Unraveling the Fungal Community Dynamics in Heat-Tolerant Coral Turbinaria sp. During Bleaching in South China Sea
Abstract
1. Introduction
2. Materials and Methods
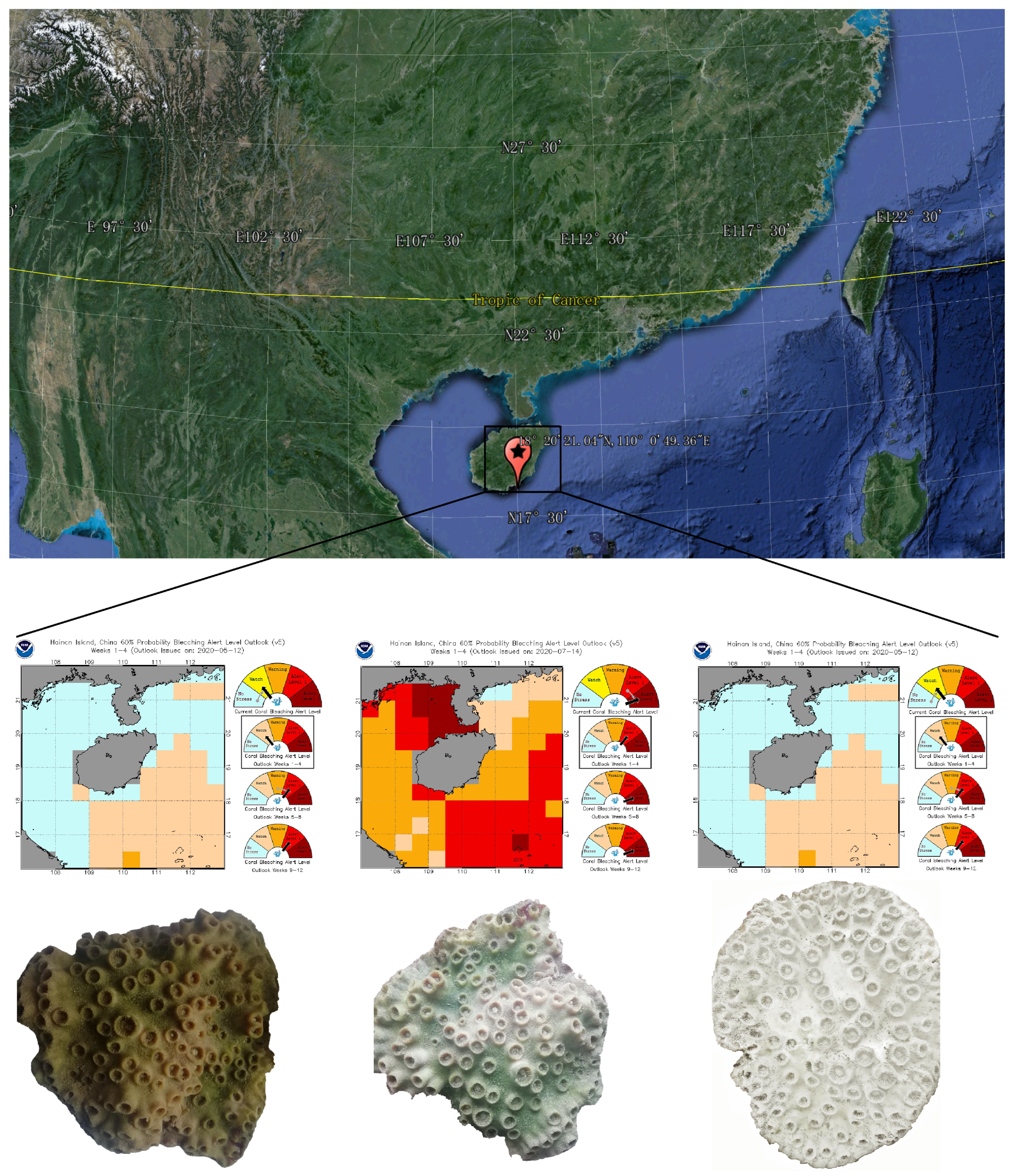
2.1. Study Sites and Sample Collection
2.2. DNA Extraction and High-Throughput Sequencing
2.3. Sequence Analysis and Taxonomic and Functional Classification
2.4. Statistical Analysis
3. Results
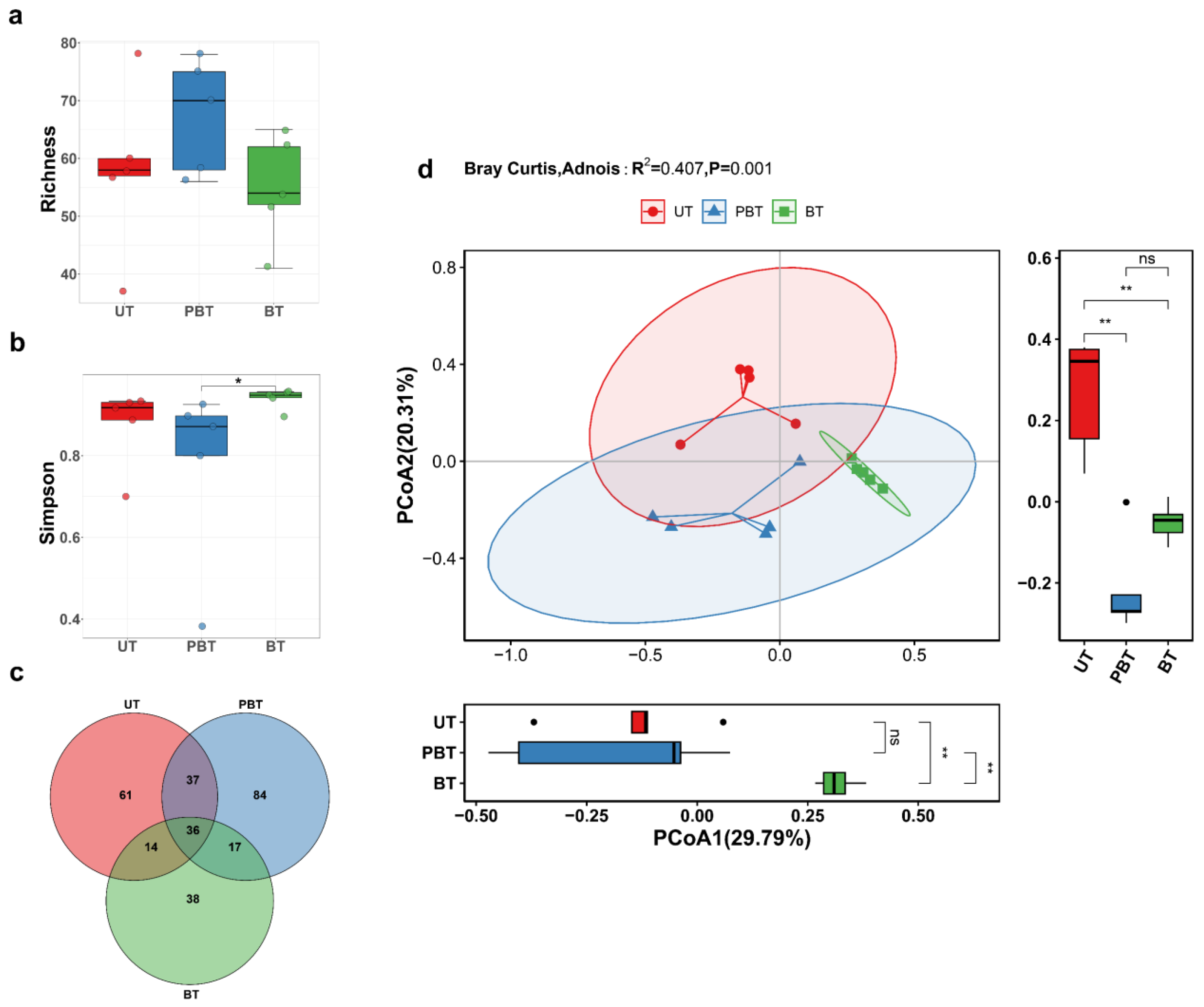
3.1. Fungal Community Diversity and Structure
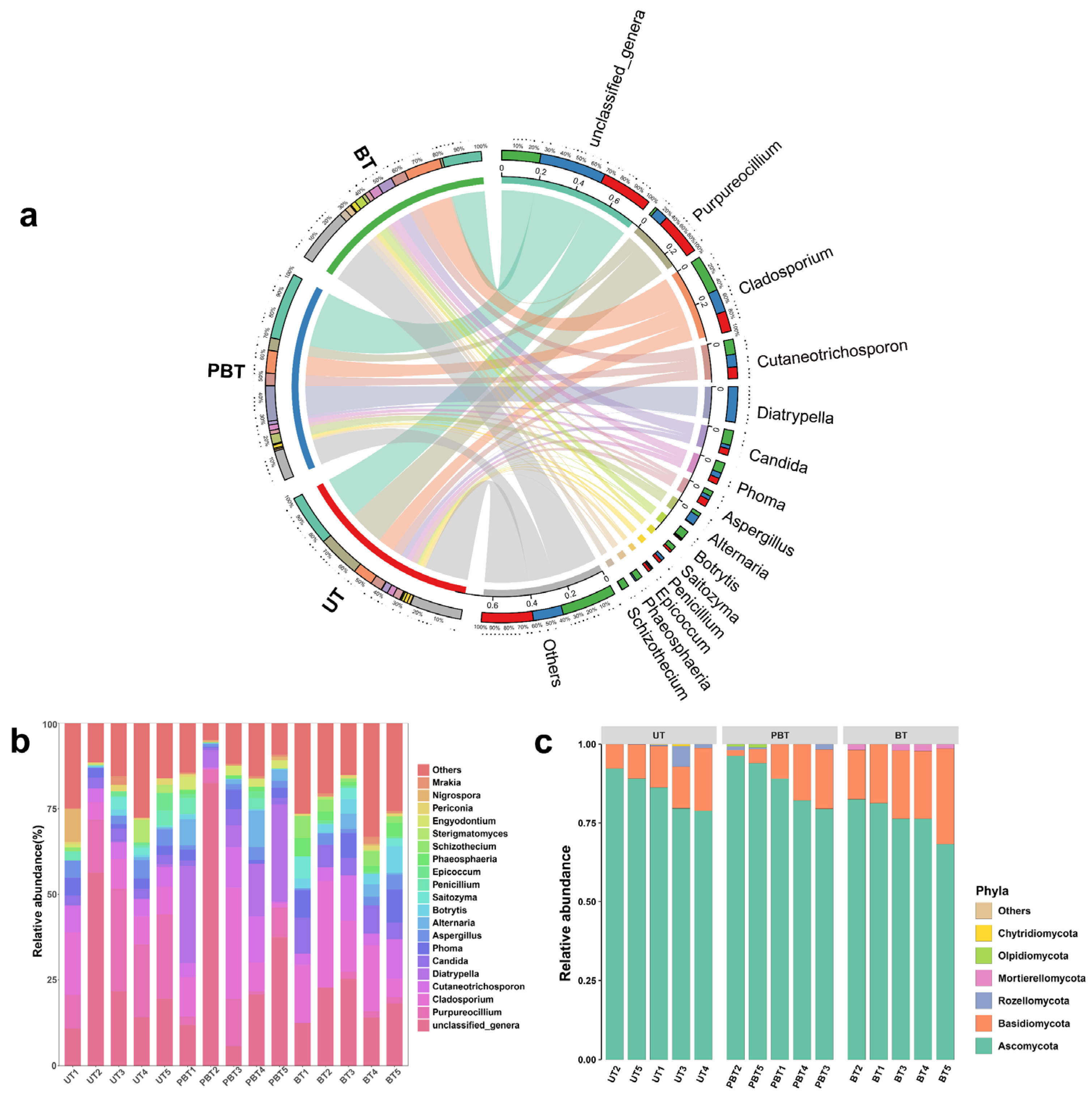
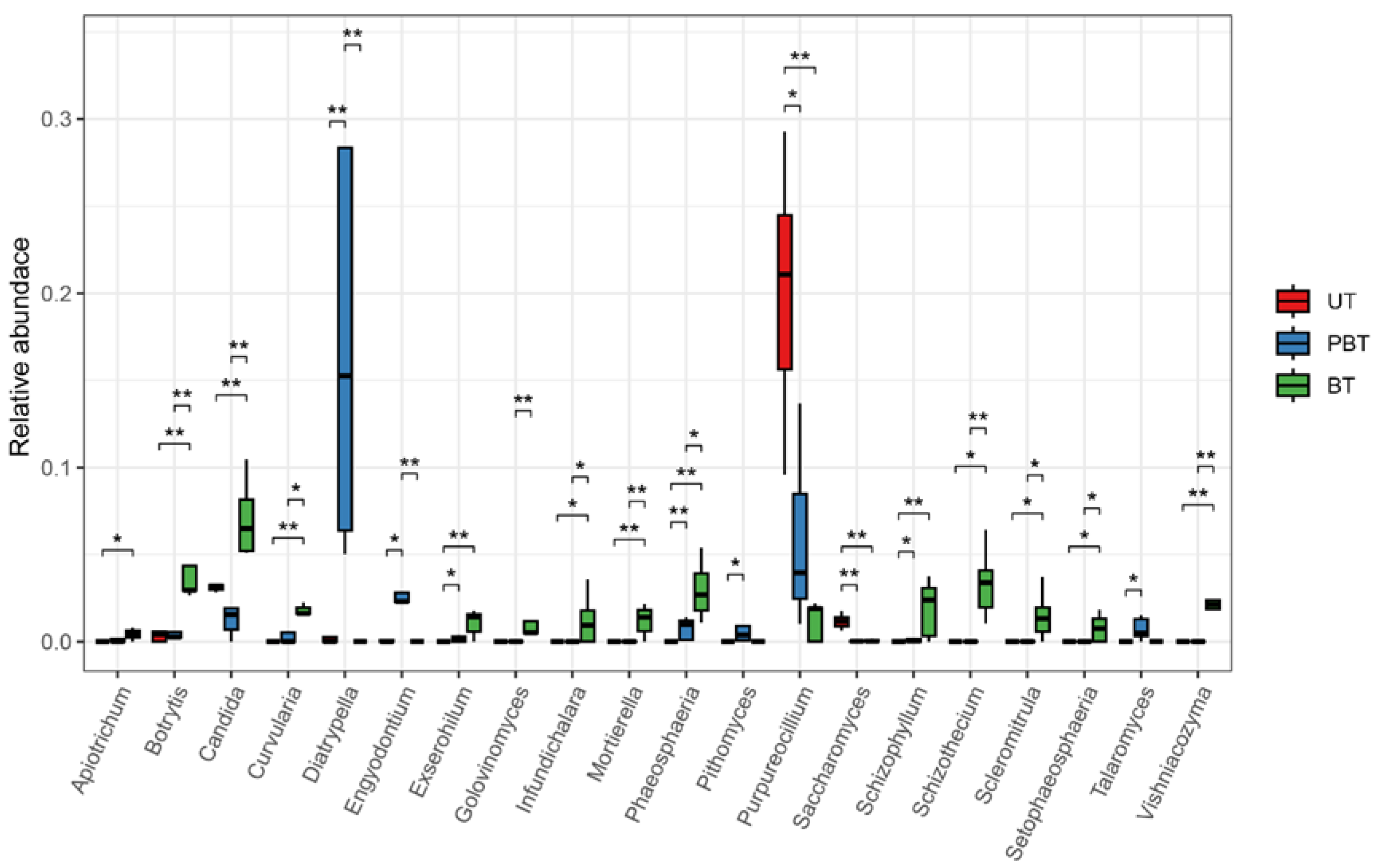
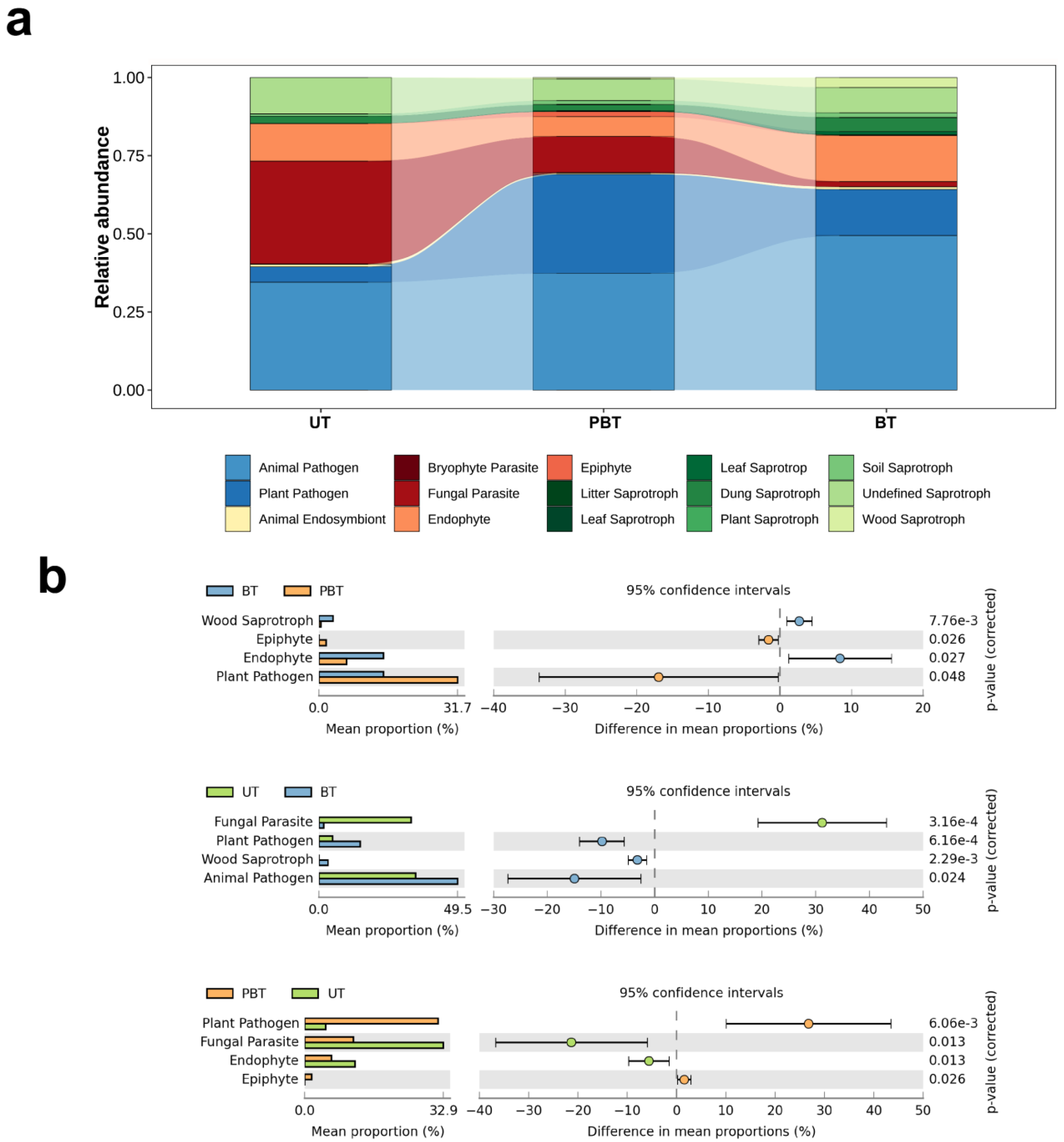
3.2. Diversity and Composition of Fungal Community
3.3. Fungal Functional Prediction
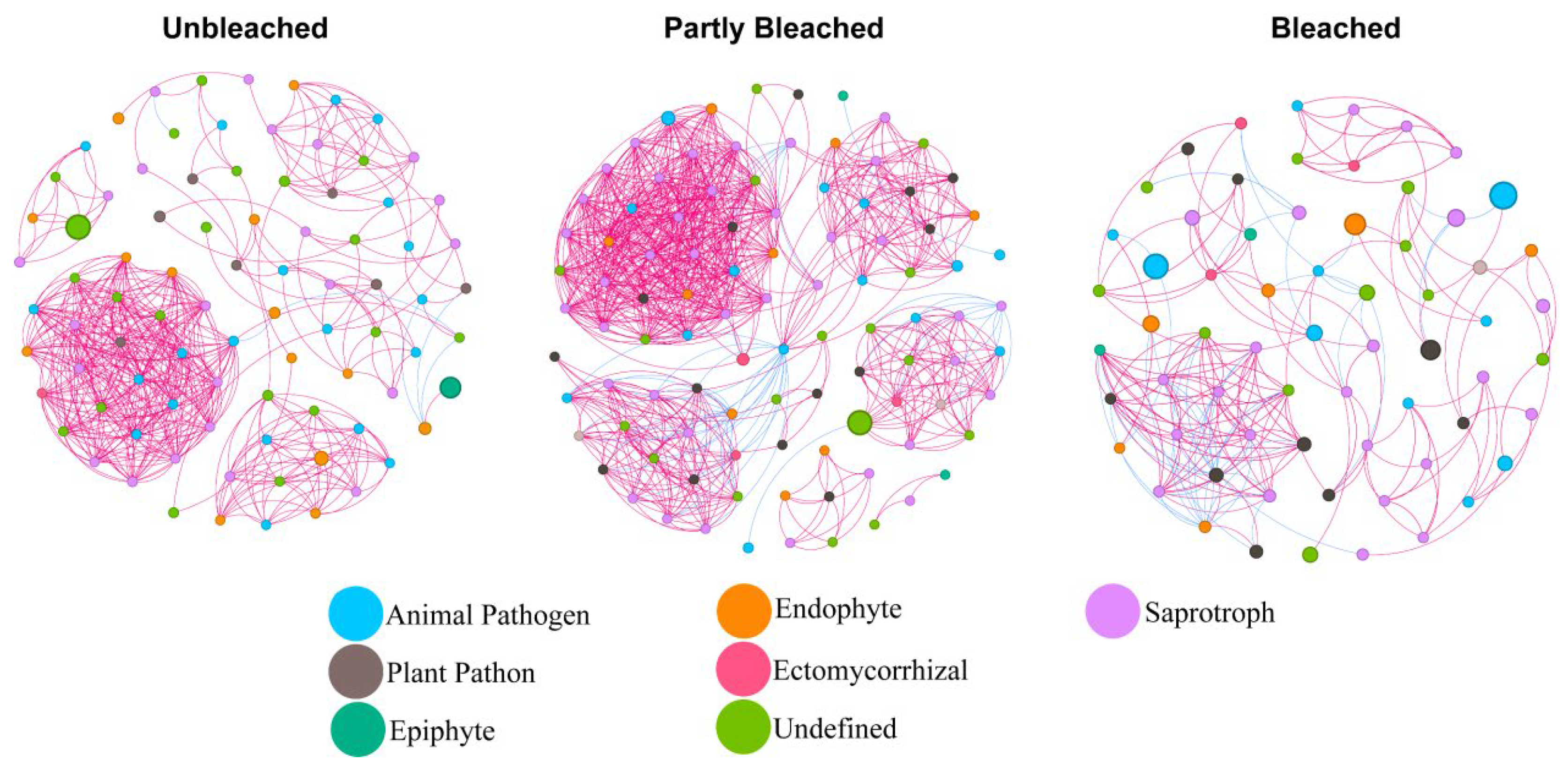
3.4. Co-Occurrence Network Analysis of Fungal Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baird, A.H.; Bhagooli, R.; Ralph, P.J.; Takahashi, S. Coral Bleaching: The Role of the Host. Trends Ecol. Evol. 2009, 24, 16–20. [Google Scholar] [CrossRef]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Bairos-Novak, K.R.; Hoogenboom, M.O.; van Oppen, M.J.; Connolly, S.R. Coral Adaptation to Climate Change: Meta-analysis Reveals High Heritability across Multiple Traits. Glob. Change Biol. 2021, 27, 5694–5710. [Google Scholar] [CrossRef] [PubMed]
- Lachs, L.; Donner, S.D.; Mumby, P.J.; Bythell, J.C.; Humanes, A.; East, H.K.; Guest, J.R. Emergent Increase in Coral Thermal Tolerance Reduces Mass Bleaching under Climate Change. Nat. Commun. 2023, 14, 4939. [Google Scholar] [CrossRef]
- Claar, D.C.; Starko, S.; Tietjen, K.L.; Epstein, H.E.; Cunning, R.; Cobb, K.M.; Baker, A.C.; Gates, R.D.; Baum, J.K. Dynamic Symbioses Reveal Pathways to Coral Survival through Prolonged Heatwaves. Nat. Commun. 2020, 11, 6097. [Google Scholar] [CrossRef]
- Palacio-Castro, A.M.; Smith, T.B.; Brandtneris, V.; Snyder, G.A.; Van Hooidonk, R.; Maté, J.L.; Manzello, D.; Glynn, P.W.; Fong, P.; Baker, A.C. Increased Dominance of Heat-Tolerant Symbionts Creates Resilient Coral Reefs in near-Term Ocean Warming. Proc. Natl. Acad. Sci. USA 2023, 120, e2202388120. [Google Scholar] [CrossRef]
- Doering, T.; Tandon, K.; Topa, S.H.; Pidot, S.J.; Blackall, L.L.; Van Oppen, M.J.H. Genomic Exploration of Coral-Associated Bacteria: Identifying Probiotic Candidates to Increase Coral Bleaching Resilience in Galaxea fascicularis. Microbiome 2023, 11, 185. [Google Scholar] [CrossRef]
- Cheng, K.; Li, X.; Tong, M.; Jong, M.-C.; Cai, Z.; Zheng, H.; Xiao, B.; Zhou, J. Integrated Metagenomic and Metaproteomic Analyses Reveal Bacterial Micro-Ecological Mechanisms in Coral Bleaching. mSystems 2023, 8, e00505-23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pang, X.; He, Y.; Lin, X.; Zhou, X.; Liu, Y.; Yang, B. Secondary Metabolites from Coral-Associated Fungi: Source, Chemistry and Bioactivities. J. Fungi 2022, 8, 1043. [Google Scholar] [CrossRef]
- Ravindran, J.; Raghukumar, C.; Raghukumar, S. Fungi in Porites lutea: Association with Healthy and Diseased Corals. Dis. Aquat. Org. 2001, 47, 219–228. [Google Scholar] [CrossRef]
- Soler-Hurtado, M.M.; Sandoval-Sierra, J.V.; Machordom, A.; Diéguez-Uribeondo, J. Aspergillus sydowii and Other Potential Fungal Pathogens in Gorgonian Octocorals of the Ecuadorian Pacific. PLoS ONE 2016, 11, e0165992. [Google Scholar] [CrossRef]
- Sweet, M.; Burn, D.; Croquer, A.; Leary, P. Characterisation of the Bacterial and Fungal Communities Associated with Different Lesion Sizes of Dark Spot Syndrome Occurring in the Coral Stephanocoenia intersepta. PLoS ONE 2013, 8, e62580. [Google Scholar] [CrossRef]
- Raghukumar, C.; Ravindran, J. Fungi and Their Role in Corals and Coral Reef Ecosystems. In Biology of Marine Fungi; Raghukumar, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 89–113. ISBN 978-3-642-23342-5. [Google Scholar]
- Raghukumar, C. Algal-Fungal Interactions in the Marine Ecosystem: Symbiosis to Parasitism; CSMCRI: Bhavnagar, India, 2006. [Google Scholar]
- Voolstra, C.R. The Coral Microbiome in Sickness, in Health and in a Changing World. Nat. Rev. Microbiol. 2024, 22, 460–475. [Google Scholar] [CrossRef]
- Chen, B.; Wei, Y.; Yu, K.; Liang, Y.; Yu, X.; Liao, Z.; Qin, Z.; Xu, L.; Bao, Z. The Microbiome Dynamics and Interaction of Endosymbiotic Symbiodiniaceae and Fungi Are Associated with Thermal Bleaching Susceptibility of Coral Holobionts. Appl. Environ. Microbiol. 2024, 90, e01939-23. [Google Scholar] [CrossRef]
- Liao, X.; Yang, J.; Zhou, Z.; Wu, J.; Xu, D.; Yang, Q.; Zhong, S.; Zhang, X. Diversity and Antimicrobial Activity of Intestinal Fungi from Three Species of Coral Reef Fish. J. Fungi 2023, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Liu, Y.; Lin, Y.; Kraithong, S.; Mo, L.; Gao, Z.; Huang, R.; Zhang, X. A New Exopolysaccharide of Marine Coral-Associated Aspergillus pseudoglaucus SCAU265: Structural Characterization and Immunomodulatory Activity. J. Fungi 2023, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.V.; Bythell, J.C.; Willis, B.L. Levels of Immunity Parameters Underpin Bleaching and Disease Susceptibility of Reef Corals. FASEB J. 2010, 24, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mao, W.; Yang, Y.; Teng, X.; Zhu, W.; Qi, X.; Chen, Y.; Zhao, C.; Hou, Y.; Wang, C.; et al. Structure and Antioxidant Activity of an Extracellular Polysaccharide from Coral-Associated Fungus, Aspergillus versicolor LCJ-5-4. Carbohydr. Polym. 2012, 87, 218–226. [Google Scholar] [CrossRef]
- Sun, F.; Yang, H.; Zhang, X.; Tan, F.; Shi, Q. Response Characteristics of Bacterial Communities in Multiple Coral Genera at the Early Stages of Coral Bleaching during El Niño. Ecol. Indic. 2022, 144, 109569. [Google Scholar] [CrossRef]
- Chavanich, S.; Kusdianto, H.; Kullapanich, C.; Jandang, S.; Wongsawaeng, D.; Ouazzani, J.; Viyakarn, V.; Somboonna, N. Microbiomes of Healthy and Bleached Corals During a 2016 Thermal Bleaching Event in the Andaman Sea of Thailand. Front. Mar. Sci. 2022, 9, 763421. [Google Scholar] [CrossRef]
- Cheng, K.; Tong, M.; Cai, Z.; Jong, M.C.; Zhou, J.; Xiao, B. Prokaryotic and Eukaryotic Microbial Communities Associated with Coral Species Have High Host Specificity in the South China Sea. Sci. Total Environ. 2023, 867, 161185. [Google Scholar] [CrossRef]
- Qin, Z.; Yu, K.; Liang, J.; Yao, Q.; Chen, B. Significant Changes in Microbial Communities Associated With Reef Corals in the Southern South China Sea During the 2015/2016 Global-Scale Coral Bleaching Event. J. Geophys. Res. Ocean. 2020, 125, e2019JC015579. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, W.; Chen, B.; Yang, E.; Meng, L.; Chen, Y.; Li, J.; Huang, X.; Liang, J.; Yap, T.-K.; et al. Genetic Structure of Turbinaria peltata in the Northern South China Sea Suggest Insufficient Genetic Adaptability of Relatively High-Latitude Scleractinian Corals to Environment Stress. Sci. Total Environ. 2021, 775, 145775. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The Vegan Package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the International AAAI Conference on Web and Social Media, San Jose, CA, USA, 17–20 May 2009; Volume 3, pp. 361–362. [Google Scholar]
- Treseder, K.K.; Marusenko, Y.; Romero-Olivares, A.L.; Maltz, M.R. Experimental Warming Alters Potential Function of the Fungal Community in Boreal Forest. Glob. Change Biol. 2016, 22, 3395–3404. [Google Scholar] [CrossRef]
- Cunning, R.; Vaughan, N.; Gillette, P.; Capo, T.R.; Mate, J.L.; Baker, A.C. Dynamic Regulation of Partner Abundance Mediates Response of Reef Coral Symbioses to Environmental Change. Ecology 2015, 96, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome Diversity: High-Throughput Sequencing and Identification of Fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Lindahl, B. Fungal Identification Biases in Microbiome Projects: Fungal Identification Biases in Microbiome Projects. Environ. Microbiol. Rep. 2016, 8, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Mattoon, E.R.; Casadevall, A.; Cordero, R.J. Beat the Heat: Correlates, Compounds, and Mechanisms Involved in Fungal Thermotolerance. Fungal Biol. Rev. 2021, 36, 60–75. [Google Scholar] [CrossRef]
- Bakar, N.A.; Karsani, S.A.; Alias, S.A. Fungal Survival under Temperature Stress: A Proteomic Perspective. PeerJ 2020, 8, e10423. [Google Scholar] [CrossRef]
- Mydlarz, L.D.; McGinty, E.S.; Harvell, C.D. What Are the Physiological and Immunological Responses of Coral to Climate Warming and Disease? J. Exp. Biol. 2010, 213, 934–945. [Google Scholar] [CrossRef]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for Plant Growth Promotion and Biocontrol against Root-Knot Nematodes Infecting Eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef]
- Hulikere, M.M.; Joshi, C.G.; Ananda, D.; Poyya, J.; Nivya, T. Antiangiogenic, Wound Healing and Antioxidant Activity of Cladosporium cladosporioides (Endophytic Fungus) Isolated from Seaweed (Sargassum wightii). Mycology 2016, 7, 203–211. [Google Scholar] [CrossRef]
- Li, X.; Li, X.-M.; Xu, G.-M.; Li, C.-S.; Wang, B.-G. Antioxidant Metabolites from Marine Alga-Derived Fungus Aspergillus wentii EN-48. Phytochem. Lett. 2014, 7, 120–123. [Google Scholar] [CrossRef]
- De Almeida, D.A.C.; Gusmão, L.F.P.; Miller, A.N. Taxonomy and Molecular Phylogeny of Diatrypaceae (Ascomycota, Xylariales) Species from the Brazilian Semi-Arid Region, Including Four New Species. Mycol. Prog. 2016, 15, 53. [Google Scholar] [CrossRef]
- Lynch, S.C.; Zambino, P.J.; Mayorquin, J.S.; Wang, D.H.; Eskalen, A. Identification of New Fungal Pathogens of Coast Live Oak in California. Plant Dis. 2013, 97, 1025–1036. [Google Scholar] [CrossRef][Green Version]
- Chen, D.; Wang, C.; Ma, X.; Chen, K.; Wang, Z.; Wang, Q.; Zhang, J.; Zhou, Q.; Shen, W. Dynamic Changes in Soil Fungal Communities and Functional Groups in Response to Sugarcane/Soybean Intercropping with Reduced Nitrogen Fertilizer Application. Biol. Fertil. Soils 2023, 59, 363–378. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Martinez, J.; Kushmaro, A.; Woodley, C.M.; Loya, Y.; Ostrander, G.K. Symbiophagy as a Cellular Mechanism for Coral Bleaching. Autophagy 2009, 5, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, K.T.; Hynson, N.A. Networks as Tools for Defining Emergent Properties of Microbiomes and Their Stability. Microbiome 2024, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Wesener, F.; Rillig, M.C.; Tietjen, B. Heat Stress Can Change the Competitive Outcome between Fungi: Insights from a Modelling Approach. Oikos 2023, 2023, e09377. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Hoogenboom, M.O.; Maynard, J.A.; Grottoli, A.G.; Middlebrook, R. Energetics Approach to Predicting Mortality Risk from Environmental Stress: A Case Study of Coral Bleaching. Funct. Ecol. 2009, 23, 539–550. [Google Scholar] [CrossRef]
- Catarecha, P.; King, E.; Díaz-González, S.; Caro, E.; Sacristán, S.; Del Pozo, J.C. Heat Stress and Soil Thermal Gradients Shape Root-Associated Fungal Community Recruitment. Front. Microbiol. 2025, 16, 1334648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liao, X.; Mo, L.; Ren, X.; Li, Y.; Hou, Q.; Mok, S.W.-F.; Huang, R.; Sun, J.; Zhang, X. Unraveling the Fungal Community Dynamics in Heat-Tolerant Coral Turbinaria sp. During Bleaching in South China Sea. J. Fungi 2025, 11, 832. https://doi.org/10.3390/jof11120832
Chen X, Liao X, Mo L, Ren X, Li Y, Hou Q, Mok SW-F, Huang R, Sun J, Zhang X. Unraveling the Fungal Community Dynamics in Heat-Tolerant Coral Turbinaria sp. During Bleaching in South China Sea. Journal of Fungi. 2025; 11(12):832. https://doi.org/10.3390/jof11120832
Chicago/Turabian StyleChen, Xinye, Xinyu Liao, Li Mo, Xumeng Ren, Yaozu Li, Qili Hou, Simon Wing-Fai Mok, Riming Huang, Jijia Sun, and Xiaoyong Zhang. 2025. "Unraveling the Fungal Community Dynamics in Heat-Tolerant Coral Turbinaria sp. During Bleaching in South China Sea" Journal of Fungi 11, no. 12: 832. https://doi.org/10.3390/jof11120832
APA StyleChen, X., Liao, X., Mo, L., Ren, X., Li, Y., Hou, Q., Mok, S. W.-F., Huang, R., Sun, J., & Zhang, X. (2025). Unraveling the Fungal Community Dynamics in Heat-Tolerant Coral Turbinaria sp. During Bleaching in South China Sea. Journal of Fungi, 11(12), 832. https://doi.org/10.3390/jof11120832






