Comparative Symbiotic Effects of Mycorrhizal Fungal Strains from Different Hosts on Seed Germination and Seedling Growth in Dendrobium officinale
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Endophytic Fungi in Dendrobium officinale Cultivation
2.2. Phylogenetic Analysis
2.3. Symbiotic Seed Germination Experiments
2.4. Effects of Fungi on Seedling Growth Under Greenhouse
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Fungal Isolation and Identification
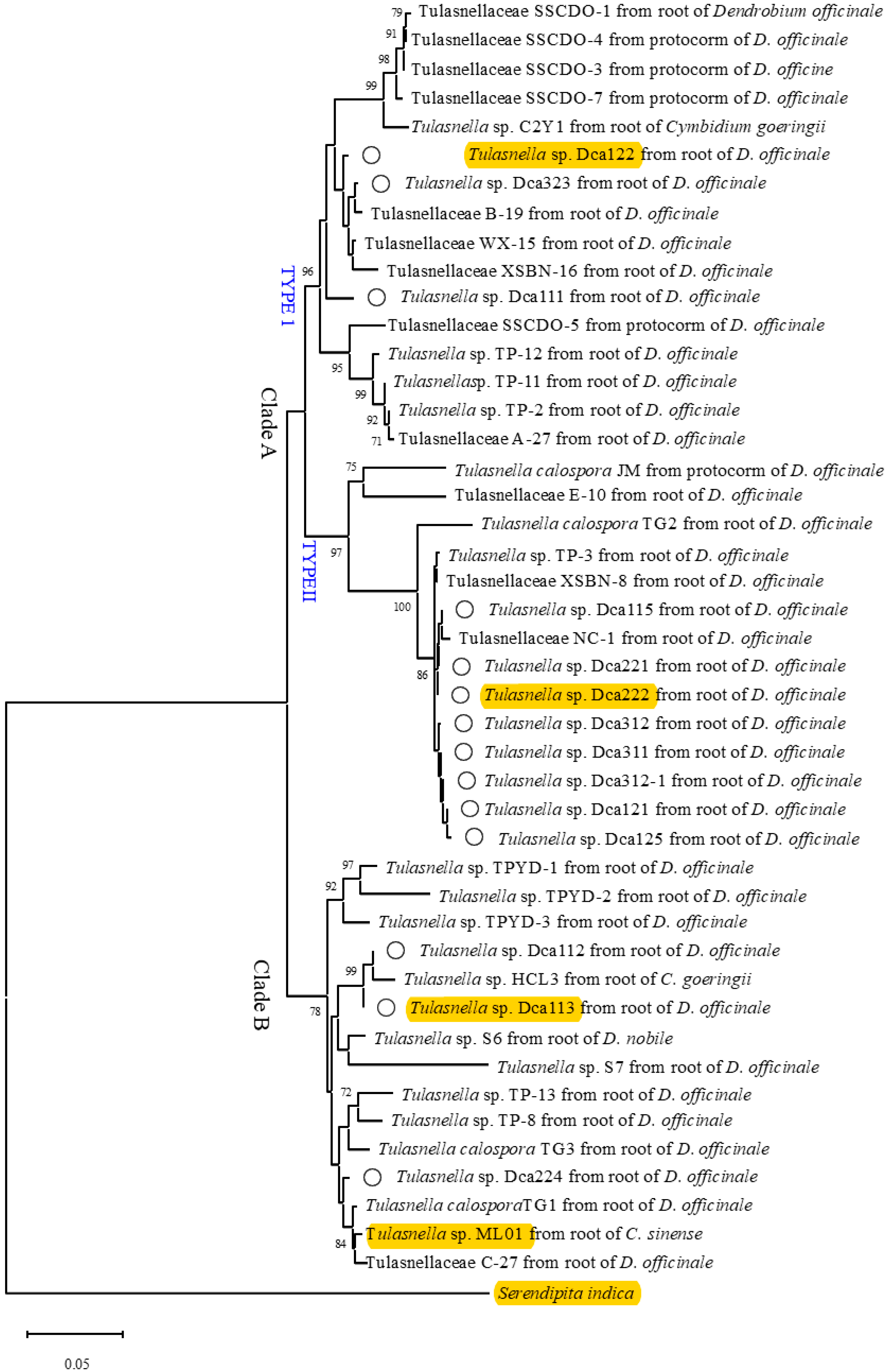
3.2. Phylogenetic Analysis
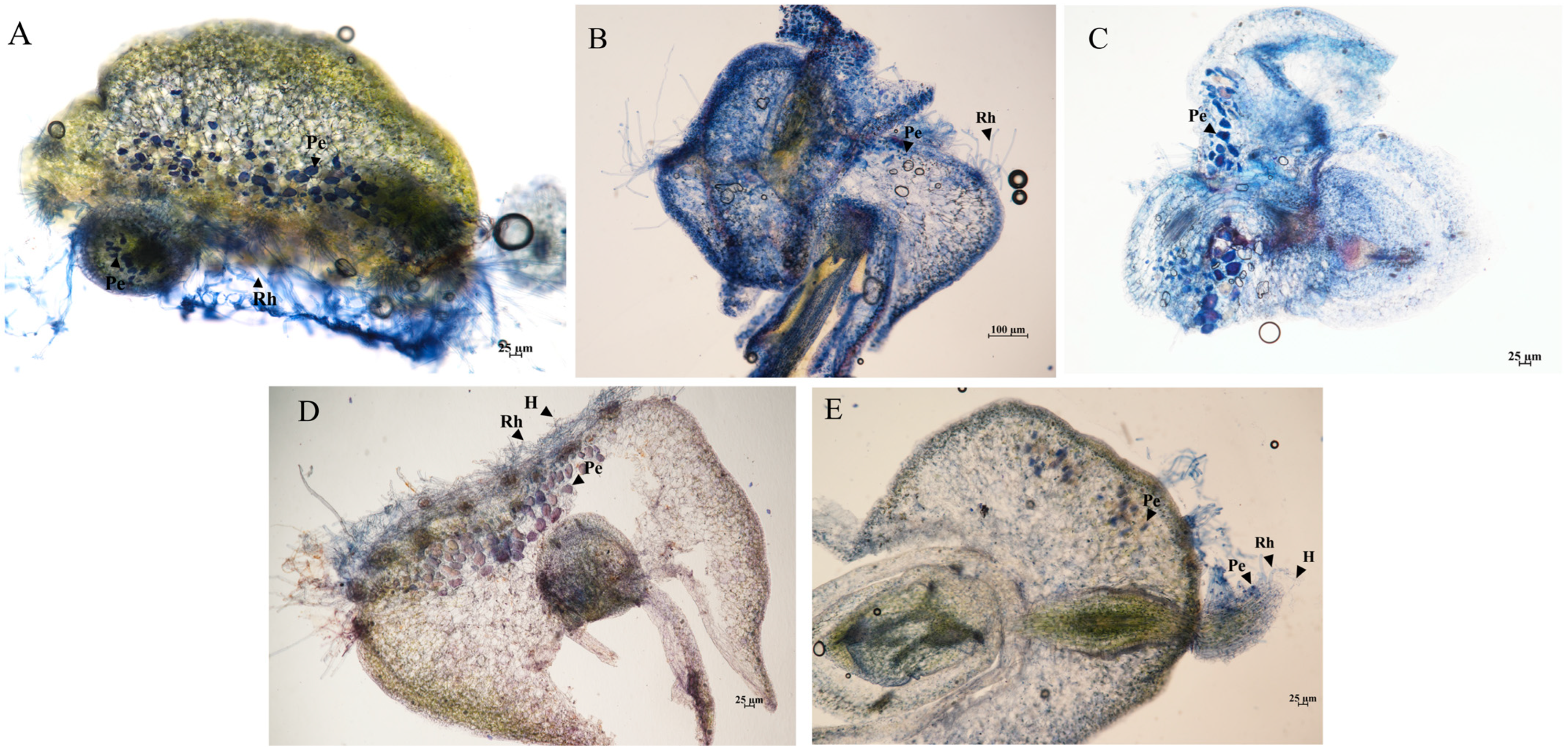
3.3. Effectiveness of Different Isolates on Seed Germination and Seedling Development in D. officinale
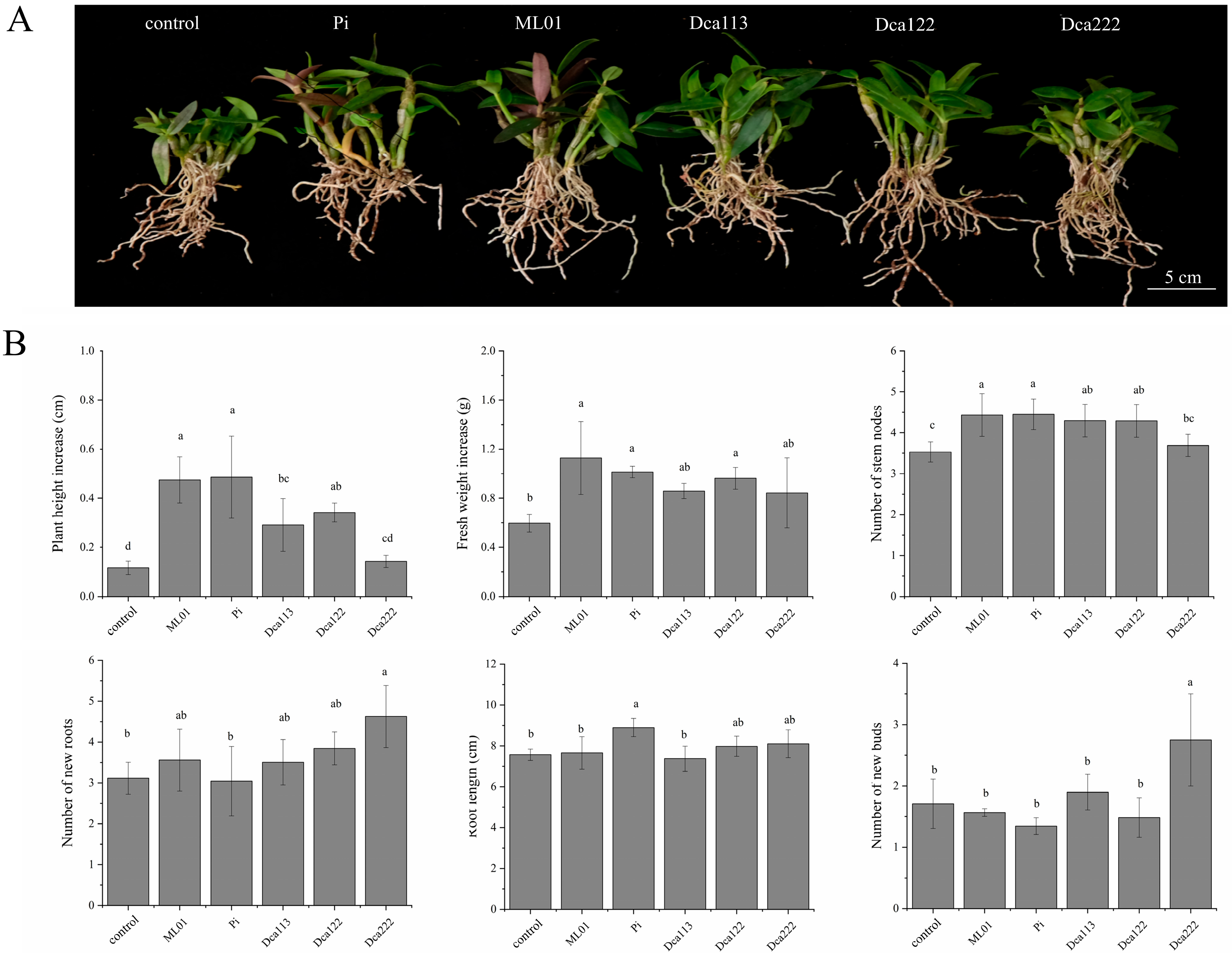
3.4. Comparative Symbiotic Effects of Different Fungal Isolates on D. officinale Seedling Growth in Pot
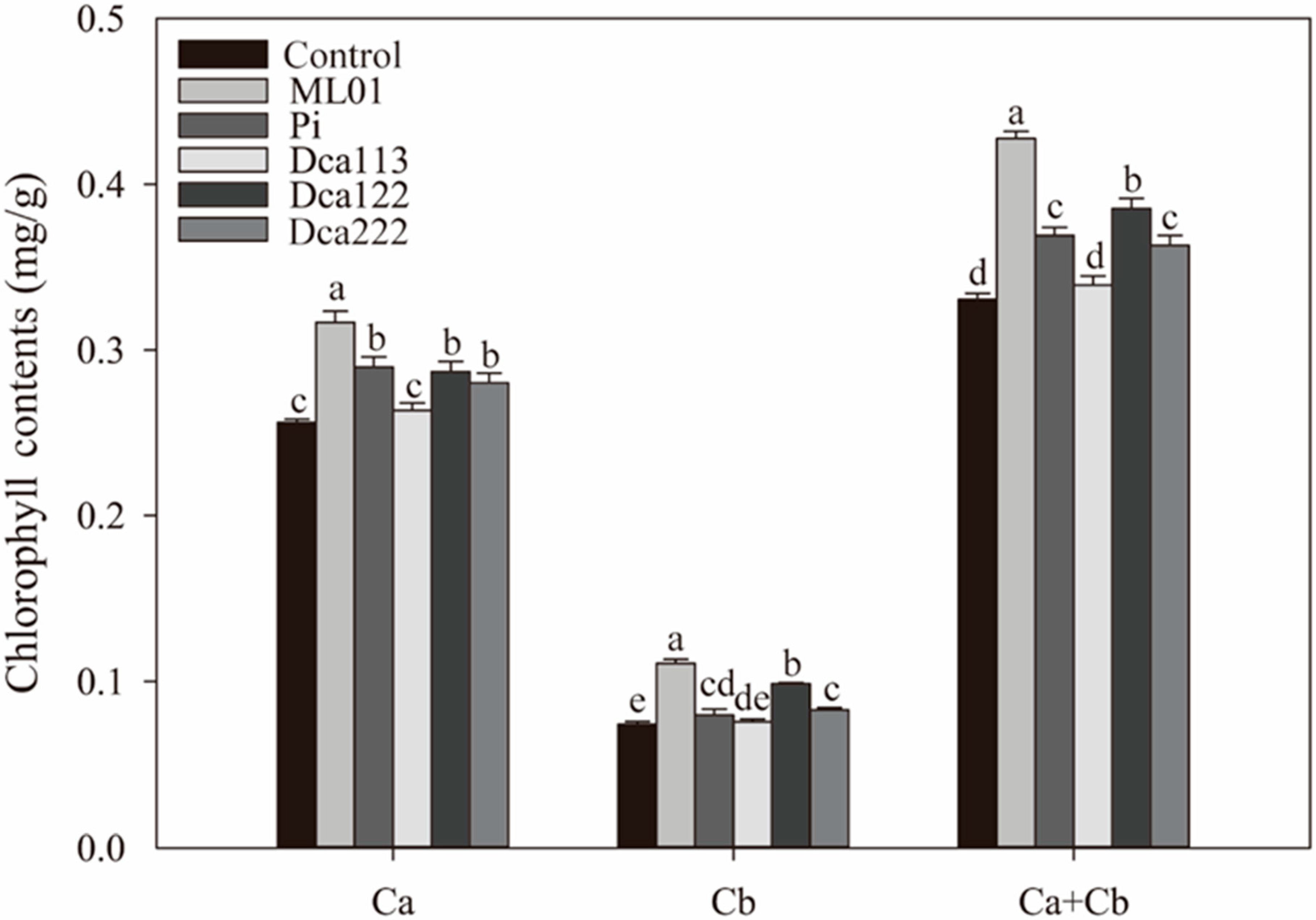
3.5. Strain-Specific Regulation of Chlorophyll Biosynthesis in D. officinale
4. Discussion
4.1. Cultivated Varieties Retain the Wild-Type Host Preference
4.2. Functional Differentiation Across Developmental Stages in Symbiotic Fungi
4.3. Mycorrhizal Fungi Shared Among Orchid Species That Are Distantly Related
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cameron, D.D.; Leake, J.R.; Read, D.J. Mutualistic Mycorrhiza in Orchids: Evidence from Plant-Fungus Carbon and Nitrogen Transfers in the Green-Leaved Terrestrial Orchid Goodyera repens. New Phytol. 2006, 171, 405–416. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Dixon, K.W.; Jersakova, J.; Tesitelova, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W.; Martos, F.; Selosse, M.A. 12 Orchid Mycorrhizas: Molecular Ecology, Physiology, Evolution and Conservation Aspects. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 207–230. [Google Scholar]
- Yang, J.W.; Chen, X.M.; Meng, Z.X.; Zhu, W.D.; Shan, T.T.; Huang, Q.; Zhou, L.S.; Guo, S.X. Effects of Tulasnella sp. S7 and its extract on Dendrobium officinale seed germination. Sci. Sin. Vitae 2020, 50, 559–570. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, D.Y.; Wang, X.J.; Li, N.Q.; Gao, J.Y. Using ex situ seedling baiting to capture seedling-associated mycorrhizal fungi in medicinal orchid Dendrobium officinale. J. Fungi 2022, 8, 1036. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, X.X.; Xing, X.K. Fungal diversity and mechanisms of symbiotic germination of orchid seeds: A review. Mycosystema 2019, 38, 1808–1825. [Google Scholar]
- Bahram, M.; Jacquemyn, H.; Kennedy, P.; Kohout, P.; Moora, M.; Oja, J.; Opik, M.; Pecoraro, L.; Tedersoo, L. Host preference and network properties in biotrophic plant-fungal associations. New Phytol. 2018, 217, 1230–1239. [Google Scholar]
- Zi, X.M.; Sheng, C.L.; Goodale, U.M.; Shao, S.C.; Gao, J.Y. In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 2014, 24, 487–499. [Google Scholar] [CrossRef]
- Meng, Y.; Shao, S.; Liu, S.; Gao, J.Y. Do the fungi associated with roots of adult plants support seed germination? A case study on Dendrobium exile (Orchidaceae). Glob. Ecol. Conserv. 2019, 17, e00582. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, X.; Liu, J.; Zhao, X.; Zhou, J.; Wang, X.; Wang, D.; Lai, C.; Xu, W.; Huang, J.; et al. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018, 9, 1615. [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Read, D.J. Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 2008, 17, 3707–3716. [Google Scholar] [CrossRef]
- Fuji, M.; Miura, C.; Yamamoto, T.; Komiyama, S.; Suetsugu, K.; Yagame, T.; Yamato, M.; Kaminaka, H. Relative effectiveness of Tulasnella fungal strains in orchid mycorrhizal symbioses between germination and subsequent seedling growth. Symbiosis 2020, 81, 53–63. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Fan, X.L.; Zhou, L.R.; Shao, S.C.; Gao, J.Y. Symbiotic fungi undergo a taxonomic and functional bottleneck during orchid seeds germination: A case study on Dendrobium moniliforme. Symbiosis 2019, 79, 205–212. [Google Scholar] [CrossRef]
- Deveautour, C.; Power, S.A.; Barnett, K.L.; Ochoa Hueso, R.; Donn, S.; Bennett, A.E.; Powell, J.R.; Mariotte, P. Temporal dynamics of mycorrhizal fungal communities and co-associations with grassland plant communities following experimental manipulation of rainfall. J. Ecol. 2020, 108, 515–527. [Google Scholar] [CrossRef]
- Freestone, M.; Reiter, N.; Swarts, N.D.; Linde, C.C. Temporal turnover of Ceratobasidiaceae orchid mycorrhizal fungal communities with ontogenetic and phenological development in Prasophyllum (Orchidaceae). Ann. Bot. 2024, 134, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Warcup, J.H. Symbiotic germination of some Australian terrestrial orchids. New Phytol. 1973, 72, 387–392. [Google Scholar] [CrossRef]
- Herrera, H.; Valadares, R.; Contreras, D.; Bashan, Y.; Arriagada, C. Mycorrhizal compatibility and symbiotic seed germination of orchids from the Coastal Range and Andes in south central Chile. Mycorrhiza 2017, 27, 175–188. [Google Scholar] [CrossRef]
- Yamamoto, T.; Miura, C.; Fuji, M.; Nagata, S.; Otani, Y.; Yagame, T.; Yamato, M.; Kaminaka, H. Quantitative evaluation of protocorm growth and fungal colonization in Bletilla striata (Orchidaceae) reveals less-productive symbiosis with a non-native symbiotic fungus. BMC Plant Biol. 2017, 17, 50. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Z.; Willian, S.; Gao, J. Characteristics of the orchid trade at public markets and implications for conservation in Xishuangbanna, Yunnan, China. Biodivers. Sci. 2017, 25, 531–539. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Ming, X.; Wang, G.; Gao, J. Isolating ecological-specific fungi and creating fungus-seed bags for epiphytic orchid conservation. Glob. Ecol. Conserv. 2021, 28, e01714. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, X.G.; Li, N.Q.; Li, T.Q.; Anbazhakan, R.; Gao, J.Y. Core Mycorrhizal Fungi Promote Seedling Growth in Dendrobium officinale: An Important Medicinal Orchid. Plants 2025, 14, 1024. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Dominant Dendrobium officinale mycorrhizal partners vary among habitats and strongly induce seed germination in vitro. Front. Ecol. Evol. 2022, 10, 994641. [Google Scholar] [CrossRef]
- Chen, X.M.; Tian, L.X.; Shan, T.T.; Sun, L.; Guo, S.X. Advances in germplasm resources and genetics and breeding of Dendrobium officinale. Acta Pharm. Sin. 2018, 53, 1493–1503. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D.; Sninsky, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Chen, X.G.; Wu, Y.H.; Li, N.Q.; Gao, J.Y. What role does the seed coat play during symbiotic seed germination in orchids: An experimental approach with Dendrobium officinale. BMC Plant Biol. 2022, 22, 375. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Li, T.; Li, N.; Gao, J. In situ seedling baiting to isolate plant growth-promoting fungi from Dendrobium officinale, an over-collected medicinal orchid in China. Glob. Ecol. Conserv. 2021, 28, e01659. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhou, D.Y.; Dai, J.; Li, Y.; Xing, Y.M.; Guo, S.X.; Chen, J. Potential Specificity Between Mycorrhizal Fungi Isolated from Widespread Dendrobium spp. and Rare D. huoshanense Seeds. Curr. Microbiol. 2022, 79, 264. [Google Scholar] [CrossRef]
- Shao, S.C.; Xi, H.P.; Mohandass, D. Symbiotic mycorrhizal fungi isolated via ex situ seed baiting induce seed germination of Dendrobium catenatum Lindl. (Orchidaceae). Appl. Ecol. Environ. Res. 2019, 17, 9753–9771. [Google Scholar] [CrossRef]
- Zhao, X.L.; Yang, J.Z.; Liu, S.; Chen, C.L.; Zhu, H.Y.; Cao, J.X. The colonization patterns of different fungi on roots of Cymbidium hybridum plantlets and their respective inoculation effects on growth and nutrient uptake of orchid plantlets. World J. Microbiol. Biotechnol. 2014, 30, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Chen, C.; Yang, J.; Zhu, H.; Liu, M.; Lv, F. Deep sequencing-based comparative transcriptional profiles of Cymbidium hybridum roots in response to mycorrhizal and non-mycorrhizal beneficial fungi. BMC Genom. 2014, 15, 747. [Google Scholar] [CrossRef]
- Liu, S.; Liu, M.; Liao, Q.G.; Lu, F.B.; Zhao, X.L. Effects of inoculated mycorrhizal fungi and non-mycorrhizal beneficial micro-organisms on plant traits, nutrient uptake and root-associated fungal community composition of the Cymbidium hybridum in greenhouse. J. Appl. Microbiol. 2021, 131, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, F.; Liao, Q.G.; Zhao, X.L. Effect of orchid mycorrhizae fungus inoculation on the growth, nutrient absorption, and nitrogen metabolism of Cymbidium hybridum under different nitrate-to-ammonium ratios. J. Plant Nutr. Fertil. 2025, 31, 578–587. [Google Scholar]
- Zuccaro, A.; Lahrmann, U.; Guldener, U.; Langen, G.; Pfiffi, S.; Biedenkopf, D.; Wong, P.; Samans, B.; Grimm, C.; Basiewicz, M.; et al. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 2011, 7, e1002290. [Google Scholar] [CrossRef]
- Xu, Z.X.; Zhu, X.M.; Yin, H.; Li, B.; Chen, X.J.; Fan, X.L.; Li, N.Q.; Selosse, M.A.; Gao, J.Y.; Han, J.J. Symbiosis between Dendrobium catenatum protocorms and Serendipita indica involves the plant hypoxia response pathway. Plant Physiol. 2023, 192, 2554–2568. [Google Scholar] [CrossRef] [PubMed]
- Arditti, J. Factors Affecting the Germination of Orchid Seeds. Bot. Rev. 1967, 33, 1–97. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, Z.L. Plant Physiology Laboratory Manual, 5th ed.; Higher Education Press: Beijing, China, 2016. [Google Scholar]
- Downing, J.L.; Liu, H.; Mccormick, M.K.; Arce, J.; Alonso, D.; Lopez-Perez, J. Generalized mycorrhizal interactions and fungal enemy release drive range expansion of orchids in southern Florida. Ecosphere 2020, 11, e03228. [Google Scholar] [CrossRef]
- Solís, K.; Barriuso, J.J.; Garcés-Claver, A.; González, V. Tulasnella tubericola (Tulasnellaceae, Cantharellales, Basidiomycota): A new Rhizoctonia-like fungus associated with mycorrhizal evergreen oak plants artificially inoculated with black truffle (Tuber melanosporum) in Spain. Phytotaxa 2017, 317, 175–187. [Google Scholar] [CrossRef]
- Davis, B.; Lim, W.; Lambers, H.; Dixon, K.W.; Read, D.J. Inorganic phosphorus nutrition in green-leaved terrestrial orchid seedlings. Ann. Bot. 2022, 129, 669–678. [Google Scholar] [CrossRef]
- Pereira, G.; Romero, C.; Suz, L.M.; Atala, C. Essential mycorrhizal partners of the endemic Chilean orchids Chloraea collicensis and C. gavilu. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 95–99. [Google Scholar] [CrossRef]
- Liu, S.; Chen, C.L.; Liu, M.; Lia, Q.G.; Zhao, X.L. Comparing the symbiotic effects of two endophytes on growth of Cymbidium hybridum. J. Huazhong Agric. Univ. 2016, 35, 43–49. [Google Scholar]
- Liu, M.; Liao, Q.G.; Chen, M.J.; Yang, J.Z.; Zhao, X.L. Cloning, expression and localization of mycorrhizal responsive CyENT3 from Cymbidium hybridum. Sci. Hortic. 2015, 197, 230–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.W.; Li, N.Q.; Jin, Q.; Anbazhakan, R.; Dai, Y.F.; Xiang, Z.X.; Gao, J.Y. Ecological specificity of fungi on seedling establishment in Dendrobium huoshanense: A narrow distributed medicinal orchid. Mycorrhiza 2025, 35, 41. [Google Scholar] [CrossRef]
- Bustam, B.M.; Dixon, K.W.; Bunn, E. In vitro propagation of temperate Australian terrestrial orchids: Revisiting asymbiotic compared with symbiotic germination: Australian Orchid Seed Germination. Bot. J. Linn. Soc. 2014, 176, 556–566. [Google Scholar] [CrossRef]
- Phillips, R.D.; Reiter, N.; Peakall, R. Orchid conservation: From theory to practice. Ann. Bot. 2020, 126, 345–362. [Google Scholar] [CrossRef]
- Cevallos, S.; Declerck, S.; Suarez, J.P. In situ Orchid Seedling-Trap Experiment Shows Few Keystone and Many Randomly Associated Mycorrhizal Fungal Species During Early Plant Colonization. Front. Plant Sci. 2018, 9, 1664. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zi, X.; Lin, H.; Gao, J.Y. Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum. J. Microbiol. 2018, 56, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, S.S.; Kohler, A.; Yan, B.; Luo, H.M.; Chen, X.M.; Guo, S.X. iTRAQ and RNA-seq analyses provide new insights into regulation mechanism of symbiotic germination of Dendrobium officinale seeds (Orchidaceae). J. Proteome Res. 2017, 16, 2174–2187. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Umehara, M.; Cao, M.; Akiyama, K.; Akatsu, T.; Seto, Y.; Hanada, A.; Li, W.; Takeda-Kamiya, N.; Morimoto, Y.; Yamaguchi, S. Structural requirements of strigolactones for shoot branching inhibition in rice and Arabidopsis. Plant Cell Physiol. 2015, 56, 1059–1072. [Google Scholar] [CrossRef]
- Verma, S.; Varma, A.; Rexer, K.H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Bütehorn, B.; Franken, P. Piriformospora indica gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Weiss, M.; Waller, F.; Zuccaro, A.; Selosse, M.A. Sebacinales—One thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
- Varma, A.; Sherameti, I.; Tripathi, S.; Prasad, R.; Ller, R.O. The symbiotic fungus Piriformospora indica: Review. In Fungal Associations; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Wang, T.; Song, Z.; Wang, X.; Xu, L.; Sun, Q.; Li, L. Functional insights into the roles of hormones in the Dendrobium officinale-Tulasnella sp. germinated seed symbiotic association. Int. J. Mol. Sci. 2018, 19, 3484. [Google Scholar] [CrossRef] [PubMed]

| Fungal Isolates Codes | GenBank Accession Numbers | Sequence Identity (%) | Closest Relatives | References |
|---|---|---|---|---|
| Dca111 | PV643361 | 99 | MT611027.1 | Downing [38] |
| Dca112 | PV643362 | 99 | KX774358.1 | Solís [39] |
| Dca113 | PV643363 | 99 | KX929166.1 | Solís [39] |
| Dca115 | PV643364 | 98 | KT601562.1 | Davis [40] |
| Dca125 | PV643365 | 98 | MN173015.1 | Meng [13] |
| Dca121 | PV643366 | 99 | MN173015.1 | Meng [13] |
| Dca122 | PV643367 | 99 | MN645108.1 | Jason [38] |
| Dca221 | PV643368 | 99 | MN173015.1 | Meng [13] |
| Dca222 | PV643369 | 99 | MN173015.1 | Meng [13] |
| Dca224 | PV643370 | 99 | KC758962.1 | Pereira [41] |
| Dca311 | PV643371 | 98 | KT601562.1 | Davis [40] |
| Dca312-1 | PV643372 | 99 | MN173015.1 | Meng [13] |
| Dca312 | PV643373 | 99 | MN173015.1 | Meng [13] |
| Dca323 | PV643374 | 98 | KT601562.1 | Davis [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.-Y.; Xie, X.-Y.; Liang, Z.-Q.; Zhang, J.-X.; Liu, S.; Zhao, X.-L. Comparative Symbiotic Effects of Mycorrhizal Fungal Strains from Different Hosts on Seed Germination and Seedling Growth in Dendrobium officinale. J. Fungi 2025, 11, 737. https://doi.org/10.3390/jof11100737
He J-Y, Xie X-Y, Liang Z-Q, Zhang J-X, Liu S, Zhao X-L. Comparative Symbiotic Effects of Mycorrhizal Fungal Strains from Different Hosts on Seed Germination and Seedling Growth in Dendrobium officinale. Journal of Fungi. 2025; 11(10):737. https://doi.org/10.3390/jof11100737
Chicago/Turabian StyleHe, Jian-Yu, Xiao-Yan Xie, Zhuo-Qi Liang, Jian-Xia Zhang, Shu Liu, and Xiao-Lan Zhao. 2025. "Comparative Symbiotic Effects of Mycorrhizal Fungal Strains from Different Hosts on Seed Germination and Seedling Growth in Dendrobium officinale" Journal of Fungi 11, no. 10: 737. https://doi.org/10.3390/jof11100737
APA StyleHe, J.-Y., Xie, X.-Y., Liang, Z.-Q., Zhang, J.-X., Liu, S., & Zhao, X.-L. (2025). Comparative Symbiotic Effects of Mycorrhizal Fungal Strains from Different Hosts on Seed Germination and Seedling Growth in Dendrobium officinale. Journal of Fungi, 11(10), 737. https://doi.org/10.3390/jof11100737




