Neurological Sequelae After Paediatric Cryptococcal Meningitis
Abstract
1. Introduction
2. Methods
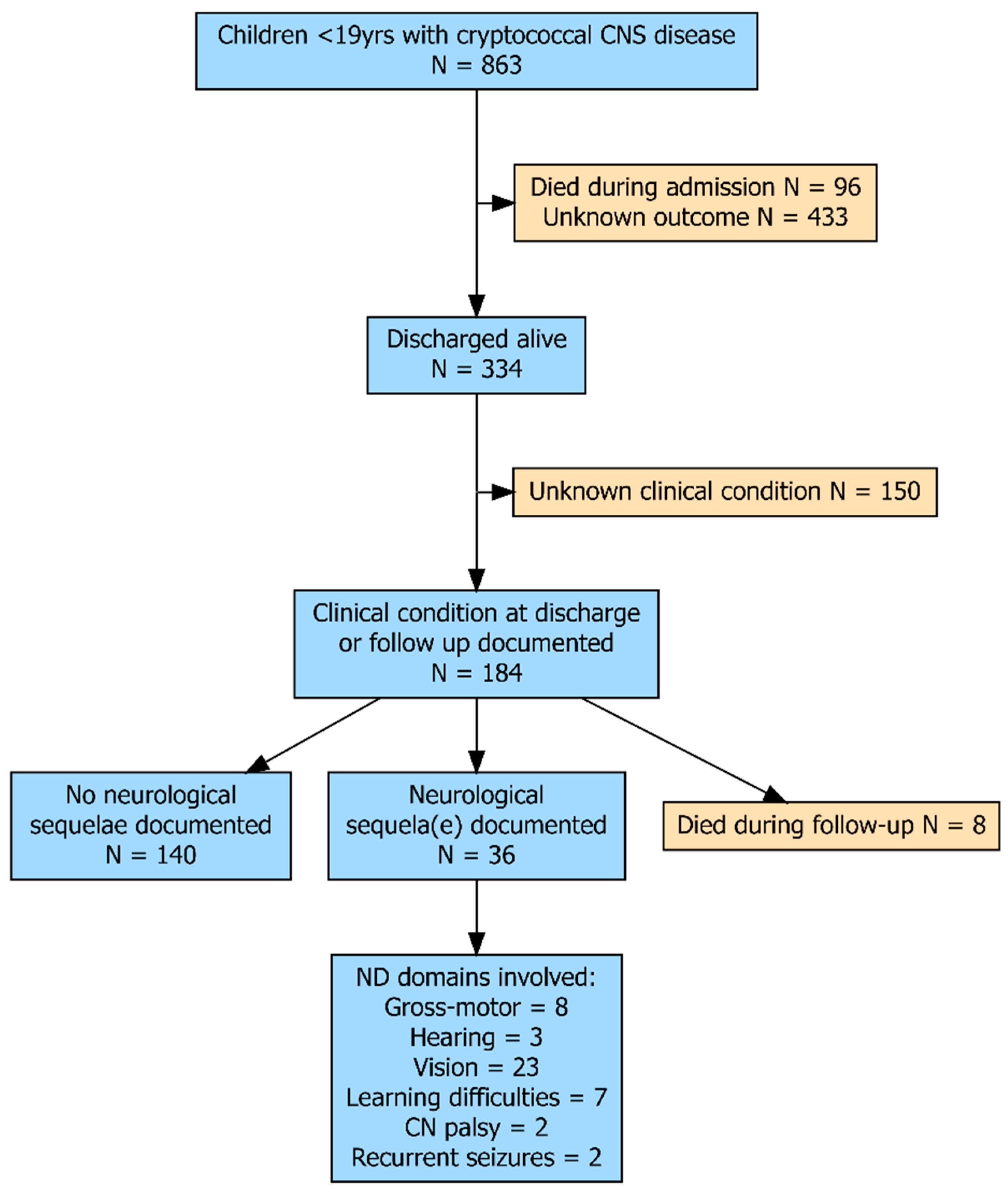
3. Results
3.1. Demographics
3.2. Mortality
3.3. Follow-Up After Discharge
3.4. Neurological or Neurodevelopmental Assessment
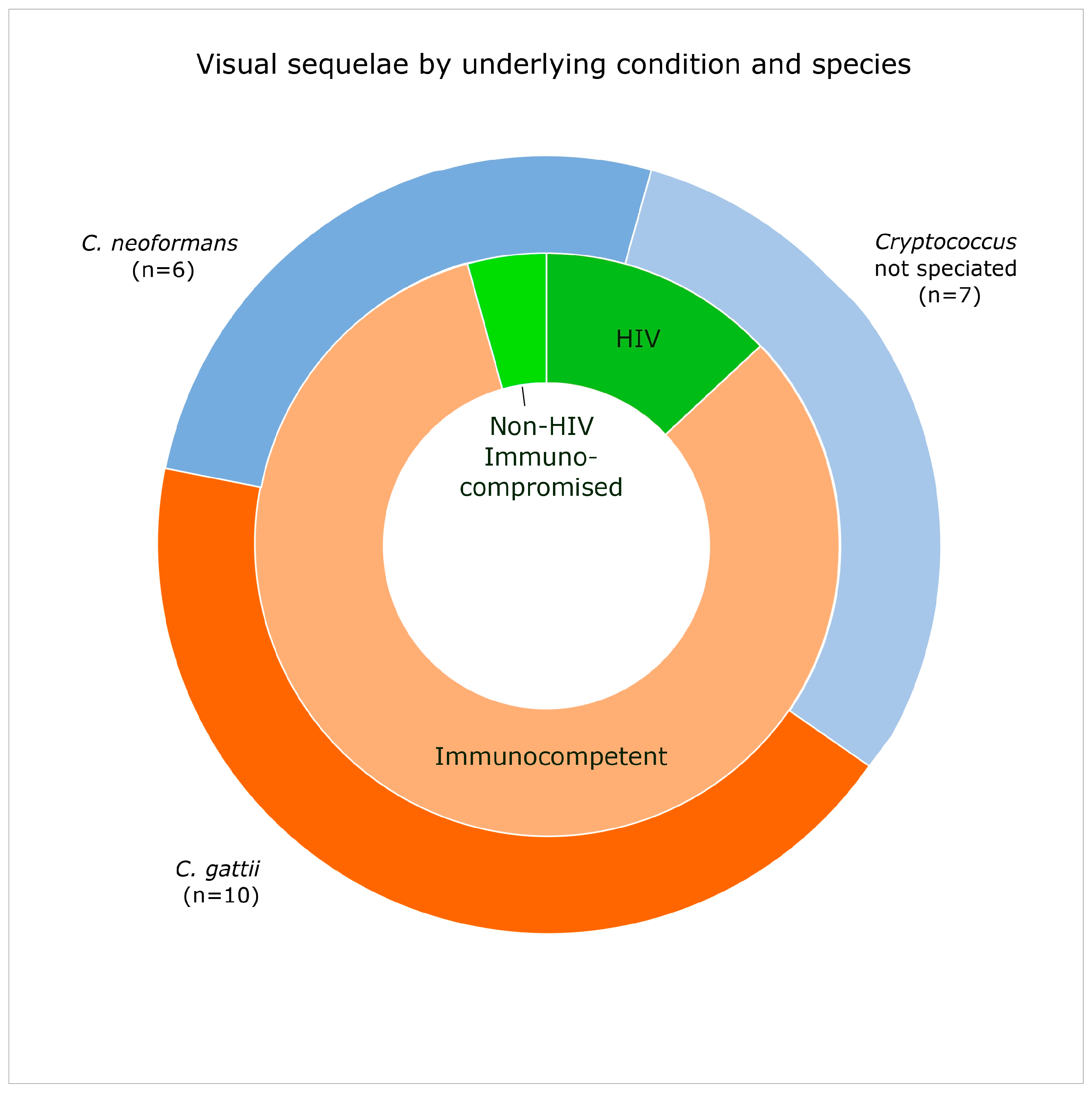
3.5. Vision
3.6. Motor Weakness
3.7. Learning Difficulties
3.8. Other Neurological Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Erice, C.; Rohlwink, U.K.; Tucker, E.W. Infections in the Developing Brain: The Role of the Neuro-Immune Axis. Front. Neurol. 2022, 13, 805786. [Google Scholar] [CrossRef]
- Chan, M.; Lye, D.; Win, M.K.; Chow, A.; Barkham, T. Clinical and microbiological characteristics of cryptococcosis in Singapore: Predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int. J. Infect. Dis. 2014, 26, 110–115. [Google Scholar] [CrossRef]
- Phillips, P.; Galanis, E.; MacDougall, L.; Chong, M.Y.; Balshaw, R.; Cook, V.J.; Bowie, W.; Steiner, T.; Hoang, L.; Morshed, M.; et al. Longitudinal clinical findings and outcome among patients with Cryptococcus gattii infection in British Columbia. Clin. Infect. Dis. 2015, 60, 1368–1376. [Google Scholar] [CrossRef]
- Chen, S.C.; Slavin, M.A.; Heath, C.H.; Playford, E.G.; Byth, K.; Marriott, D.; Kidd, S.E.; Bak, N.; Currie, B.; Hajkowicz, K.; et al. Clinical manifestations of Cryptococcus gattii infection: Determinants of neurological sequelae and death. Clin. Infect. Dis. 2012, 55, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.D.; Rolfes, M.A.; Birkenkamp, K.E.; Nakasujja, N.; Rajasingham, R.; Meya, D.B.; Boulware, D.R. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: A prospective cohort study. Metab. Brain Dis. 2014, 29, 269–279. [Google Scholar] [CrossRef]
- Gifford, A.; Jayawardena, N.; Carlesse, F.; Lizarazo, J.M.; McMullan, B.B.; Groll, A.H.; Warris, A. Pediatric Cryptococcosis. Pediatr. Infect. Dis. J. 2024, 43, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zainel, A.; Mitchell, H.; Sadarangani, M. Bacterial Meningitis in Children: Neurological Complications, Associated Risk Factors, and Prevention. Microorganisms 2021, 9, 535. [Google Scholar] [CrossRef]
- Chiang, S.S.; Khan, F.A.; Milstein, M.B.; Tolman, A.W.; Benedetti, A.; Starke, J.R.; Becerra, M.C. Treatment outcomes of childhood tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 947–957. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Meningitis (Bacterial) and Meningococcal Disease: Recognition, Diagnosis and Management (NG240). 2024. Available online: https://www.nice.org.uk/guidance/ng240/resources/meningitis-bacterial-and-meningococcal-disease-recognition-diagnosis-and-management-pdf-66143949881029 (accessed on 10 February 2025).
- National Institute for Commutable Disease. GERMS-SA: Annual Surveillance Review 2022. Available online: www.nicd.ac.za/wp-content/uploads/2024/02/NICD-GERMS-Annual-Report-2022.pdf (accessed on 10 February 2025).
- South African National Department of Health. Paediatric STGs and EML 2023 Edition Updated October 2024. 2024. Available online: https://www.health.gov.za/nhi-edp-stgs-eml/ (accessed on 10 February 2025).
- Boyles, T.H.; Bamford, C.; Bateman, K.; Blumberg, L.; Dramowski, A.; Karstaedt, A.; Korsman, S.; le Roux, D.; Maartens, G.; Madhi, S.; et al. Guidelines for the management of acute meningitis in children and adults in South Africa. S. Afr. J. Epidemiol. Infect. 2013, 28, 5–15. [Google Scholar] [CrossRef]
- Western Cape Government. Draft Protocol: Meningitis in Children: Hospital Level. 2019. Available online: https://d7.westerncape.gov.za/assets/departments/health/FP/meningitis_management_protocol_-_paediatrics_and_family_medicine_final_november_2017.pdf (accessed on 10 February 2025).
- Ahmadian, D.; Young, K.; Gallego, C.; Miller, M. Cochlear Implantation in Post-Meningitis Deafness: Audiological, Imaging, and Postoperative Outcomes: A Systematic Review With Qualitative Synthesis. Otol. Neurotol. 2024, 45, 840–848. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 10 February 2025).
- Lizarazo, J.; Escandón, P.; Agudelo, C.I.; Castañeda, E. Cryptococcosis in Colombian children and literature review. Mem. Inst. Oswaldo Cruz 2014, 109, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.-T.; Xu, Y.-C.; Wang, H.-Z.; Li, T.-S. Molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii in China between 2007 and 2013 using multilocus sequence typing and the DiversiLab system. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.L.; Coutinho, J.V.S.C.; Ferreira Filho, L.A.; Marques, S.M.; Borges, M.A.S.B. Cranial base pachymeningitis in children: Beyond tuberculosis. Pediatr. Infect. Dis. J. 2022, 41, e175–e177. [Google Scholar] [CrossRef]
- Padmanabha, H.; Kasinathan, A.; Kumar, A.; Zaman, K.; Suthar, R.; Suri, D.; Vyas, S.; Sankhyan, N. Vision Loss in an 8-Year-Old Immunocompetent Boy with Cryptococcal Meningitis. Pediatr. Infect. Dis. J. 2018, 37, e230–e232. [Google Scholar] [CrossRef]
- Kocabas, B.; Emin Parlak, M.; Özhak Baysan, B.; Karaali, K.; Bingöl, A.; Haspolat, S. Disseminated Cryptococcosis With Severe Increased Intracranial Pressure Complicated With Cranial Nerve Palsy in a Child. Pediatr. Infect. Dis. J. 2018, 37, 373–375. [Google Scholar] [CrossRef]
- Chimowa, T.; King, I.; Tam, P.; Gonzalez-Martinez, C. Cryptococcal meningitis in a previously healthy child. Malawi Med. J. 2017, 29, 330–331. [Google Scholar] [CrossRef]
- Pinto Junior, V.L.; Pone, M.V.d.S.; Pone, S.M.; Campos, J.M.S.; Garrido, J.R.P.; Barros, A.C.M.W.d.; Trilles, L.; Barbosa, G.G.; Morales, B.P.; Bezerra, C.d.C.F.; et al. Cryptococcus gattii molecular type VGII as agent of meningitis in a healthy child in Rio de Janeiro, Brazil: Report of an autochthonous case. Rev. Soc. Bras. Med. Trop. 2010, 43, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Sirinavin, S.; Intusoma, U.; Tuntirungsee, S. Mother-to-child transmission of Cryptococcus neoformans. Pediatr. Infect. Dis. J. 2004, 23, 278–279. [Google Scholar] [CrossRef]
- Schoeman, J.F.; Honey, E.M.; Loock, D.B. Raised ICP in a child with cryptococcal meningitis: CT evidence of a distal CSF block. Childs Nerv. Syst. 1996, 12, 568–571. [Google Scholar] [CrossRef]
- Woodall, W.C., III; Bertorini, T.E.; Bakhtian, B.J.; Gelfand, M.S. Spinal arachnoiditis with Cryptococcus neoformans in a nonimmunocompromised child. Pediatr. Neurol. 1990, 6, 206–208. [Google Scholar] [CrossRef]
- Kaur, H.; Gupta, P.; Pilania, R.; Suri, D.; Singh, S.; Ghosh, A.; Rudramurthy, S.M. Trend of pediatric cryptococcosis in a tertiary care centre and review of literature. Indian. J. Med. Microbiol. 2023, 43, 18–29. [Google Scholar] [CrossRef]
- Enicker, B.; Aldous, C. Cerebrospinal Fluid Shunting in Children with Hydrocephalus and Increased Intracranial Pressure Secondary to Human Immunodeficiency Virus-Related Cryptococcal Meningitis. World Neurosurg. 2022, 168, e530–e537. [Google Scholar] [CrossRef]
- Bouille, J.G.d.; Epelboin, L.; Henaff, F.; Migaud, M.; Abboud, P.; Blanchet, D.; Aznar, C.; Djossou, F.; Lortholary, O.; Elenga, N.; et al. Invasive cryptococcosis in French Guiana: Immune and genetic investigation in six non-HIV patients. Front. Immunol. 2022, 13, 881352. [Google Scholar] [CrossRef] [PubMed]
- Nguefack, S.; Taguebue, J.; Wandji, Y.; Kago, D.; Bate, B.; Chelo, D.; Ndombo, P.O.K. Neuromeningeal cryptococcosis in children: Clinical and prognostic aspects in a pediatric hospital in Yaounde–Cameroon. Pediatr. Oncall. 2020, 17, 77–81. [Google Scholar] [CrossRef]
- Grimshaw, A.; Palasanthiran, P.; Huynh, J.; Marais, B.; Chen, S.; McMullan, B. Cryptococcal infections in children: Retrospective study and review from Australia. Future Microbiol. 2019, 14, 1531–1544. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Ford, T.J.; Currie, B.J.; Francis, J.R. Cryptococcus gattii infection complicated by immune reconstitution inflammatory syndrome in three apparently immunocompetent children. J. Paediatr. Child. Health. 2019, 55, 943–947. [Google Scholar] [CrossRef]
- Gao, L.; Jiao, A.; Wu, X.; Zhao, S.; Ma, Y.; Liu, G.; Yin, J.; Xu, B.; Shen, K. Clinical characteristics of disseminated cryptococcosis in previously healthy children in China. BMC Infect. Dis. 2017, 17, 359. [Google Scholar] [CrossRef]
- Luo, F.; Tao, Y.; Wang, Y.; Li, H. Clinical study of 23 pediatric patients with cryptococcosis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3801–3810. [Google Scholar]
- Hassan, H.; Cotton, M.F.; Rabie, H. Complicated and protracted cryptococcal disease in HIV-infected children. Pediatr. Infect. Dis. J. 2015, 34, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, J.; Zhang, S.; Zhang, X.; Li, J.; Sun, Y.; Qi, S. A case-control study of risk factors for HIV-negative children with cryptococcal meningitis in Shi Jiazhuang, China. BMC Infect. Dis. 2012, 12, 376. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Xu, N.; Wang, X.; Wen, H.; Laio, W.; Gu, J. Cryptococcal meningitis in immunocompetent children. Mycoses 2012, 55, 168–171. [Google Scholar]
- Huang, K.; Huang, Y.; Hung, I.; Lin, T. Cryptococcosis in nonhuman immunodeficiency virus-infected children. Pediatr. Neurol. 2010, 42, 267–270. [Google Scholar] [CrossRef]
- Correa, M.d.P.S.C.; Severo, L.C.; Oliveira, F.d.M.; Irion, K.; Londero, A.T. The spectrum of computerized tomography (CT) findings in central nervous system (CNS) infection due to Cryptococcus neoformans var. gattii in immunocompetent children. Rev. Inst. Med. Trop. Sao Paulo 2002, 44, 283–287. [Google Scholar] [CrossRef]
- Laurenson, I.; Trevett, A.; Lalloo, D.; Nwokolo, N.; Naraqi, S.; Black, J.; Tefurani, N.; Saweri, A.; Mavo, B.; Igo, J. Meningitis caused by Cryptococcus neoformans var. gattii and var. neoformans in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 57–60. [Google Scholar] [CrossRef]
- Bateson, E.M. Computed tomography of intracranial torulosis in the Australian aboriginal. Australas. Radiol. 1986, 30, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Moodley, A.; Rae, W.; Bhigjee, A.; Connolly, C.; Devparsad, N.; Michowicz, A.; Harrison, T.; Loyse, A. Early clinical and subclinical visual evoked potential and Humphrey’s visual field defects in cryptococcal meningitis. PLoS ONE 2012, 7, e52895. [Google Scholar] [CrossRef] [PubMed]
- Seaton, R.A.; Verma, N.; Naraqi, S.; Wembri, J.P.; Warrell, D.A. Visual loss in immunocompetent patients with Cryptococcus neoformans var. gattii meningitis. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [CrossRef]
- Molloy, S.F.; Ross, B.; Kanyama, C.; Mfinanga, S.; Lesikari, S.; Heyderman, R.S.; Kalata, N.; Ellis, J.; Kouanfack, C.; Chanda, D.; et al. Fungal Burden and Raised Intracranial Pressure Are Independently Associated With Visual Loss in Human Immunodeficiency Virus-Associated Cryptococcal Meningitis. Open Forum Infect. Dis. 2021, 8, ofab066. [Google Scholar] [CrossRef]
- Chang, C.C.; Harrison, T.S.; Bicanic, T.A.; Chayakulkeeree, M.; Sorrell, T.C.; Warris, A.; Hagen, F.; Spec, A.; Oladele, R.; Govender, N.P.; et al. Global guideline for the diagnosis and management of cryptococcosis: An initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect. Dis. 2024, 24, e495–e512. [Google Scholar] [CrossRef] [PubMed]
- Miglia, K.J.; Govender, N.P.; Rossouw, J.; Meiring, S.; Mitchell, T.G. Analyses of pediatric isolates of Cryptococcus neoformans from South Africa. J. Clin. Microbiol. 2011, 49, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Evidence Review for Long-Term Complications and Follow-Up for Bacterial Meningitis: Neonatal Infection: Antibiotics for Prevention and Treatment: Evidence Review O; (NICE Guideline, No. 195); National Institute for Health and Care Excellence (NICE): London, UK, March 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK604350/ (accessed on 10 February 2025).
| Total (N = 863) † | HIV (N = 555) | Non-HIV Immunocompromised (N = 67) | Immuno- Competent (N = 204) | p * | |
|---|---|---|---|---|---|
| Males | 61% (250/411) | 60% (102/170) | 48% (24/50) | 66% (109/166) | n.s |
| Age in years (IQR) | 9 (5–13) | 9.5 (8–10) | 12.5 (8–15) | 8 (3–12) | n.s |
| Continent: | |||||
| Africa | 57% (494/862) | 88% (489/554) | 0% | 2% (5/204) | |
| Asia | 22% (193/862) | 4% (21/554) | 34% (23/67) | 73% (148/204) | |
| Europe | 1% (7/862) | <1% (1/554) | 7% (5/67) | <1% (1/204) | |
| North America | 8% (68/862) | 6% (34/554) | 37% (25/67) | 4% (9/204) | |
| South America | 9% (80/862) | 2% (9/554) | 12% (8/67) | 13% (27/204) | |
| Oceania | 2% (20/862) | 0% | 9% (6/67) | 7% (14/204) | |
| Cryptococcus species: | <0.001 | ||||
| neoformans | 89% (464/522) | 95% (333/351) | 80% (41/51) | 76% (84/111) | |
| gattii | 10% (54/522) | 4% (15/351) | 18% (9/51) | 24% (27/111) | |
| other # | 1% (4/522) | 1% (3/351) | 2% (1/51) | 0% | |
| Inpatient mortality | 22% (96/430) | 28% (49/172) | 22% (14/65) | 17% (33/192) | 0.04 |
| General follow-up (% of survivors) | 50% (168/334) | 28% (34/123) | 57% (29/51) | 66% (105/159) | <0.001 |
| Duration follow-up months (IQR) | 12 (6–24) | 8 (4–61) | 18 (12–29) | 12 (6–12) | n.s |
| Death during follow-up | 2% (8/334) | 2% (3/123) | 10% (5/51) | 0% (0/159) | <0.001 |
| Any documented clinical condition at discharge or follow-up | 55% (184/334) | 36% (44/123) | 80% (41/51) | 62% (99/159) | <0.001 |
| Neurological sequelae | 20% (36/184) | 25% (11/44) | 5% (2/41) | 23% (23/99) | 0.01 |
| Domains involved: | |||||
| Gross-motor | 4% (8/184) | 5% (2/44) | 2% (1/41) | 5% (5/99) | n.s |
| Hearing | 2% (3/184) | 0 | 2% (1/41) | 2% (2/99) | n.s |
| Vision | 13% (23/184) | 7% (3/44) | 2% (1/41) | 19% (19/99) | 0.01 |
| Learning difficulties | 4% (7/184) | 11% (5/44) | 2% (1/41) | 1% (1/99) | 0.01 |
| CN palsy | 1% (2/184) | 2% (1/44) | 0 | 1% (1/99) | n.s |
| Recurrent seizures | 1% (2/184) | 0 | 0 | 2% (2/99) | n.s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gifford, A.; Patel, S.A.; Matlakala, M.; Dangarembizi, R.; Warris, A. Neurological Sequelae After Paediatric Cryptococcal Meningitis. J. Fungi 2025, 11, 767. https://doi.org/10.3390/jof11110767
Gifford A, Patel SA, Matlakala M, Dangarembizi R, Warris A. Neurological Sequelae After Paediatric Cryptococcal Meningitis. Journal of Fungi. 2025; 11(11):767. https://doi.org/10.3390/jof11110767
Chicago/Turabian StyleGifford, Alison, Simran Atulkumar Patel, Masilo Matlakala, Rachael Dangarembizi, and Adilia Warris. 2025. "Neurological Sequelae After Paediatric Cryptococcal Meningitis" Journal of Fungi 11, no. 11: 767. https://doi.org/10.3390/jof11110767
APA StyleGifford, A., Patel, S. A., Matlakala, M., Dangarembizi, R., & Warris, A. (2025). Neurological Sequelae After Paediatric Cryptococcal Meningitis. Journal of Fungi, 11(11), 767. https://doi.org/10.3390/jof11110767






