin. 2011, 54, 1281–1287. [Google Scholar]
- Xu, W.; Sui, L.; Gao, P.; Zhang, R.; Wang, Z. Study and Application of Wettable Powder of Beauveria bassiana to Control Corn Borer. Chin. J. Biol. Control 2020, 36, 862–865. [Google Scholar]
- Chen, J.; Li, W.; Wu, C.; Wu, S.; Tong, Y. Analysis of the Effects of Beauveria bassiana Appressorium Formation on Insect Cuticle Metabolism Based on LC-MS. J. Fungi 2025, 11, 595. [Google Scholar] [CrossRef]
- Zhang, J. Identification of the Genes Regulated by BbMPK1 MAPK Involved in the Penetration of Insect Cuticle in Beauveria bassiana. Master’s Thesis, Southwest University, Chongqing, China, 2009. [Google Scholar]
- Qiu, W. Comprehensive Mycology; Science Press: Beijing, China, 1998. [Google Scholar]
- Luo, F.; Li, S.; Chen, L.; Zhang, W.; Wang, B. Identification of spore germination and virulence related biomarkers from Beauveria bassiana using an LC-MS-based metabolomic technique. Acta Microbiol. Sin. 2014, 54, 33–41. [Google Scholar]
- Fan, Y.; Cao, Z.; Gu, S.; Dong, J. Effect of Different Induction Factors on Appressorium of Setosphaeria turcica. Sci. Agric. Sin. 2004, 37, 769–772. [Google Scholar]
- Wang, H.; Lin, F.; Wang, Z. Mechanical Penetrating Force of Appressoria of Plant Pathogenic Fungi. Mycosystema 2004, 23, 151–157. [Google Scholar]
- Kleemann, J.; Takahara, H.; Stüber, K.; O’Connell, R. Identification of soluble secreted proteins from appressoria of Colletotrichum higginsianum by analysis of expressed sequence tags. Microbiology 2008, 154, 1204–1217. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kawano, T.; Li, D.; Kolattukudy, P.E. A mitogen-activated protein kinase kinase required for induction of cytokinesis and appressorium formation by host signals in the conidia of Colletotrichum gloeosporioides. Plant Cell 2000, 12, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Boulesteix, A.L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Briefings Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zeng, X.; Wang, M.; Qin, L.; Tan, C.; Wu, J. Enrichment analysis of differentially expressed genes in chronic heart failure. Ann. Palliat. Med. 2021, 10, 9049056. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; Hu, J.; Ding, Z.; Liang, Q.; Zhang, Y.; Wang, H. Mineral and metabolic profiles in tea leaves and flowers during flower development. Plant Physiol. Biochem. 2016, 106, 316–326. [Google Scholar] [CrossRef]
- Lin, H.; Rao, J.; Shi, J.; Hu, C.; Cheng, F.; Wilson, Z.A.; Zhang, D.; Quan, S. Seed metabolomic study reveals significant metabolite variations and correlations among different soybean cultivars. J. Integr. Plant Biol. 2014, 56, 826–836. [Google Scholar] [CrossRef]
- Rao, J.; Cheng, F.; Hu, C.; Quan, S.; Lin, H.; Wang, J.; Chen, G.; Zhao, X.; Alexander, D.; Guo, L.; et al. Metabolic map of mature maize kernels. Metabolomics 2014, 10, 775–787. [Google Scholar] [CrossRef]
- Li, W. Physical and Chemical Factors Affecting Appressorium Formation of Metarhizium anisopliae and Beauveria bassiana. Master’s Thesis, Southwest University, Chongqing, China, 2003. [Google Scholar]
- Li, W.; Wang, Z.; Yin, Y.; Xia, Y.; Peng, G. Effection of Physical and Chemical Factors on Appressorium Formation of Beauveria bassiana. J. Chongqing Univ. (Natural Sci. Ed.) 2004, 27, 102–106. [Google Scholar]
- Valero-Jiménez, C.A.; Faino, L.; Spring in’t Veld, D.; Smit, S.; Zwaan, B.J.; van Kan, J.A. Comparative genomics of Beauveria bassiana: Uncovering signatures of virulence against mosquitoes. BMC Genom. 2016, 17, 986. [Google Scholar] [CrossRef]
- Lengeler, K.B.; Davidson, R.C.; D’souza, C.; Harashima, T.; Shen, W.C.; Wang, P.; Pan, X.; Waugh, M.; Heitman, J. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 2000, 64, 746–785. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.P.; Nicole, M.C.; Sritubtim, S.; Morency, M.J.; Ellis, M.; Ehlting, J.; Beaudoin, N.; Barbazuk, B.; Klessig, D.; Lee, J.; et al. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006, 11, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Recombined Microbial Fermentation for L-Carnitine Production. Master’s Thesis, Dalian Polytechnic University, Dalian, China, 2015. [Google Scholar]
- Jin, K.; Zhang, Y.; Fang, W.; Luo, Z.; Zhou, Y.; Pei, Y. Carboxylate transporter gene JEN1 from the entomopathogenic fungus Beauveria bassiana is involved in conidiation and virulence. Appl. Environ. Microbiol. 2010, 76, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lovett, B.; Leger, R.J.S. The insect pathogens. The fungal kingdom 2017, 5, 923–943. [Google Scholar]
- Zhu, J.; Ying, S.H.; Feng, M.G. The Pal pathway required for ambient pH adaptation regulates growth, conidiation, and osmotolerance of Beauveria bassiana in a pH-dependent manner. Appl. Microbiol. Biotechnol. 2016, 100, 4423–4433. [Google Scholar] [CrossRef]
- Joseph, E.; Cario, S.; Simon, A.; Wörle, M.; Mazzeo, R.; Junier, P.; Job, D. Protection of metal artifacts with the formation of metal–oxalates complexes by Beauveria bassiana. Front. Microbiol. 2012, 2, 270. [Google Scholar] [CrossRef]
- Zhao, C.; Bu, H.; Zhu, J.; Wang, Y.; Oliver, K.M.; Hu, F.; Huang, B.; Li, Z.; Peng, F. Integration of untargeted metabolomics with transcriptomics provides insights into beauvericin biosynthesis in Cordyceps chanhua under H2O2-induced oxidative stress. J. Fungi 2022, 8, 484. [Google Scholar] [CrossRef]
- Xie, L.; Yin, W.; Li, W.; Gao, W. Advance on natural products and their pharmacological activities and biosynthesis from Beauveria species. Nat. Prod. Res. Dev. 2020, 32, 150–164. [Google Scholar]
- Liu, K.; Li, G.; Wu, S.; Zhang, Y.; Zhou, R.; Lv, C.; Yue, X.; Zhu, Z.; Zhou, T.; Huang, B.; et al. Competitive exclusion of insect parasitic fungi: Enhanced metabolite biosynthesis in Beauveria bassiana and fatty acid production in Metarhizium robertsii during host coinfection. Pestic. Biochem. Physiol. 2025, 214, 106561. [Google Scholar] [CrossRef]
- Feng, P.; Shang, Y.; Cen, K.; Wang, C. Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 11365–11370. [Google Scholar] [CrossRef]
- Campbell, R.K.; Barnes, G.L.; Butt, T.M. Growth and sporulation of Beauveria bassiana and Metarhizium anisopliae on media containing various amino acids. J. Invertebr. Pathol. 1978, 31, 289–295. [Google Scholar] [CrossRef]
- Pham, T.A.; Kim, J.S.; Kim, K.W.; Kim, J.J. Production of blastospore of entomopathogenic Beauveria bassiana in a submerged batch culture. Mycobiology 2009, 37, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, S.; Chen, X.; Li, Z.; Feng, P.; Ying, S.; Feng, M. Ubr1-mediated ubiquitylation orchestrates asexual development, polar growth, and virulence-related cellular events in Beauveria bassiana. Appl. Microbiol. Biotechnol. 2021, 105, 2747–2758. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, S.; Li, Y.; Chen, H.; Zhang, S.; Wang, D.; Feng, M. Arginine accumulation suppresses heat production during fermentation of the biocontrol fungus Beauveria bassiana. Appl. Environ. Microbiol. 2025, 91, e02134-24. [Google Scholar] [CrossRef]
- Proietti, S.; Bertini, L.; Falasca, G.P.; Altamura, M.M.; Benvenuto, E.; Luziatelli, F.; Cardinali, G.; Reverberi, M. Beauveria bassiana rewires molecular mechanisms related to growth and defense in tomato. J. Exp. Bot. 2023, 74, 4225–4243. [Google Scholar] [CrossRef]
- Safavi, S.A.; Shah, F.A.; Pakdel, A.K.; Rasoulian, G.R.; Bandani, A.R.; Butt, T.M. Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana. Fems Microbiol. Lett. 2007, 270, 116–123. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Xue, J.; Li, B. Isolation and identification of toxins inhibiting Dentrolimus tabulaeformis from an antagonistic strain of Beauveria. Acta Microbiol. Sin. 2008, 48, 596–601. [Google Scholar]
- Huang, B.; Tang, R.; Chen, G.; Li, X. The Potential Defense of Calcium Oxalate Crystals Against Rove Beetle in Inflorescence of Amorphophallus albus. J. Southwest For. Univ. (Natural Sci.) 2019, 39, 92–97. [Google Scholar]
- Zhang, L.; Fasoyin, O.E.; Molnár, I.; Xu, Y. Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Nat. Prod. Rep. 2020, 37, 1181–1206. [Google Scholar] [CrossRef]
- Cerstiaens, A.; Huybrechts, J.; Kotanen, S.; Lebeau, I.; Meylaers, K.; De Loof, A.; Schoofs, L. Neurotoxic and neurobehavioral effects of kynurenines in adult insects. Biochem. Biophys. Res. Commun. 2003, 312, 1171–1177. [Google Scholar] [CrossRef]
- Liu, J. Study on Molecular Mechanism Involved in Pathogenicity Differences of the Beauveria bassiana to Different Insect Hosts and Function of Allergen Protein Bb-f2. Ph.D. Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar]
- Toopaang, W.; Bunnak, W.; Srisuksam, C.; Wattananukit, W.; Tanticharoen, M.; Yang, Y.L.; Amnuaykanjanasin, A. Microbial polyketides and their roles in insect virulence: From genomics to biological functions. Nat. Prod. Rep. 2022, 39, 2008–2029. [Google Scholar] [CrossRef] [PubMed]
- Toopaang, W.; Panyawicha, K.; Srisuksam, C.; Hsu, W.C.; Lin, C.C.; Tanticharoen, M.; Yang, Y.L.; Amnuaykanjanasin, A. Metabolomic analysis demonstrates the impacts of polyketide synthases PKS14 and PKS15 on the production of beauvericins, bassianolide, enniatin A, and ferricrocin in entomopathogen Beauveria bassiana. Metabolites 2023, 13, 425. [Google Scholar] [CrossRef]
- Toopaang, W.; Phonghanpot, S.; Punya, J.; Panyasiri, C.; Klamchao, K.; Wasuwan, R.; Srisuksam, C.; Sangsrakru, D.; Sonthirod, C.; Tangphatsornruang, S.; et al. Targeted disruption of the polyketide synthase gene pks15 affects virulence against insects and phagocytic survival in the fungus Beauveria bassiana. Fungal Biol. 2017, 121, 664–675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Cai, H.; Wu, C.; Wang, D.; Ni, J.; Wu, S.; Tong, Y. Effects of Insect Cuticular Compounds on Appressorium Formation and Metabolic Activity in Beauveria bassiana. J. Fungi 2025, 11, 833. https://doi.org/10.3390/jof11120833
Chen J, Cai H, Wu C, Wang D, Ni J, Wu S, Tong Y. Effects of Insect Cuticular Compounds on Appressorium Formation and Metabolic Activity in Beauveria bassiana. Journal of Fungi. 2025; 11(12):833. https://doi.org/10.3390/jof11120833
Chicago/Turabian StyleChen, Jiarui, Huaxin Cai, Canxia Wu, Dongxu Wang, Jingyang Ni, Songqing Wu, and Yinghua Tong. 2025. "Effects of Insect Cuticular Compounds on Appressorium Formation and Metabolic Activity in Beauveria bassiana" Journal of Fungi 11, no. 12: 833. https://doi.org/10.3390/jof11120833
APA StyleChen, J., Cai, H., Wu, C., Wang, D., Ni, J., Wu, S., & Tong, Y. (2025). Effects of Insect Cuticular Compounds on Appressorium Formation and Metabolic Activity in Beauveria bassiana. Journal of Fungi, 11(12), 833. https://doi.org/10.3390/jof11120833





