Arbuscular Mycorrhizal Fungi as Core Engineers in Synthetic Microbial Communities: Boosting Plant Growth and Soil Health for Sustainable Agriculture
Abstract
1. Introduction
2. Design and Preparation of AMF-Based SynCom
2.1. Taxonomic Basis of AMF to Engage in SynCom
| Class | Order | Family | Genera |
|---|---|---|---|
| Archaeosporomycetes | Archaeosporales | Ambisporaceae | Ambispora |
| Archaeosporaceae | Archaeospora | ||
| Geosiphonaceae | Geosiphon | ||
| Polonosporaceae | Polonospora | ||
| Glomeromycetes | Diversisporales | Acaulosporaceae | Acaulospora |
| Diversisporaceae | Corymbiglomus, Desertispora, Diversispora, Otospora, Redeckera, Sieverdingia and Tricispora | ||
| Gigasporaceae | Bulbospora, Cetraspora, Dentiscutata, Fuscutata, Gigaspora, Intraornatospora, Paradentiscutata, Racocetra and Scutellospora | ||
| Pacisporaceae | Pacispora | ||
| Sacculosporaceae | Sacculospora | ||
| Entrophosporales | Entrophosporaceae | Entrophospora, Albahupha and Alborhynchus | |
| Glomerales | Glomeraceae | Complexispora, Dominikia, Epigeocarpum, Funneliformis, Funneliglomus, Glomus, Halonatospora, Kamienskia, Silvaspora, Microdominikia, Microkamienskia, Oehlia, Nanoglomus, Orientoglomus, Rhizoglomus, Rhizophagus, Sclerocarpum, Sclerocystis and Septoglomus | |
| Paraglomeromycetes | Paraglomerales | Paraglomerales | Innospora and Paraglomus |
| Pervetustaceae | Pervetustus |
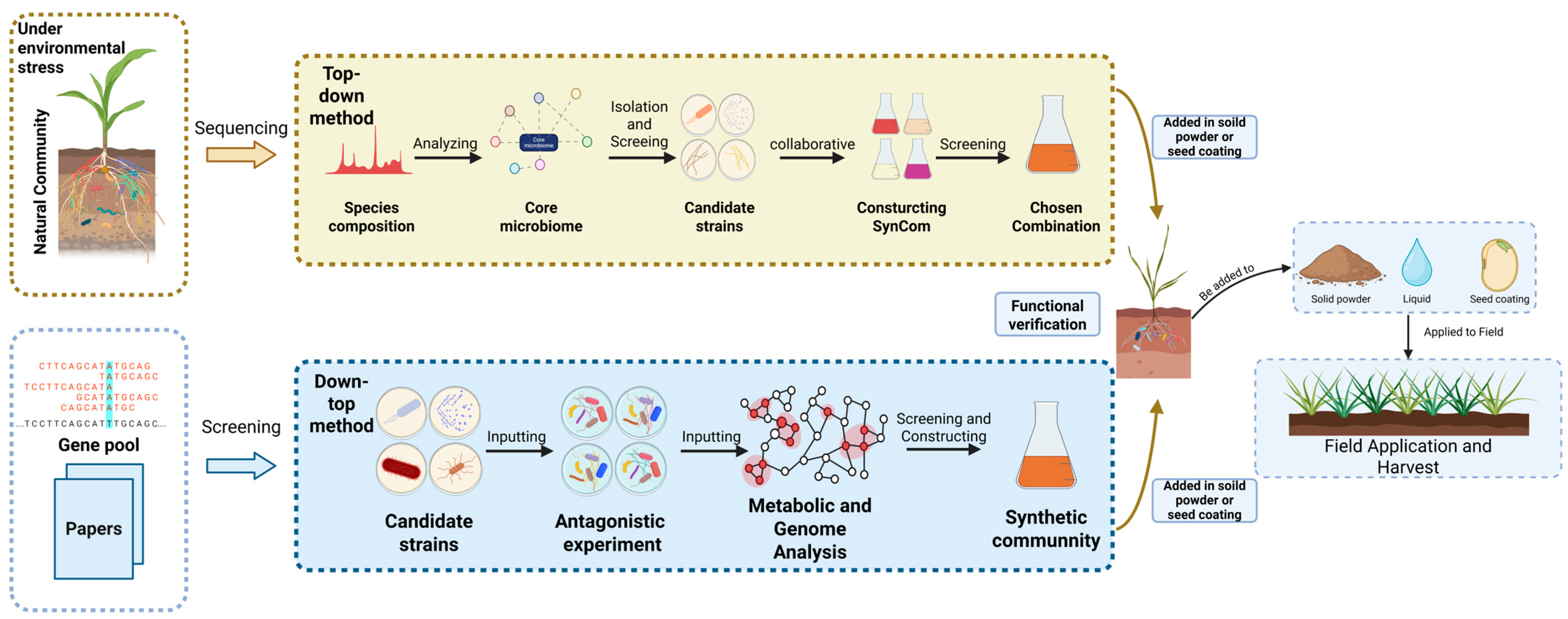
2.2. Design and Construction of AMF-Based SynCom and Large-Scale Production Approaches for AMF-Based SynCom
2.2.1. Design and Construction of AMF-Based SynCom
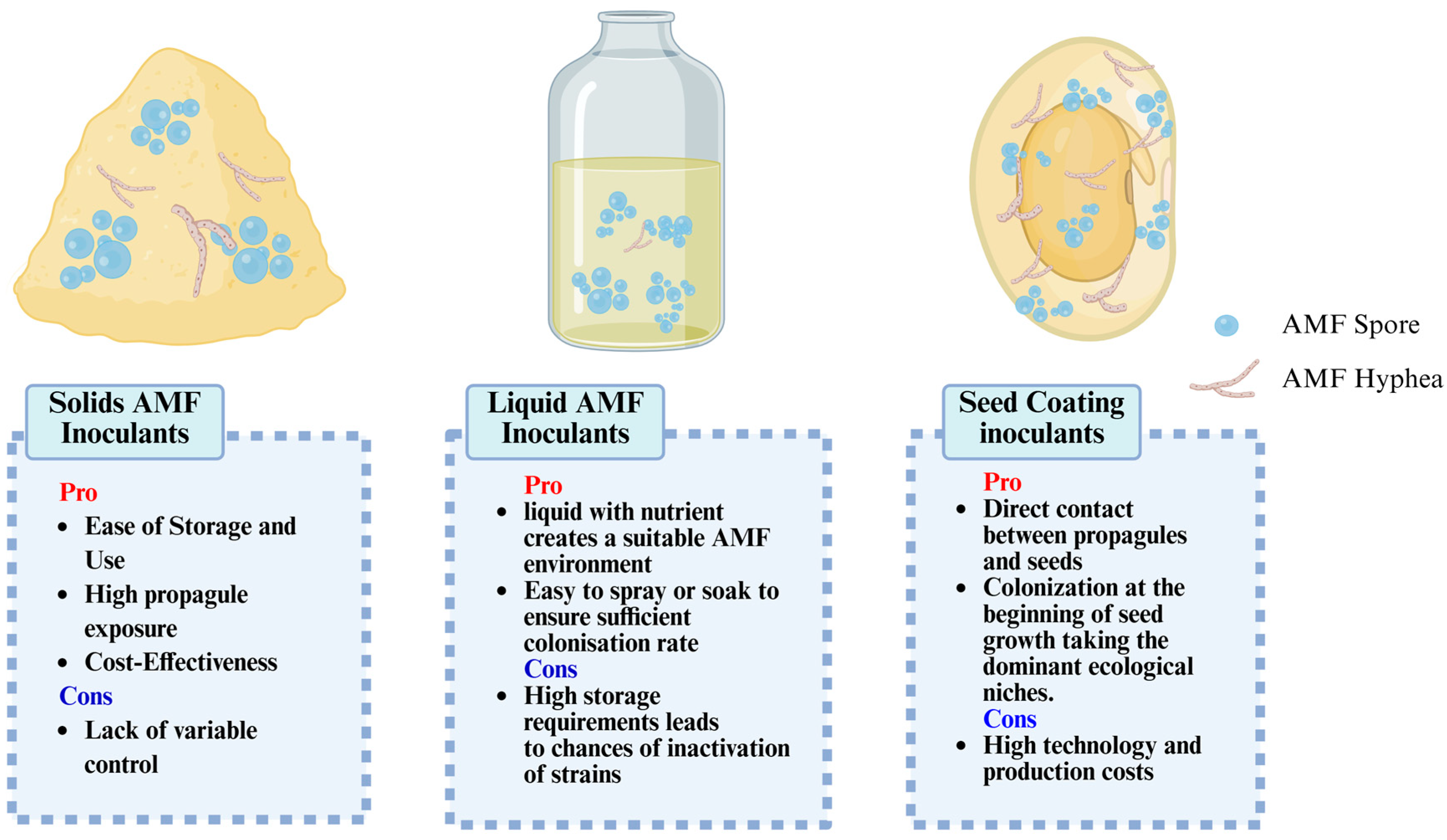
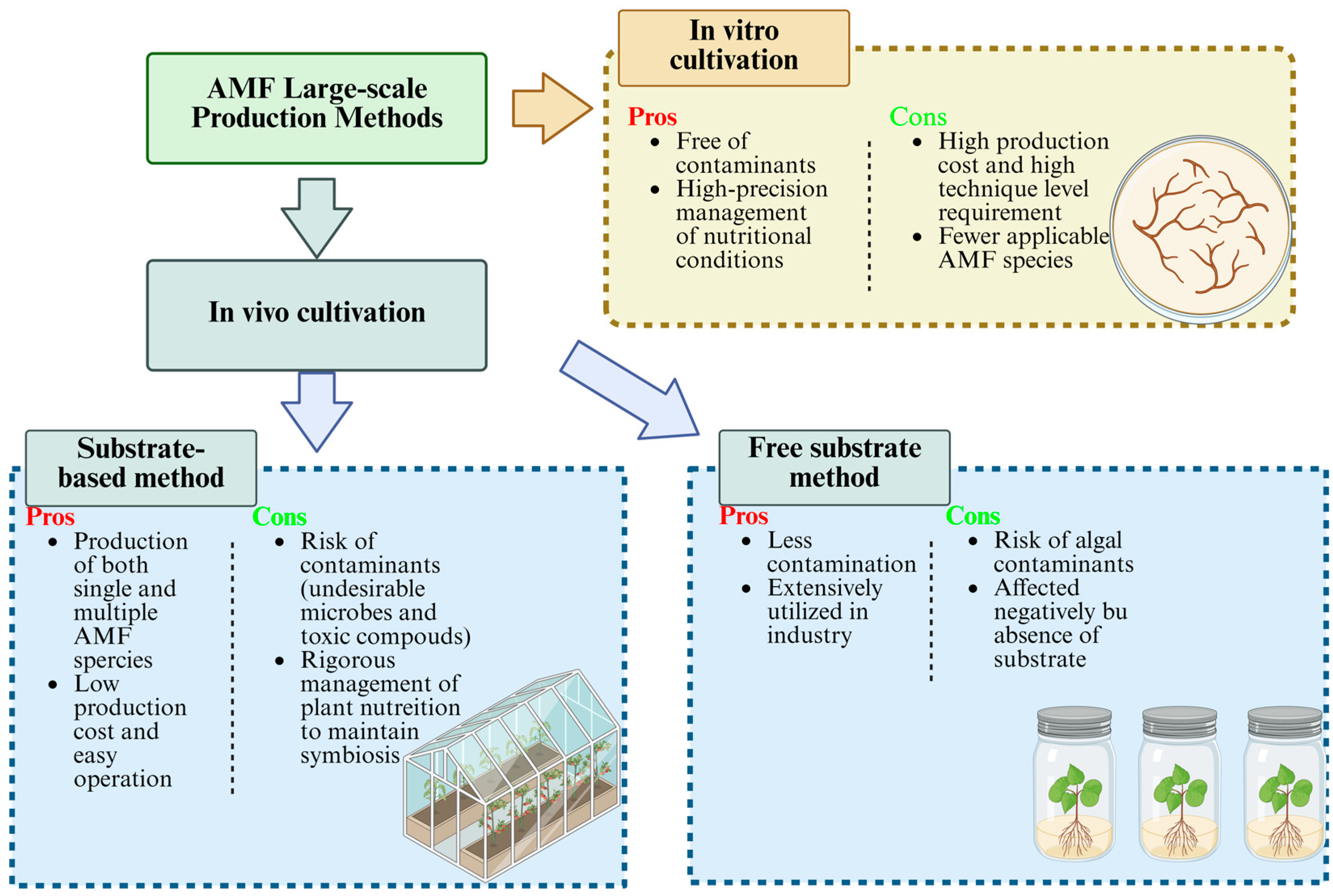
2.2.2. AMF-Based SynCom Agent and Production Methods
2.2.3. Co-Culture Strategy: Essential for Synchronized Amplification of AMF and Companion Bacteria
3. AMF-Based SynCom Interaction and Plant Growth Promotion
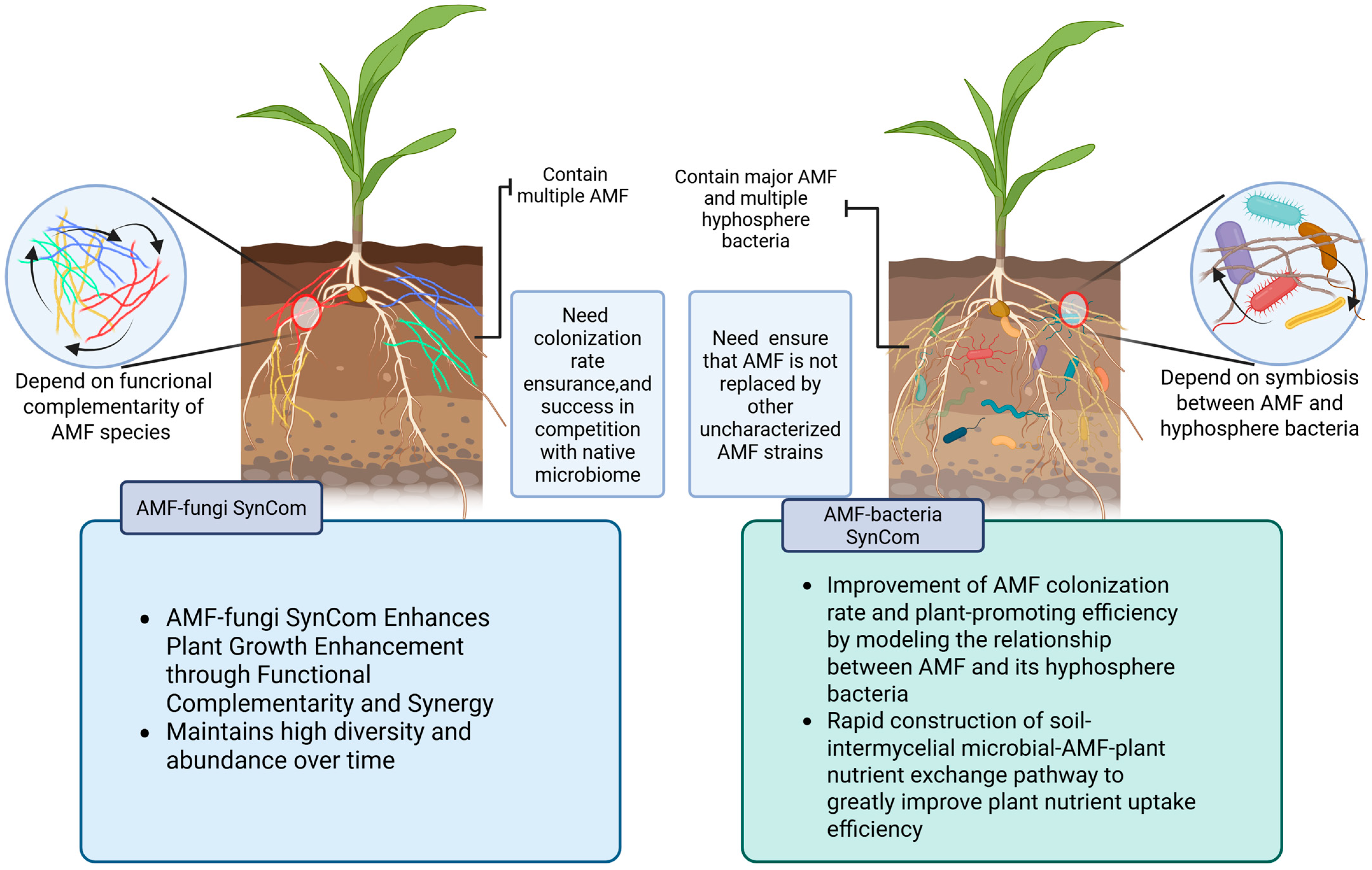
3.1. Interactions in AMF-Based SynCom Constructed by Fungi and Its Plant Growth Promotion Effects
Trichoderma
3.2. Interactions Between AMF and Bacteria and Their Effects on Promoting Plant Growth
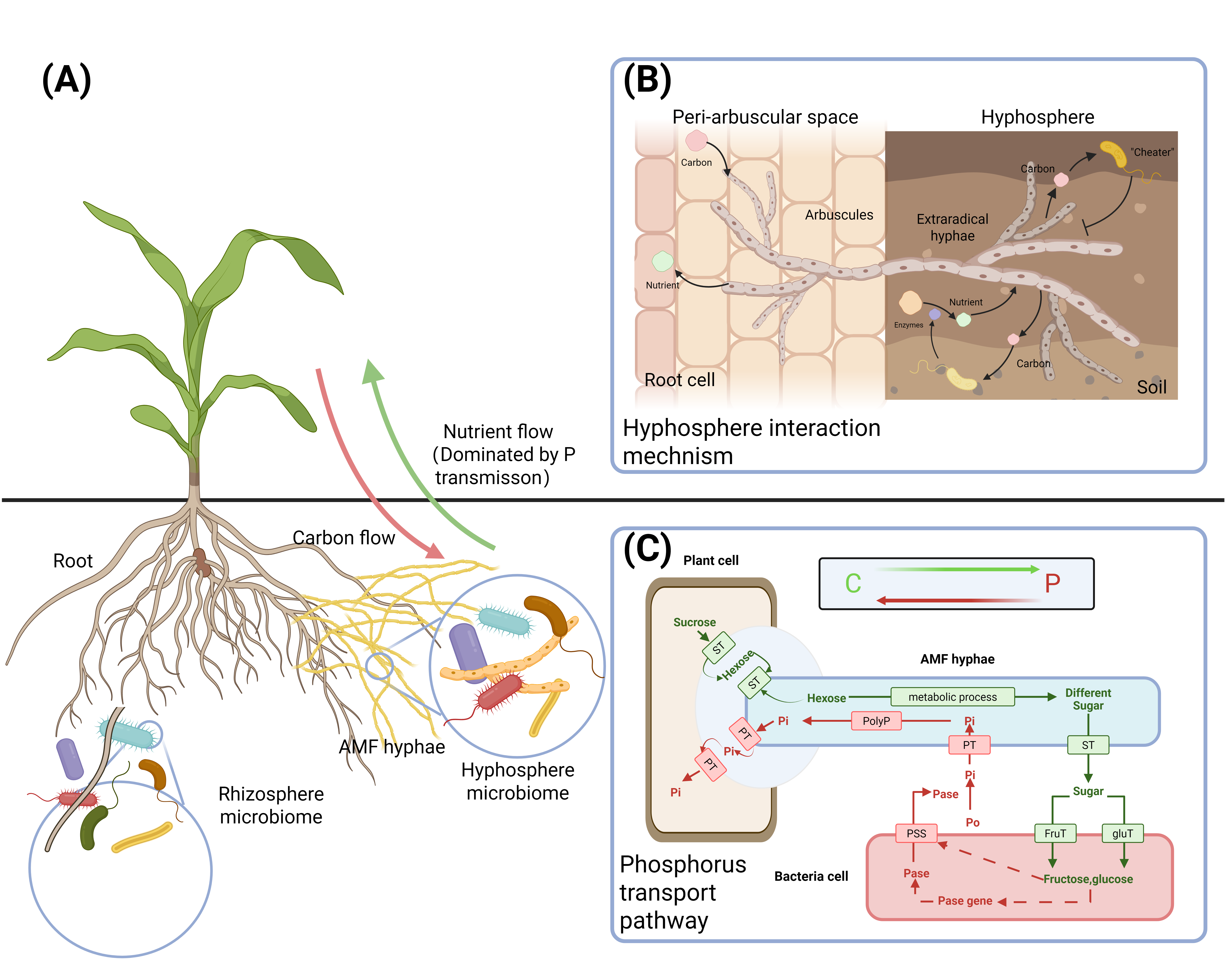
3.2.1. Streptomyces
3.2.2. Pseudomonas
| [Species Name | Main Effect for Plant-Promoting |
|---|---|
| Pseudomonas flurescens |
|
| Pseudomonas putida |
|
| Pseudomonas aerginosa |
|
| Pseudomonas nitroreducens |
|
| Pseudomonas protegens |
|
3.2.3. Rhizobia
3.2.4. Bacillus spp.
3.3. Synergistic Effects of AMF with Multiple Microbes and Their Combined Effects on Plants
4. Limitations of AMF-Based SynCom Research and Application
4.1. Limitation in AMF and SynCom Field
4.2. Perspectives
5. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Fan, Z.; Hu, Z.; Li, Z.; Fu, Y.; Wang, Q.; Lin, X.; Feng, Y. Synthetic Communities Derived from the Core Endophytic Microbiome of Hyperaccumulators and Their Role in Cadmium Phytoremediation. Microbiome 2024, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.S.; Creevey, C.J.; Santana, M.F. Designing a Synthetic Microbial Community through Genome Metabolic Modeling to Enhance Plant–Microbe Interaction. Environ. Microbiome 2023, 18, 81. [Google Scholar] [CrossRef]
- Russ, D. The Plant Microbiome: From Ecology to Reductionism and Beyond. Annu. Rev. Microbiol. 2020, 74, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Mazylyte, R.; Kailiuviene, J.; Mazoniene, E.; Orola, L.; Kaziuniene, J.; Mazylyte, K.; Lastauskiene, E.; Gegeckas, A. The Co-Inoculation Effect on Triticum Aestivum Growth with Synthetic Microbial Communities (Syncoms) and Their Potential in Agrobiotechnology. Plants 2024, 13, 1716. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing Causality: Opportunities of Synthetic Communities for Plant Microbiome Research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef]
- Mehlferber, E.C.; Arnault, G.; Joshi, B.; Partida-Martinez, L.P.; Patras, K.A.; Simonin, M.; Koskella, B. A Cross-Systems Primer for Synthetic Microbial Communities. Nat. Microbiol. 2024, 9, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Case, N.T.; Gurr, S.J.; Fisher, M.C.; Blehert, D.S.; Boone, C.; Casadevall, A.; Chowdhary, A.; Cuomo, C.A.; Currie, C.R.; Denning, D.W.; et al. Fungal Impacts on Earth’s Ecosystems. Nature 2025, 638, 49–57. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Liu, F.; Liang, J.; Zhao, P.; Tsui, C.K.M.; Cai, L. Cross-Kingdom Synthetic Microbiota Supports Tomato Suppression of Fusarium Wilt Disease. Nat. Commun. 2022, 13, 7890. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, S.; Peng, H.; Zhang, L.; Li, S.; Berendsen, R.L.; Yang, T.; Dong, C.; Wei, Z.; Xu, Y.; et al. Synergic Interactions between Trichoderma and the Soil Microbiomes Improve Plant Iron Availability and Growth. NPJ Biofilms Microbiomes 2025, 11, 56. [Google Scholar] [CrossRef]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y.; et al. Core Microbiomes for Sustainable Agroecosystems. Nat. Plants 2018, 4, 247–257. [Google Scholar] [CrossRef]
- Luo, J.; Gu, S.; Guo, X.; Liu, Y.; Tao, Q.; Zhao, H.-P.; Liang, Y.; Banerjee, S.; Li, T. Core Microbiota in the Rhizosphere of Heavy Metal Accumulators and Its Contribution to Plant Performance. Environ. Sci. Technol. 2022, 56, 12975–12987. [Google Scholar] [CrossRef]
- Janoušková, M.; Krak, K.; Wagg, C.; Štorchová, H.; Caklová, P.; Vosátka, M. Effects of Inoculum Additions in the Presence of a Preestablished Arbuscular Mycorrhizal Fungal Community. Appl. Environ. Microbiol. 2013, 79, 6507–6515. [Google Scholar] [CrossRef]
- Harrison, M.J. Signaling in the Arbuscular Mycorrhizal Symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef]
- Hoosein, S.; Neuenkamp, L.; Trivedi, P.; Paschke, M.W. Am Fungal-Bacterial Relationships: What Can They Tell Us About Ecosystem Sustainability and Soil Functioning? Front. Fungal Biol. 2023, 4, 1141963. [Google Scholar] [CrossRef]
- Davison, J.; García De León, D.; Zobel, M.; Moora, M.; Bueno, C.G.; Barceló, M.; Gerz, M.; León, D.; Meng, Y.; Pillar, V.D.; et al. Plant Functional Groups Associate with Distinct Arbuscular Mycorrhizal Fungal Communities. New Phytol. 2020, 226, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Gadkar, V.; Yarden, O.; Kapulnik, Y. Analysis of Quantitative Interactions between Two Species of Arbuscular Mycorrhizal Fungi, Glomus mosseae and G. intraradices, by Real-Time Pcr. Appl. Environ. Microbiol. 2006, 72, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Beurden, B.V. The Cry for Help: How Plants Orchestrate Interactions with the Rhizosphere Microbiome. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2024. [Google Scholar]
- Koziol, L.; McKenna, T.P.; Bever, J.D. Meta-Analysis Reveals Globally Sourced Commercial Mycorrhizal Inoculants Fall Short. New Phytol. 2025, 246, 821–827. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Flagella, Z.; Pellegrino, E.; Ercoli, L. Effect of Arbuscular Mycorrhizal Fungal Seed Coating on Grain Protein and Mineral Composition of Old and Modern Bread Wheat Genotypes. Agronomy 2022, 12, 2418. [Google Scholar] [CrossRef]
- Liu, R.; Li, M.; Guo, S.; Chen, Y. The Role of Amf Community Composition, Diversity, and Distribution in Sustainable Agroecosystems. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Inoculum Production and Application; Springer: Singapore, 2024; pp. 281–317. [Google Scholar]
- Delaeter, M.; Magnin-Robert, M.; Randoux, B.; Lounès-Hadj Sahraoui, A. Arbuscular Mycorrhizal Fungi as Biostimulant and Biocontrol Agents: A Review. Microorganisms 2024, 12, 1281. [Google Scholar] [CrossRef]
- Sikes, B.A.; Cottenie, K.; Klironomos, J.N. Plant and Fungal Identity Determines Pathogen Protection of Plant Roots by Arbuscular Mycorrhizas. J. Ecol. 2009, 97, 1274–1280. [Google Scholar] [CrossRef]
- Malik, R.J.; Dixon, M.H.; Bever, J.D. Mycorrhizal Composition Can Predict Foliar Pathogen Colonization in Soybean. Biol. Control. 2016, 103, 46–53. [Google Scholar] [CrossRef]
- Maherali, H.; Klironomos, J.N. Influence of Phylogeny on Fungal Community Assembly and Ecosystem Functioning. Science 2007, 316, 1746–1748. [Google Scholar] [CrossRef]
- Sikes, B.A. When Do Arbuscular Mycorrhizal Fungi Protect Plant Roots from Pathogens? Plant Signal. Behav. 2010, 5, 763–765. [Google Scholar] [CrossRef]
- Nadieline, C.V.; Quéré, A.l.; Ndiaye, C.; Diène, A.A.; Rego, F.D.; Sadio, O.; Thioye, Y.I.; Neyra, M.; Kébé, C.M.F.; Wade, T.K. Development of on-Farm Amf Inoculum Production for Sustainable Agriculture in Senegal. PLoS ONE 2024, 19, e0310065. [Google Scholar] [CrossRef]
- Ramalakshmi, A.; Mythili, M.; Karthikeyan, R.; Senthil, A.; Sivakumar, U.; Balakrishnan, M. Seed Coating of Native Rhizophagus irregularis and Funneliformis Sp. For Improved Production and Moisture Stress Mitigation in Finger Millet, Eleusine Coracana. J. Soil Sci. Plant Nutr. 2023, 23, 5810–5826. [Google Scholar] [CrossRef]
- Deja-Sikora, E.; Werner, K.; Hrynkiewicz, K. Amf Species Do Matter: Rhizophagus irregularis and Funneliformis mosseae Affect Healthy and Pvy-Infected Solanum tuberosum L. in a Different Way. Front. Microbiol. 2023, 14, 1127278. [Google Scholar] [CrossRef]
- Singh, R.; Soni, S.K.; Bajpai, A. Cooperative Interaction of Glomus Intraradices with Plant Growth-Promoting Rhizobacteria Promotes Plant Development and Essential Oil Yield of Pogostemon Cablin and Reduces Disease Occurrence under Organic Field Conditions. Australas. Plant Pathol. 2023, 52, 595–607. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Posta, K.; Chmura, D.; Piotrowska-Seget, Z. Differences in the effects of single and mixed species of AMF on the growth and oxidative stress defense in Lolium perenne exposed to hydrocarbons. Ecotoxicol. Environ. Saf. 2021, 217, 112252. [Google Scholar] [CrossRef]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; De Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; Van Velzen, R.; et al. Synthetic Bacterial Community Derived from a Desert Rhizosphere Confers Salt Stress Resilience to Tomato in the Presence of a Soil Microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef]
- Lawson, C.E.; Harcombe, W.R.; Hatzenpichler, R.; Lindemann, S.R.; Löffler, F.E.; O’Malley, M.A.; García Martín, H.; Pfleger, B.F.; Raskin, L.; Venturelli, O.S.; et al. Common Principles and Best Practices for Engineering Microbiomes. Nat. Rev. Microbiol. 2019, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Amit, G.; Bashan, A. Top-down Identification of Keystone Taxa in the Microbiome. Nat. Commun. 2023, 14, 3951. [Google Scholar] [CrossRef]
- Bittleston, L.S. Connecting Microbial Community Assembly and Function. Curr. Opin. Microbiol. 2024, 80, 102512. [Google Scholar] [CrossRef]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and Representative Bacterial Community of Maize Roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef]
- Genomics, F.L.; Fletcher, L. The Power of Multi-Omics: Understanding the Omes; Front Line Genomics: Hampshire, UK, 2025. [Google Scholar]
- Wu, D.; Daugherty, S.C.; Van Aken, S.E.; Pai, G.H.; Watkins, K.L.; Khouri, H.; Tallon, L.J.; Zaborsky, J.M.; Dunbar, H.E.; Tran, P.L.; et al. Metabolic Complementarity and Genomics of the Dual Bacterial Symbiosis of Sharpshooters. PLoS Biol. 2006, 4, e188. [Google Scholar] [CrossRef]
- Nayfach, S.; Roux, S.; Seshadri, R.; Udwary, D.; Varghese, N.; Schulz, F.; Wu, D.; Paez-Espino, D.; Chen, I.M.; Huntemann, M.; et al. A Genomic Catalog of Earth’s Microbiomes. Nat. Biotechnol. 2020, 39, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.F.; Dhakan, D.B.; Serra, L.; Francisco, A.P.; Carvalho-Santos, Z.; Baltazar, C.; Elias, A.P.; Anjos, M.; Zhang, T.; Maddocks, O.D.K.; et al. Metabolic Cross-Feeding in Imbalanced Diets Allows Gut Microbes to Improve Reproduction and Alter Host Behaviour. Nat. Commun. 2020, 11, 4236. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yang, Y.; Wang, D.; Cheng, S.H.; Zheng, C.; Zhang, T. Charting the Complexity of the Activated Sludge Microbiome through a Hybrid Sequencing Strategy. Microbiome 2021, 9, 205. [Google Scholar] [CrossRef]
- Jiao, J.Y.; Fu, L.; Hua, Z.S.; Liu, L.; Salam, N.; Liu, P.F.; Lv, A.P.; Wu, G.; Xian, W.D.; Zhu, Q.; et al. Insight into the Function and Evolution of the Wood-Ljungdahl Pathway in Actinobacteria. ISME J. 2021, 15, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Osimiri, L.C.; Chevalier, M.; Bugaj, L.J.; Nguyen, T.H.; Greenstein, R.A.; Ng, A.H.; Stewart-Ornstein, J.; Neves, L.T.; El-Samad, H. El-Samad. Optogenetic Control Reveals Differential Promoter Interpretation of Transcription Factor Nuclear Translocation Dynamics. Cell Syst. 2020, 11, 336–353 e24. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Khan, Z.; Asad, M.; D’amato, R.; Alsahli, A.A.; Ahmad, P. Synthetic Bacterial Community Derived from Astragalus Mongholicus and Plant-Plant Interactions Inhibit Cadmium Uptake by Modulating Gene Expression, Antioxidant System and Carbohydrate Metabolism under Cadmium Contaminated Soil. J. Environ. Chem. Eng. 2024, 12, 111619. [Google Scholar] [CrossRef]
- Schwab, S.; De Souza Pires, A.; Candido, G.Z.; Saggin Júnior, O.J.; Reis, V.M.; Cruz, L.M. Analysis of the Endophytic Microbiota of Roots and Culms of Two Commercial Sugarcane Cultivars Inoculated with a Synthetic Microbial Community. Appl. Soil Ecol. 2024, 195, 105235. [Google Scholar] [CrossRef]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of Arbuscular Mycorrhizal Fungal Inoculant Benchmarks. Microorganisms 2020, 9, 81. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of Arbuscular Mycorrhiza on Organic Solutes in Maize Leaves under Salt Stress. Mycorrhiza 2011, 21, 423–430. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, L.; Xiao, Y. The Combined Use of Arbuscular Mycorrhizal Fungi, Biochar and Nitrogen Fertilizer Is Most Beneficial to Cultivate Cichorium intybus L. In Cd-Contaminated Soil. Ecotoxicol. Environ. Saf. 2021, 217, 112154. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering Natural Microbiomes toward Enhanced Bioremediation by Microbiome Modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef]
- Declerck, S.; Strullu, D.G.; Fortin, J.A. (Eds.) In Vitro Culture of Mycorrhizas; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 978-3-540-24027-3. [Google Scholar]
- IJdo, M.; Cranenbrouck, S.; Declerck, S. Methods for Large-Scale Production of Am Fungi: Past, Present, and Future. Mycorrhiza 2011, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, D.M.; Kaonongbua, W.; Bever, J.D. Isolation, Culture, and Detection of Arbuscular Mycorrhizal Fungi. In Manual of Environmental Microbiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 627–637. [Google Scholar]
- Aziem, S.; Negi, A.K. A Review on the Science of Growing Crops without Soil (Soilless Culture)—A Novel Alternative for Growing Crops. Int. J. Agric. Crop Sci. (IJACS) 2014, 7, 833–842. [Google Scholar]
- Kinnunen, M.; Dechesne, A.; Proctor, C.; Hammes, F.; Johnson, D.; Quintela-Baluja, M.; Graham, D.; Daffonchio, D.; Fodelianakis, S.; Hahn, N.; et al. A Conceptual Framework for Invasion in Microbial Communities. ISME J. 2016, 10, 2773–2779. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Wang, X.; Liu, S.; Luo, L.; Zeng, Q.; Wu, Y.; Zeng, Y.; Yang, Z.; Sheng, G.; et al. Emerging Nanochitosan for Sustainable Agriculture. Int. J. Mol. Sci. 2024, 25, 12261. [Google Scholar] [CrossRef]
- Wright, R.J.; Gibson, M.I.; Christie-Oleza, J.A. Artificial Selection of Microbial Communities to Enhance Degradation of Recalcitrant Polymers. bioRxiv 2018. [Google Scholar] [CrossRef]
- Martiny, J.B.H.; Martiny, A.C.; Weihe, C.; Lu, Y.; Berlemont, R.; Brodie, E.L.; Goulden, M.L.; Treseder, K.K.; Allison, S.D. Microbial Legacies Alter Decomposition in Response to Simulated Global Change. ISME J. 2017, 11, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Al-Tameemi, Z.; Rodríguez-Verdugo, A. Microbial Diversification Is Maintained in an Experimentally Evolved Synthetic Community. mSystems 2024, 9, e0105324. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Z.; He, Y.; Li, G.; Lv, X.; Zhuang, L. High-Throughput Sequencing Analysis of the Rhizosphere Arbuscular Mycorrhizal Fungi (Amf) Community Composition Associated with Ferula sinkiangensis. BMC Microbiol. 2020, 20, 335. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are There Benefits of Simultaneous Root Colonization by Different Arbuscular Mycorrhizal Fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef]
- Faghihinia, M.; Jansa, J.; Halverson, L.J.; Staddon, P.L. Hyphosphere Microbiome of Arbuscular Mycorrhizal Fungi: A Realm of Unknowns. Biol. Fertil. Soils 2023, 59, 17–34. [Google Scholar] [CrossRef]
- Weber, S.E.; Bascompte, J.; Kahmen, A.; Niklaus, P.A. Amf Diversity Promotes Plant Community Phosphorus Acquisition and Reduces Carbon Costs Per Unit of Phosphorus. New Phytol. 2025, 248, 886–896. [Google Scholar] [CrossRef]
- Jansa, J.; Mozafar, A.; Frossard, E. Phosphorus Acquisition Strategies within Arbuscular Mycorrhizal Fungal Community of a Single Field Site. Plant Soil 2005, 276, 163–176. [Google Scholar] [CrossRef]
- Koide, R.T. Functional Complementarity in the Arbuscular Mycorrhizal Symbiosis. New Phytol. 2000, 147, 233–235. [Google Scholar] [CrossRef]
- Zoubi, B.; Mokrini, F.; Houssayni, S.; Benkebboura, A.; Akachoud, O.; Ghoulam, C.; Housseini, A.I.; Qaddoury, A. Effectiveness of the Arbuscular Mycorrhizal Fungi Funneliformis mossae and Rhizophagus irregularis as Biological Control Agent of the Citrus Nematode Tylenchulus semipenetrans. J. Nat. Pestic. Res. 2025, 11, 100104. [Google Scholar] [CrossRef]
- Thonar, C.; Frossard, E.; Šmilauer, P.; Jansa, J. Competition and Facilitation in Synthetic Communities of Arbuscular Mycorrhizal Fungi. Mol. Ecol. 2014, 23, 733–746. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The Multifaceted Roles of Arbuscular Mycorrhizal Fungi in Peanut Responses to Salt, Drought, and Cold Stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of Single and Multiple Species Inocula of Arbuscular Mycorrhizal Fungi on the Salinity Tolerance of a Bangladeshi Rice (Oryza sativa L.) Cultivar. Mycorrhiza 2020, 30, 431–444. [Google Scholar] [CrossRef]
- Hardin, G. The Competitive Exclusion Principle: An Idea That Took a Century to be Born Has Implications in Ecology, Economics, and Genetics. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef]
- Alley, T.R. Competition Theory, Evolution, and the Concept of an Ecological Niche. Acta Biotheor. 1982, 31, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Anca, I.-A. Plants, Mycorrhizal Fungi, and Bacteria: A Network of Interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wu, R.; Qi, D.; Xu, T.; Chang, W.; Li, K.; Fang, X.; Song, F. The Effect of Amf Combined with Biochar on Plant Growth and Soil Quality under Saline-Alkali Stress: Insights from Microbial Community Analysis. Ecotoxicol. Environ. Saf. 2024, 281, 116592. [Google Scholar] [CrossRef]
- Fricker, M.; Boddy, L.; Bebber, D. Network Organisation of Mycelial Fungi. In Biology of the Fungal Cell; Howard, R.J., Gow, N.A.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 309–330. [Google Scholar]
- Aguilar-Trigueros, C.A.; Boddy, L.; Rillig, M.C.; Fricker, M.D. Network Traits Predict Ecological Strategies in Fungi. ISME Commun. 2022, 2, 2. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X. Does Arbuscular Mycorrhizal Fungi Inoculation Influence Soil Carbon Sequestration? Biol. Fertil. Soils 2024, 60, 213–225. [Google Scholar] [CrossRef]
- Guzman-Guzman, P.; Etesami, H.; Santoyo, G. Trichoderma: A Multifunctional Agent in Plant Health and Microbiome Interactions. BMC Microbiol. 2025, 25, 434. [Google Scholar] [CrossRef]
- Sorahinobar, M.; Yusefieh, N.; Rezayian, M.; Shahbazi, S. Multifaceted Role of Trichoderma harzianum Isolates in Mitigating Drought Stress and Promoting Adaptive Responses in Barley Cultivars. Sci. Rep. 2025, 15, 26552. [Google Scholar] [CrossRef]
- Yağmur, A.; Demir, S.; Canpolat, S.; Rezaee Danesh, Y.; Farda, B.; Djebaili, R.; Pace, L.; Pellegrini, M. Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum. Plants 2024, 13, 386. [Google Scholar] [CrossRef]
- Al-Asbahi, A.A.S. Arbuscular Mycorrhizal Protein Mrna over-Expression in Bread Wheat Seedlings by Trichoderma harzianum Raifi (Krl-Ag2) Elicitation. Gene 2012, 494, 209–213. [Google Scholar] [CrossRef]
- Sivaprakasam Padmanaban, P.B.; Stange, P.; Weber, B.; Ghirardo, A.; Pritsch, K.; Karl, T.; Benz, J.P.; Rosenkranz, M.; Schnitzler, J.P. Strain and Contact-Dependent Metabolomic Reprogramming Reveals Distinct Interaction Strategies between Laccaria bicolor and Trichoderma. Fungal Biol. Biotechnol. 2025, 12, 13. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Pascual, J.A.; Lloret, E.; Roldán, A. Interactions between Arbuscular Mycorrhizal Fungi and Trichoderma harzianum and Their Effects on Fusarium Wilt in Melon Plants Grown in Seedling Nurseries. J. Sci. Food Agric. 2009, 89, 1843–1850. [Google Scholar] [CrossRef]
- Lahrach, Z.; Legeay, J.; Ahmed, B.; Hijri, M. The Composition of the Arbuscular Mycorrhizal Fungal Bacteriome Is Species Dependent. Environ. Microbiome 2024, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Interactions between Arbuscular Mycorrhizal Fungi and Soil Bacteria. Appl. Microbiol. Biotechnol. 2011, 89, 917–930. [Google Scholar] [CrossRef]
- Li, X.; Song, C.; Kang, X.; Chen, F.; Li, A.; Wang, Y.; Zou, J.; Yin, J.; Li, Y.; Sun, Z.; et al. Assembly and Functional Profile of Rhizosphere Microbial Community during the Salix Viminalis-Amf Remediation of Polycyclic Aromatic Hydrocarbon Polluted Soils. J. Environ. Manag. 2024, 370, 122503. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Groten, K. The Costs and Benefits of Plant–Arbuscular Mycorrhizal Fungal Interactions. Annu. Rev. Plant Biol. 2022, 73, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, Y.; Zhou, T.; Lu, Y.; Yang, X.; Tang, K.; Liu, F. Synergy between Arbuscular Mycorrhizal Fungi and Rhizosphere Bacterial Communities Increases the Utilization of Insoluble Phosphorus and Potassium in the Soil by Maize. J. Agric. Food Chem. 2024, 72, 23631–23642. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Hobbie, E.A. Carbon Allocation to Ectomycorrhizal Fungi Correlates with Belowground Allocation in Culture Studies. Ecology 2006, 87, 563–569. [Google Scholar] [CrossRef]
- El-Ghany, M.F.A.; El–Kherbawy, M.I.; Mohamed, D.A. Mitigation Adverse Effects of Salinity Stress on Wheat Plants by Co-Inoculation of Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Compost Amendment. Int. J. Health Sci. 2022, 6, 12467–12488. [Google Scholar] [CrossRef]
- Yang, M.; Song, Y.; Ma, H.; Li, Z.; Ding, J.; Yin, T.; Niu, K.; Sun, S.; Qi, J.; Lu, G.; et al. Unveiling the Hidden World: How Arbuscular Mycorrhizal Fungi and Its Regulated Core Fungi Modify the Composition and Metabolism of Soybean Rhizosphere Microbiome. Environ. Microbiome 2024, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.; Linderman, R.G.; Bethlenfalvay, G.J. Bacterial Associations with the Mycorrhizosphere and Hyphosphere of the Arbuscular Mycorrhizal Fungus Glomus Mosseae. Plant Soil 1998, 202, 79–87. [Google Scholar] [CrossRef]
- Frey, S.D. Mycorrhizal Fungi as Mediators of Soil Organic Matter Dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Zeng, Q.; Wang, X.; Liu, S.; Wang, E.; Wu, Y.; Zeng, Y.; He, M.; Wang, Y.; et al. Chitosan Hydrogel Microspheres Loaded with Bacillus Subtilis Promote Plant Growth and Reduce Chromium Uptake. Int. J. Biol. Macromol. 2025, 286, 138401. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.; Tang, J.; Hu, L.; Chen, X. Arbuscular Mycorrhizal Fungi Affect Root Morphology Locally but Not Systemically through Altering Nutrient- and Phytohormone-Related Gene Expressions at Low Soil P Level. Plant Soil 2024, 512, 1279–1296. [Google Scholar] [CrossRef]
- Qiu, L.-X.; Guan, D.-X.; Liu, Y.-W.; Luo, Y.; Teng, H.H.; Kuzyakov, Y.; Ma, L.Q. Interactions of Silicon and Arbuscular Mycorrhizal Fungi on Phosphorus Uptake during Rice Vegetative Growth. Geoderma 2025, 454, 117184. [Google Scholar] [CrossRef]
- Jin, Z.; Jiang, F.; Wang, L.; Declerck, S.; Feng, G.; Zhang, L. Arbuscular Mycorrhizal Fungi and Streptomyces: Brothers in Arms to Shape the Structure and Function of the Hyphosphere Microbiome in the Early Stage of Interaction. Microbiome 2024, 12, 83. [Google Scholar] [CrossRef]
- Worthen, D.B. Streptomyces in Nature and Medicine: The Antibiotic Makers. J. Hist. Med. Allied Sci. 2008, 63, 273–274. [Google Scholar] [CrossRef]
- Montes-Montes, G.; González-Escobedo, R.; Muñoz-Castellanos, L.N.; Avila-Quezada, G.D.; Ramírez-Sánchez, O.; Borrego-Loya, A.; Ortiz-Aguirre, I.; Muñoz-Ramírez, Z.Y. Whole Genome Analysis of Streptomyces Spp. Strains Isolated from the Rhizosphere of Vitis vinifera L. Reveals Their Role in Nitrogen and Phosphorus Metabolism. Nitrogen 2024, 5, 301–314. [Google Scholar] [CrossRef]
- Santos-Beneit, F. Genome Sequencing Analysis of Streptomyces Coelicolor Mutants That Overcome the Phosphate-Depending Vancomycin Lethal Effect. BMC Genom. 2018, 19, 457. [Google Scholar] [CrossRef]
- Daynes, C.N.; Field, D.J.; Saleeba, J.A.; Cole, M.A.; McGee, P.A. Development and Stabilisation of Soil Structure Via Interactions between Organic Matter, Arbuscular Mycorrhizal Fungi and Plant Roots. Soil Biol. Biochem. 2013, 57, 683–694. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P.; Das, P.; Solanki, R.; Kapur, M.K. Proactive Role of Streptomyces Spp. in Plant Growth Stimulation and Management of Chemical Pesticides and Fertilizers. Int. J. Environ. Sci. Technol. 2022, 19, 10457–10476. [Google Scholar] [CrossRef]
- Zhao, W.; Du, E.; Luo, R.; Chen, Y.; Sun, Z.; Gui, F. Arbuscular Mycorrhizal Fungus and Pseudomonas Bacteria Affect Tomato Response to Tuta Absoluta (Lepidoptera: Gelechiidae) Herbivory. BMC Plant Biol. 2024, 24, 1236. [Google Scholar] [CrossRef]
- Siasou, E.; Standing, D.; Killham, K.; Johnson, D. Mycorrhizal Fungi Increase Biocontrol Potential of Pseudomonas Fluorescens. Soil Biol. Biochem. 2009, 41, 1341–1343. [Google Scholar] [CrossRef]
- Yadav, A.; Saini, I.; Kaushik, P.; Ahmad Ansari, M.; Rashid Khan, M.; Haq, N. Effects of Arbuscular Mycorrhizal Fungi and P-Solubilizing Pseudomonas Fluorescence (Atcc-17400) on Morphological Traits and Mineral Content of Sesame. Saudi J. Biol. Sci. 2021, 28, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, S.; Pirzad, A. Arbuscular Mycorrhizal Fungi and Pseudomonas in Reduce Drought Stress Damage in Flax (Linum usitatissimum L.): A Field Study. Mycorrhiza 2017, 27, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Berta, G.; Massa, N.; Glick, B.R.; Lingua, G. Synergistic Interactions between the Acc Deaminase-Producing Bacterium Pseudomonas Putida Uw4 and the Am Fungus Gigaspora Rosea Positively Affect Cucumber Plant Growth: Synergistic Interactions between Pseudomonas putida Uw4 and Gigaspora rosea. FEMS Microbiol. Ecol. 2008, 64, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, L.; Wang, J.; Qin, L.; Sun, X.; Liu, J.; Han, Y.; Chen, S. Chemotaxis of Rhizosphere Pseudomonas Sp. Induced by Foliar Spraying of Lanthanum Reduces Cadmium Uptake by Pakchoi. J. Hazard. Mater. 2025, 482, 136625. [Google Scholar] [CrossRef] [PubMed]
- Neeraj; Singh, K. Organic Amendments to Soil Inoculated Arbuscular Mycorrhizal Fungi and Pseudomonas Fluorescens Treatments Reduce the Development of Root-Rot Disease and Enhance the Yield of Phaseolus vulgaris L. Eur. J. Soil Biol. 2011, 47, 288–295. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Odibo, F.J.C. Effects of Carbon and Nitrogen Sources on Rhamnolipid Biosurfactant Production by Pseudomonas nitroreducens Isolated from Soil. World J. Microbiol. Biotechnol. 2011, 28, 937–942. [Google Scholar] [CrossRef]
- Lalucat, J.; Gomila, M.; Mulet, M.; Zaruma, A.; García-Valdés, E. Past, Present and Future of the Boundaries of the Pseudomonas Genus: Proposal of Stutzerimonas Gen. Nov. Syst. Appl. Microbiol. 2022, 45, 126289. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, M.; Liu, C.; Wang, E.; Bai, Y.; Yuan, M.M.; Shi, S.; Zhou, J.; Ding, J.; Xie, Y.; et al. Quinolone-Mediated Metabolic Cross-Feeding Develops Aluminium Tolerance in Soil Microbial Consortia. Nat. Commun. 2024, 15, 10148. [Google Scholar] [CrossRef]
- Dahal, S.; Alvarez, S.; Balboa, S.J.; Hicks, L.M.; Rojas, C.M. Defining the Secondary Metabolites in the Pseudomonas protegens Pbl3 Secretome with Antagonistic Activity against Burkholderia glumae. Phytopathology 2024, 114, 2481–2490. [Google Scholar] [CrossRef]
- Topalovic, O.; Kudjordjie, E.N.; Sheikh, S.; Kenou, G.B.; Bak, F.; Ekelund, F.; Vestergard, M. Meloidogyne incognita Parasitism Is Affected by Pseudomonas protegens Cha0 and Its Effects on Tomato-Associated Microbiota. Environ. Microbiome 2025, 20, 79. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Q.; Chen, F.; Yan, X.; Liao, H. Effects of Co-Inoculation with Arbuscular Mycorrhizal Fungi and Rhizobia on Soybean Growth as Related to Root Architecture and Availability of N and P. Mycorrhiza 2011, 21, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Jiang, H.; Tian, J.; Zhao, J.; Liao, H. Rhizobia Enhance Acquisition of Phosphorus from Different Sources by Soybean Plants. Plant Soil 2011, 349, 25–36. [Google Scholar] [CrossRef]
- Wang, X.; Fang, L.; Beiyuan, J.; Cui, Y.; Peng, Q.; Zhu, S.; Wang, M.; Zhang, X. Improvement of Alfalfa Resistance against Cd Stress through Rhizobia and Arbuscular Mycorrhiza Fungi Co-Inoculation in Cd-Contaminated Soil. Environ. Pollut. 2021, 277, 116758. [Google Scholar] [CrossRef]
- Omirou, M.; Fasoula, D.A.; Ioannides, I.M. Bradyrhizobium Inoculation Alters Indigenous Amf Community Assemblages and Interacts Positively with Amf Inoculum to Improve Cowpea Performance. Appl. Soil Ecol. 2016, 108, 381–389. [Google Scholar] [CrossRef]
- Antunes, P.M.; Deaville, D.; Goss, M.J. Effect of Two Amf Life Strategies on the Tripartite Symbiosis with Bradyrhizobium japonicum and Soybean. Mycorrhiza 2006, 16, 167–173. [Google Scholar] [CrossRef]
- Zhu, R.F.; Tang, F.L.; Liu, J.L.; Liu, F.Q.; Deng, X.Y.; Chen, J.S. Co-Inoculation of Arbusculr Mycorrhizae and Nitrogen Fixing Bacteria Enhance Alfalfa Yield under Saline Conditions. Pak. J. Bot. 2016, 48, 763–769. [Google Scholar]
- Gao, X.; Lu, X.; Wu, M.; Zhang, H.; Pan, R.; Tian, J.; Li, S.; Liao, H. Co-Inoculation with Rhizobia and Amf Inhibited Soybean Red Crown Rot: From Field Study to Plant Defense-Related Gene Expression Analysis. PLoS ONE 2012, 7, e33977. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, A.; Wang, F.; Han, X.; Wang, D.; Li, S. Arbuscular Mycorrhizal Fungi and Rhizobium Facilitate Nitrogen Uptake and Transfer in Soybean/Maize Intercropping System. Front. Plant Sci. 2015, 6, 339. [Google Scholar] [CrossRef]
- Cheeke, T.E.; Darby, H.; Rosenstiel, T.N.; Bever, J.D.; Cruzan, M.B. Effect of Bacillus Thuringiensis (Bt) Maize Cultivation History on Arbuscular Mycorrhizal Fungal Colonization, Spore Abundance and Diversity, and Plant Growth. Agric. Ecosyst. Environ. 2014, 195, 29–35. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Hnini, M.; Rabeh, K.; Oubohssaine, M. Interactions between Beneficial Soil Microorganisms (Pgpr and Amf) and Host Plants for Environmental Restoration: A Systematic Review. Plant Stress 2024, 11, 100391. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Guesmi, A.; Hamadi, N.B.; Khezami, L.; Soltani, T.; Attia, F.; Chehab, H. Enhancing Olive Tree (Olea europaea) Rhizosphere Dynamics: Co-Inoculation Effects of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria in Field Experiments. Appl. Soil Ecol. 2024, 202, 105596. [Google Scholar] [CrossRef]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The Interaction between Arbuscular Mycorrhizal Fungi and Endophytic Bacteria Enhances Plant Growth of Acacia Gerrardii under Salt Stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Almutairi, K.F.; Alshaikh, N.A.; Kumar, A.; Wu, Q.-S.; Ibramimova, U.; Abd-Allah, E.F. 8—Role of Arbuscular Mycorrhizal Fungi in Plant Growth Promotion and Biotic Stress Management. In Management of Mycorrhizal Symbiosis for Mycoremediation and Phytostabilization; Wu, Q.-S., Ed.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 145–155. [Google Scholar]
- Eng, A.; Borenstein, E. Microbial Community Design: Methods, Applications, and Opportunities. Curr. Opin. Biotechnol. 2019, 58, 117–128. [Google Scholar] [CrossRef]
- Wang, X.Y.; Teng, Y.; Wang, X.; Xu, Y.; Li, R.; Sun, Y.; Dai, S.; Hu, W.; Wang, H.; Li, Y.; et al. Nitrogen Transfer and Cross-Feeding between Azotobacter chroococcum and Paracoccus aminovorans Promotes Pyrene Degradation. ISME J. 2023, 17, 2169–2181. [Google Scholar] [CrossRef]
- Gilbert, E.S.; Walker, A.W.; Keasling, J.D. A Constructed Microbial Consortium for Biodegradation of the Organophosphorus Insecticide Parathion. Appl. Microbiol. Biotechnol. 2003, 61, 77–81. [Google Scholar] [CrossRef]
- He, T.; Xu, Z.-M.; Wang, J.-F.; Zhang, K.; Wang, F.-P.; Li, W.-L.; Tian, P.; Li, Q.-S. Inoculation of Escherichia Coli Enriched the Key Functional Bacteria That Intensified Cadmium Accumulation by Halophyte Suaeda Salsa in Saline Soils. J. Hazard. Mater. 2023, 458, 131922. [Google Scholar] [CrossRef]
- Jagmann, N.; Philipp, B. Design of Synthetic Microbial Communities for Biotechnological Production Processes. J. Biotechnol. 2014, 184, 209–218. [Google Scholar] [CrossRef]
- Li, C.; Han, Y.; Zou, X.; Zhang, X.; Ran, Q.; Dong, C. A Systematic Discussion and Comparison of the Construction Methods of Synthetic Microbial Community. Synth. Syst. Biotechnol. 2024, 9, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Mataigne, V.; Vannier, N.; Vandenkoornhuyse, P.; Hacquard, S. Multi-Genome Metabolic Modeling Predicts Functional Inter-Dependencies in the Arabidopsis Root Microbiome. Microbiome 2022, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Raj, S.; Sreenikethanam, A.; Maddheshiya, R.; Kumari, S.; Han, S.; Kapoor, K.K.; Bhaskar, R.; Bajhaiya, A.K.; Gahlot, D.K. Multi-Omics Approaches in Plant–Microbe Interactions Hold Enormous Promise for Sustainable Agriculture. Agronomy 2023, 13, 1804. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, M.; Wang, H.; Zhou, Z.; Yin, Y.; Wang, R.; Xing, B.; Yang, X.; Cai, Y.; Zhu, Z.-J. Charting Unknown Metabolic Reactions by Mass Spectrometry-Resolved Stable-Isotope Tracing Metabolomics. Nat. Commun. 2025, 16, 5059. [Google Scholar] [CrossRef]
- Dakal, T.C.; Xu, C.; Kumar, A. Advanced Computational Tools, Artificial Intelligence and Machine-Learning Approaches in Gut Microbiota and Biomarker Identification. Front. Med. Technol. 2024, 6, 1434799. [Google Scholar] [CrossRef]
- Karkaria, B.D.; Fedorec, A.J.H.; Barnes, C.P. Automated Design of Synthetic Microbial Communities. Nat. Commun. 2021, 12, 672. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.W.; Tao, Z.; Wang, T.; Zuo, W.; Zeng, Y.; Liu, Y.Y.; Dai, L. Data-Driven Prediction of Colonization Outcomes for Complex Microbial Communities. Nat. Commun. 2024, 15, 2406. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival. Cell 2018, 175, 973–983.e14. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Fu, R.; Luo, J.; Hou, X.; Gao, K.; Su, L.; Xu, Y.; Miao, Y.; Liu, Y.; Xu, Z.; et al. Listening to Plant’s Esperanto via Root Exudates: Reprogramming the Functional Expression of Plant Growth-Promoting Rhizobacteria. New Phytol. 2023, 239, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fu, R.; Feng, H.; Jiang, G.; Finkel, O.; Sun, T.; Liu, M.; Huang, B.; Li, S.; Wang, X.; et al. Rin Enhances Plant Disease Resistance via Root Exudate-Mediated Assembly of Disease-Suppressive Rhizosphere Microbiota. Mol. Plant 2023, 16, 1379–1395. [Google Scholar] [CrossRef] [PubMed]
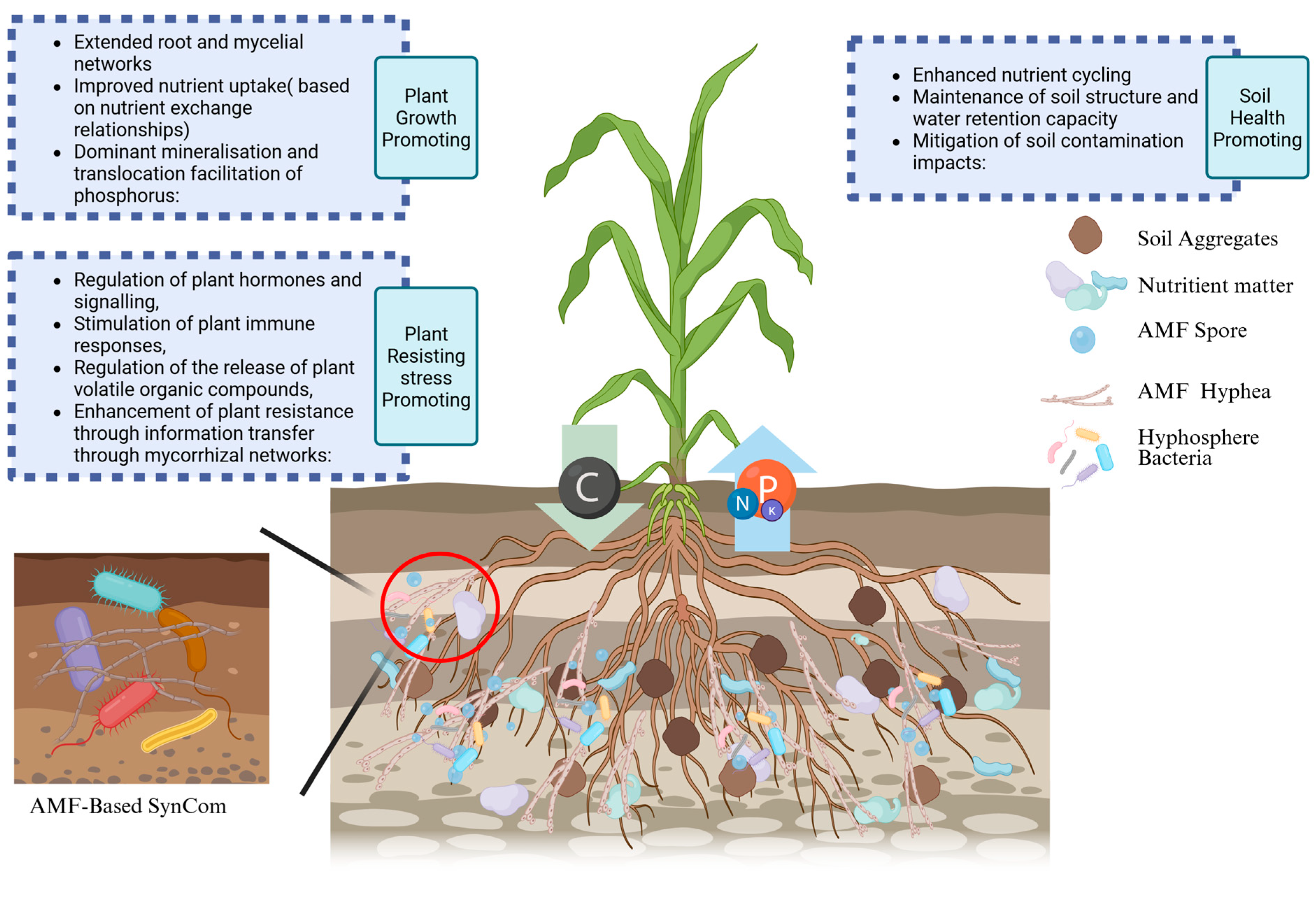
| Type of SynCom | Design and Construction Methods | Inoculation | Mechanisms and Effects |
|---|---|---|---|
| Suppressing maize seedling blight SynCom [35] | 7 species selected from maize rhizosphere via selective media + 16S rRNA sequencing; assembled based on antagonism to Fusarium | Root drench at 107 CFU/mL in sterile MS medium |
|
| Suppressing tomato wilt SynCom [8] | 93 bacteria + 74 fungi screened from tomato rhizosphere using metagenomics, NetShift + random forest modeling; cross-validated by 16S/ITS sequencing | Soil inoculation at 107 CFU/g soil in ½ MS medium |
|
| Mycorrhizal fungal-bacterial SynCom [61] | 5 genera (Pseudomonas, Flavisolibacter, etc.) isolated from maize rhizosphere; co-inoculated with AMF | Soil inoculation at 108 CFU/mL + AMF spores. |
|
| Salt stress-tolerant SynCom [31] | 15 strains from Indigofera argentea rhizosphere; selected for halotolerance via NaCl gradient plating | Seed coating in equimolar mixture |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Wang, Y.; Wang, X.; Jing, X.; Shu, X.; Ren, P.; Liu, W.; Ye, Q.; Fu, W.; Hao, Z.; et al. Arbuscular Mycorrhizal Fungi as Core Engineers in Synthetic Microbial Communities: Boosting Plant Growth and Soil Health for Sustainable Agriculture. J. Fungi 2025, 11, 769. https://doi.org/10.3390/jof11110769
Zeng Y, Wang Y, Wang X, Jing X, Shu X, Ren P, Liu W, Ye Q, Fu W, Hao Z, et al. Arbuscular Mycorrhizal Fungi as Core Engineers in Synthetic Microbial Communities: Boosting Plant Growth and Soil Health for Sustainable Agriculture. Journal of Fungi. 2025; 11(11):769. https://doi.org/10.3390/jof11110769
Chicago/Turabian StyleZeng, Yinan, Yan Wang, Xueli Wang, Xuemin Jing, Xiangyang Shu, Ping Ren, Weijia Liu, Qinxin Ye, Wei Fu, Zhipeng Hao, and et al. 2025. "Arbuscular Mycorrhizal Fungi as Core Engineers in Synthetic Microbial Communities: Boosting Plant Growth and Soil Health for Sustainable Agriculture" Journal of Fungi 11, no. 11: 769. https://doi.org/10.3390/jof11110769
APA StyleZeng, Y., Wang, Y., Wang, X., Jing, X., Shu, X., Ren, P., Liu, W., Ye, Q., Fu, W., Hao, Z., Zhang, X., Chen, B., & Wang, X. (2025). Arbuscular Mycorrhizal Fungi as Core Engineers in Synthetic Microbial Communities: Boosting Plant Growth and Soil Health for Sustainable Agriculture. Journal of Fungi, 11(11), 769. https://doi.org/10.3390/jof11110769