Serendipita indica Enhances Drought Tolerance in Phoebe sheareri Seedlings by Improving Photosynthetic Efficiency, Stimulating the Antioxidant Defense System, and Modulating Hormone Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Fungal Preparation and Inoculation
2.3. Experimental Design
2.4. Measurements
2.4.1. Determination of Fungal Colonization and Biomass
2.4.2. Measurement of Gas Exchange Parameters
2.4.3. Measurement of Physiological and Biochemical Indicators
2.5. Data Analysis
3. Results
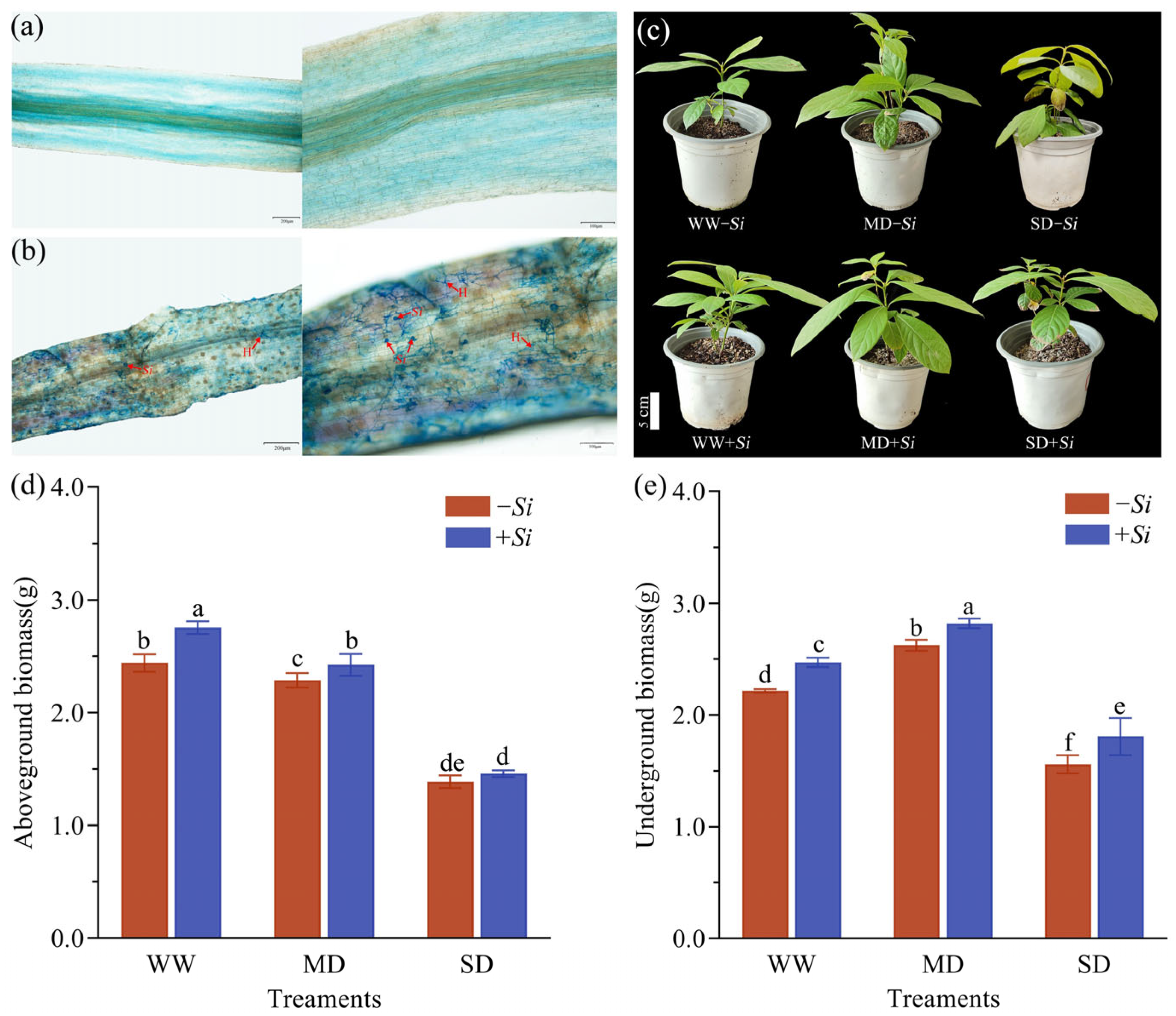
3.1. Colonization of S. indica on the Roots of P. sheareri Seedlings and Its Effects on Plant Growth and Biomass Under Drought Stress
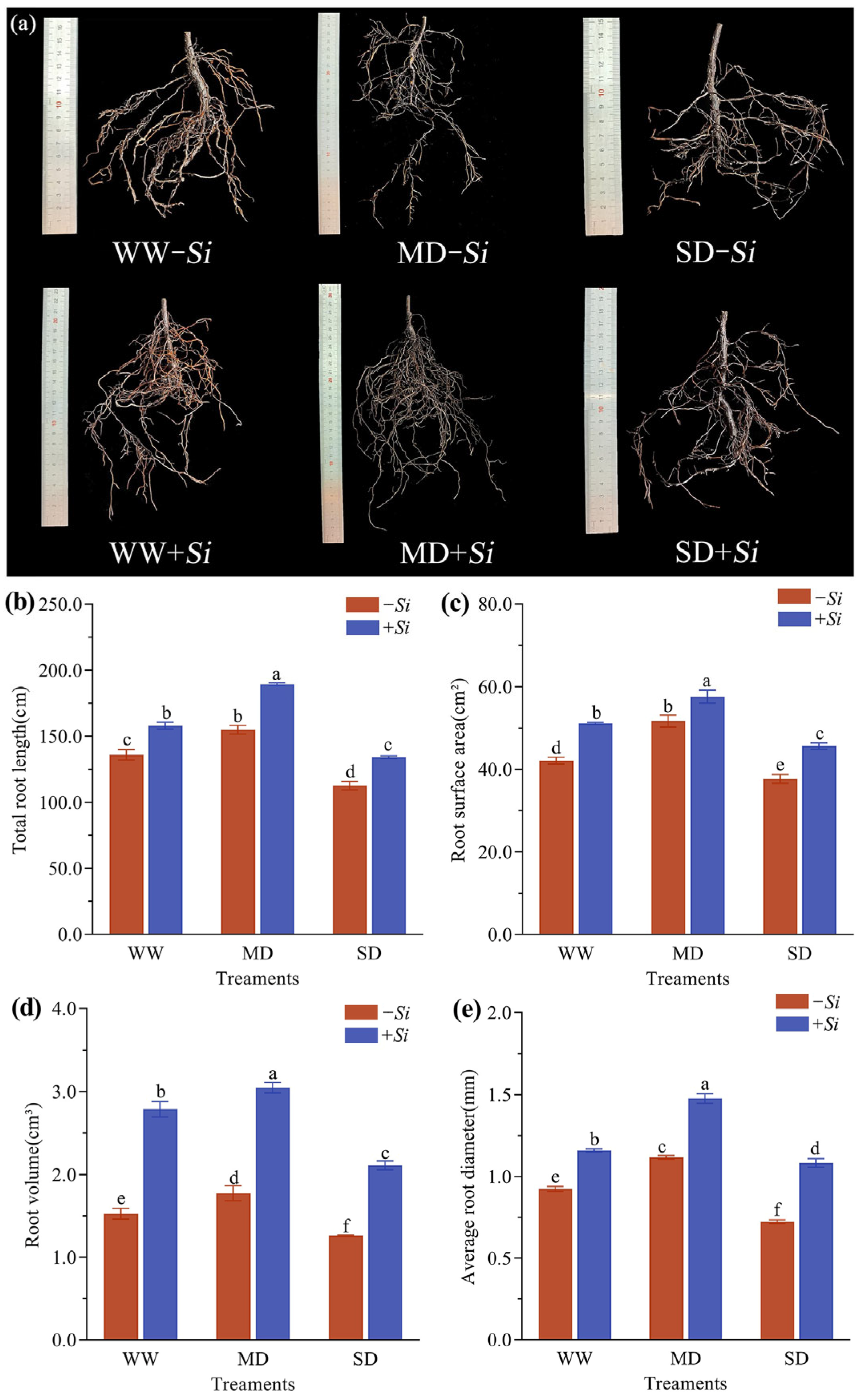
3.2. Effects of S. indica on Root Growth of P. sheareri Seedlings Under Drought Stress
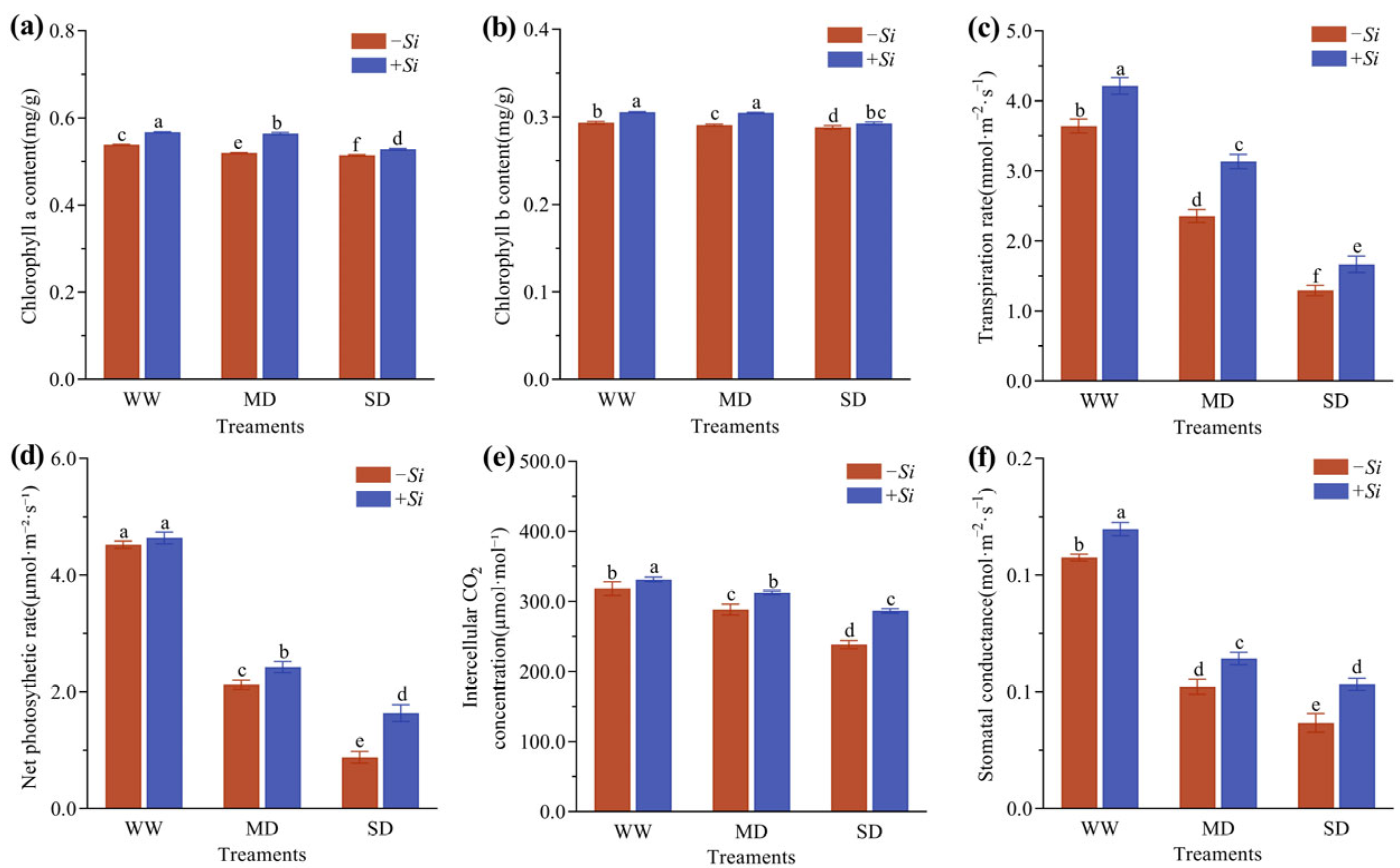
3.3. Effects of S. indica on Chlorophyll Content and Photosynthetic Efficiency in P. sheareri Seedlings Under Drought Stress
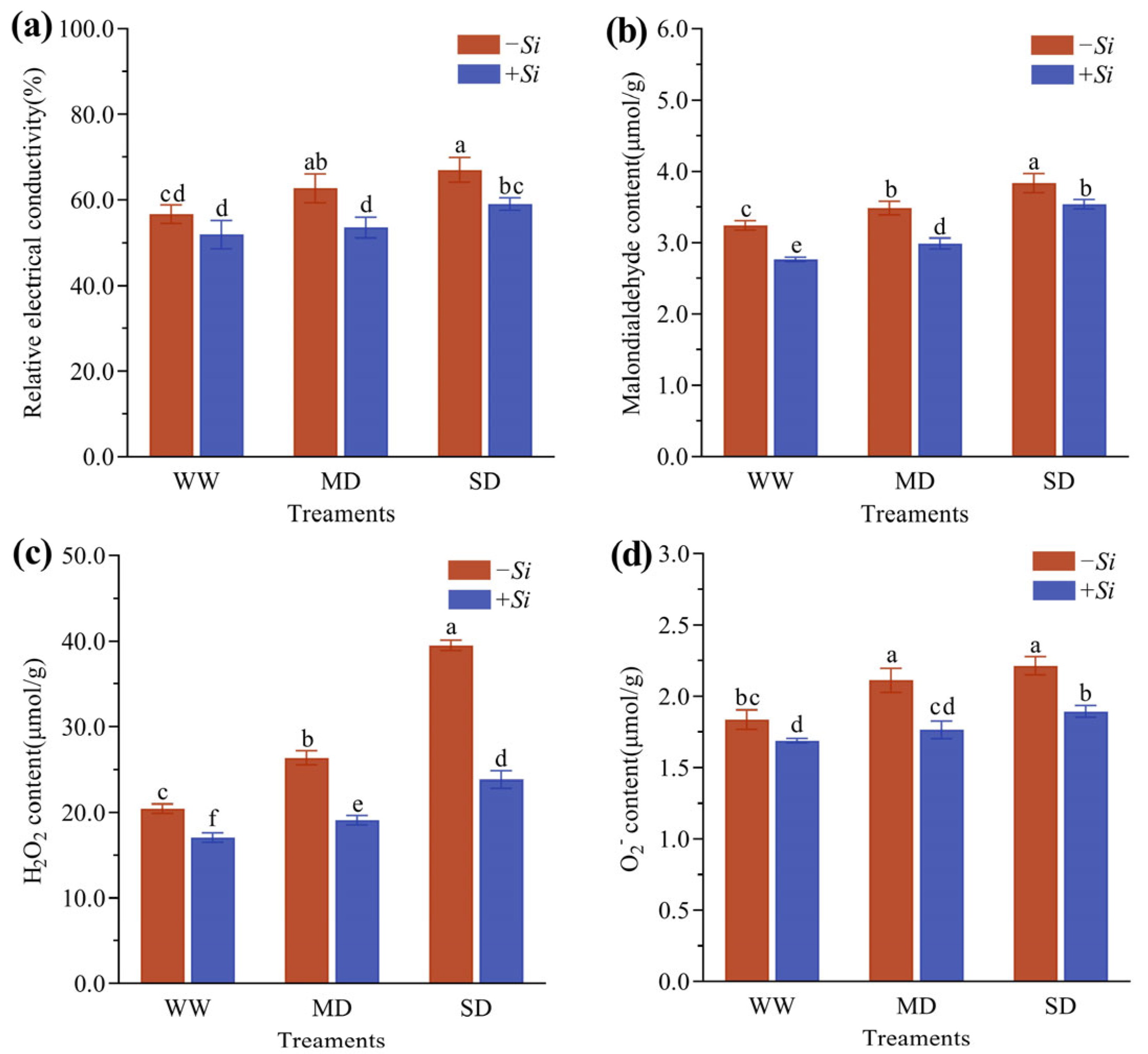
3.4. Effects of S. indica on Cell Membrane Permeability and Reactive Oxygen Species Metabolism in P. sheareri Seedlings Under Drought Stress
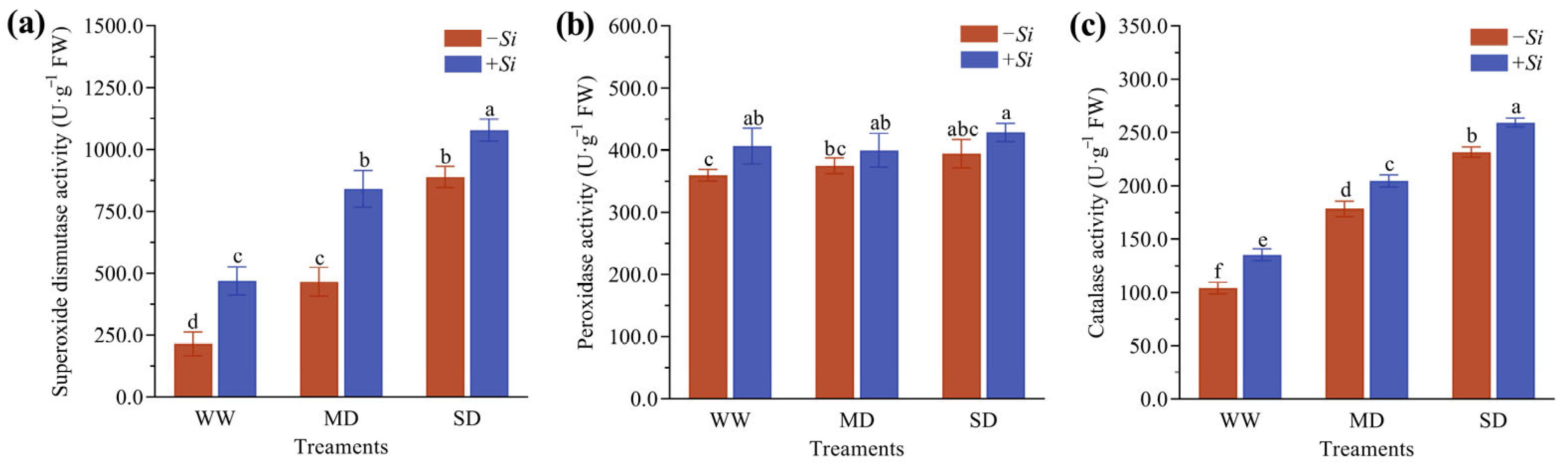
3.5. Effects of S. indica on Antioxidant Enzyme Activities in P. sheareri Seedlings Under Drought Stress
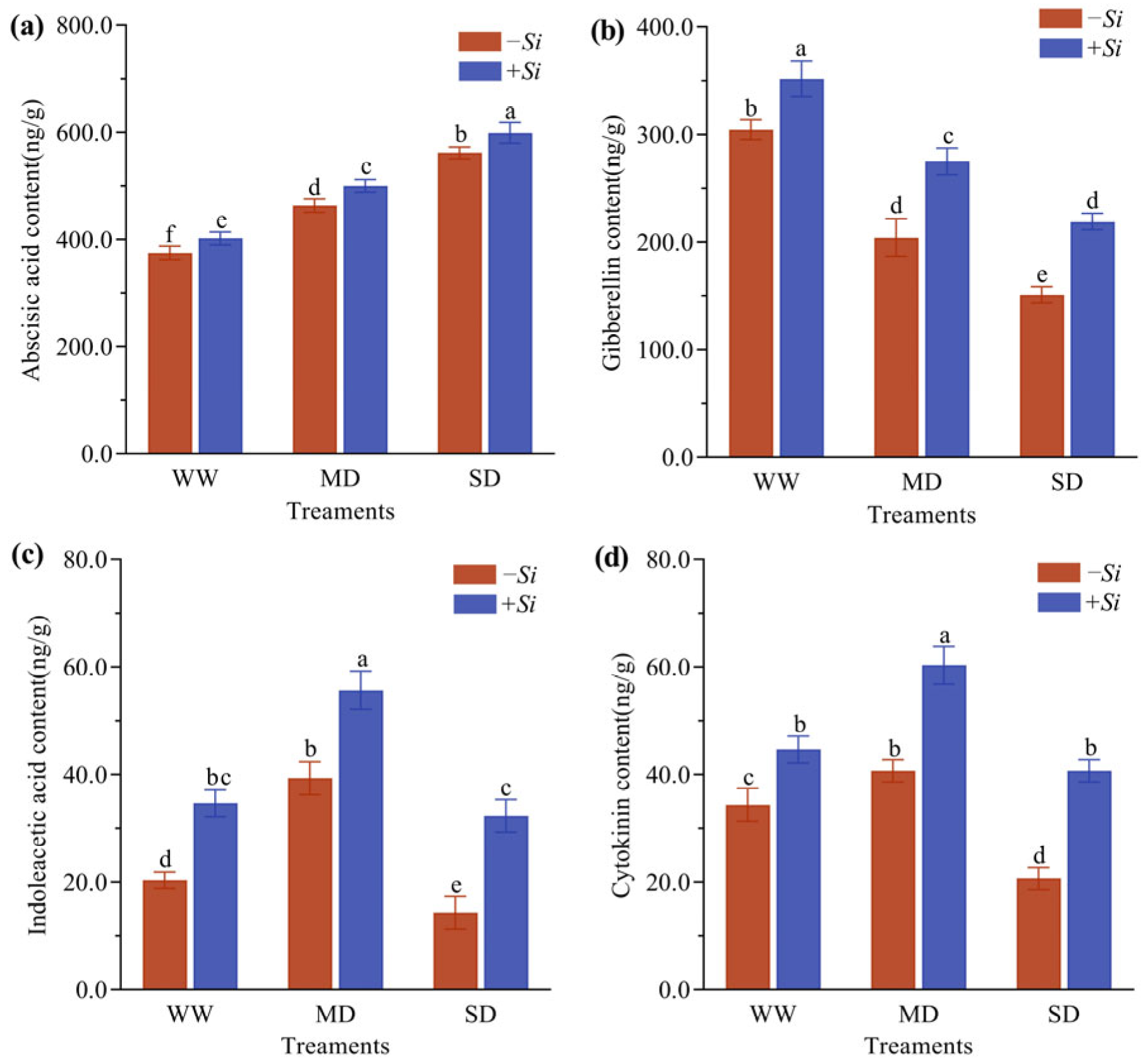
3.6. Effects of S. indica on Hormone Content in P. sheareri Seedlings Under Drought Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takács, G.; Gergely, I.; Ördög, V.; Vörös, L.; Iváncsics, J. Approaches to studying wheat and maize drought stress responses. Plant Soil 2025, 513, 1–18. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Kumari, A.; Jagadesh, M.; Singh, S.K.; Bhatt, R.; Singh, M.; Seth, C.S.; Li, Y.R. Climate change adaptation: Challenges for agricultural sustainability. Plant Cell Environ. 2024, 48, 2522–2533. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.P.C.; Silva, D.D.D.A.; Moreira, M.C.; Passos, J.B.M.C.; Coelho, C.D.; Elesbon, A.A.A. Development of an annual drought classification system based on drought severity indexes. An. Da Acad. Bras. De Cienc. 2019, 91, e20180188. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Ma, A.F.; Feng, Q.; Gong, Z.Z. Research advances in plant drought resistance. Chin. Sci. Bull. 2025, 70, 4297–4314. (In Chinese) [Google Scholar] [CrossRef]
- Shi, F.; Pan, Z.; Dai, P.; Shen, Y.; Lu, Y.; Han, B. Effect of Water logging Stress on Leaf Anatomical Structure and Ultrastructure of Phoebe sheareri Seedlings. Forests 2023, 14, 1294. [Google Scholar] [CrossRef]
- Song, Y.; Yao, X.; Tan, Y.; Gan, Y.; Yang, J.; Corlett, R.T. Comparative analysis of complete chloroplast genome sequences of two subtropical trees, Phoebe sheareri and Phoebe omeiensis (Lauraceae). Tree Genet. Genomes 2017, 13, 120. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Tang, W.; Li, M.L.; Qian, Y.Y.; Ning, L.P. Wood structural characteristics of Phoebe sheareri. J. Northeast For. Univ. 2017, 45, 53–56. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Wen, S.; Yang, L.; Sun, W.; He, G.; Zhang, D. The cross talk of sesquiterpenes and phenylpropanes mediated by the shikimic acid pathway affects essential oil content in Phoebe sheareri leaves. Ind. Crops Prod. 2024, 216, 118791. [Google Scholar] [CrossRef]
- Fan, Y.; Lou, Y.K.; Ku, W.P.; Dai, Q.L.; Wang, Z.Q.; Zhao, M.S.; Yu, S.Q. Age structure and spatial point pattern of Phoebe sheareri population in Mount Tianmu. J. Zhejiang AF Univ. 2020, 37, 1027–1035. [Google Scholar]
- Wang, Y.; Ma, X.H.; Lu, Y.F.; Hu, X.E.; Lou, L.H.; Tong, Z.K.; Zhang, J.H. Assessing the current genetic structure of 21 remnant populations and predicting the impacts of climate change on the geographic distribution of Phoebe shearer in southern China. Sci. Total Environ. 2022, 846, 157391. [Google Scholar] [CrossRef]
- Li, L.; Feng, Y.; Qi, F.Y.; Hao, R.Y. Research Progress of Piriformospora indica in Improving Plant Growth and Stress Resistance to Plant. J. Fungi 2023, 9, 965. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Yadav, B.G.; Kumar, S.G.; Kumar, R.; Kogel, K.H.; Kumar, S. Piriformospora indica and Azotobacter chroococcum Consortium Facilitates Higher Acquisition of N, P with Improved Carbon Allocation and Enhanced Plant Growth in Oryza sativa. J. Fungi 2022, 8, 453. [Google Scholar] [CrossRef]
- Shrivastava, N.; Mahajan, S.; Jain, A.; Sharma, P.; Kharakwal, A.C.; Varma, A. Mutualistic Interaction of Piriformospora indica (Serendipita indica) with Aloe vera, the Wonder Plant for Modern Living. Am. J. Plant Sci. 2019, 10, 2002–2011. [Google Scholar] [CrossRef][Green Version]
- Das, A.; Kamal, S.; Shakil, N.A.; Sherameti, I.; Oelmüller, R.; Dua, M.; Tuteja, N.; Johri, A.K.; Varma, A. The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal. Behav. 2012, 7, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Qu, P.Y.; Wang, D.M.; Yan, S.T.; Gong, Q.H.; Yang, R.; Hu, Y.; Liu, N.R.; Cheng, C.Z.; Wang, P.F.; et al. The Influence of Piriformospora indica Colonization on the Root Development and Growth of Cerasus humilis Cuttings. Plants 2024, 13, 1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Zong, F.Q.; Lin, W.; Tang, X.X.; Xuan, S.K.; He, B.Z.; Wu, B.H.; Guo, L.J. The effects of Piriformospora indica on the growth of cuttings from three species of woody ornamental plants. Ind. Crops Prod. 2025, 223, 120127. [Google Scholar] [CrossRef]
- Achatz, B.; von Rüden, S.; Andrade, D.; Neumann, E.; Pons-Kühnemann, J.; Kogel, K.H.; Franken, P.; Waller, F. Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil 2010, 333, 59–70. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Shi, F.; Li, H.H.; Ding, Y.W.; Chang, W.; Ping, Y.; Song, F.Q. Piriformospora indica alleviates soda saline-alkaline stress in Glycine max by modulating plant metabolism. Front. Plant Sci. 2024, 15, 1406542. [Google Scholar] [CrossRef]
- Xu, L.; Wang, A.A.; Wang, J.; Wei, Q.; Zhang, W.Y. Piriformospora indica confers drought tolerance on Zea may L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017, 5, 251–258. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef]
- Wu, W.J.; Liu, R.C.; Xiao, Z.Y.; Alqahtani, M.D.; Wang, F.L.; Almaabadi, A.D.; Kuca, K.; Zou, Y.N.; Wu, Q.S. Non-targeted metabolomics reveals hormonal mechanisms regarding arbuscular mycorrhizal fungi- and Serendipita indica-mediated plant growth response in Camellia oleifera. Sci. Hortic. 2024, 337, 113544. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, M.Y.; Zhang, W.Y.; Xu, L.; Chen, J.F.; Pan, R.; Tian, X.H. Effect of the endophytic fungus Piriformospora indica on the growthand drought tolerance of rice seedling under drought stress. Chin. J. Ecol. 2018, 37, 2642–2648. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuca, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Barranco, D.; Ruiz, N.; Gómez-del Campo, M. Frost tolerance of eight olive cultivars. Hortscience 2005, 40, 558–560. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, L.; Liu, S.Y.; Zhao, M.Y.; Liu, J.M.; Lin, L.J.; Liu, K.D. Physiological and transcriptomic analysis of IAA-induced antioxidant defense and cell wall metabolism in postharvest mango fruit. Food Res. Int. 2023, 174, 113504. [Google Scholar] [CrossRef]
- Liu, R.C.; Gao, W.Q.; Srivastava, A.K.; Zou, Y.N.; Kuca, K.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.S. Differential Effects of Exogenous Glomalin-Related Soil Proteins on Plant Growth of Trifoliate Orange Through Regulating Auxin Changes. Front. Plant Sci. 2021, 12, 745402. [Google Scholar] [CrossRef]
- Guo, C.C.; Bao, X.Y.; Sun, H.C.; Zhu, L.X.; Zhang, Y.J.; Zhang, K.; Bai, Z.Y.; Zhu, J.J.; Liu, X.Q.; Li, A.C.; et al. Optimizing root system architecture to improve cotton drought tolerance and minimize yield loss during mild drought stress. Field Crops Res. 2024, 308, 109305. [Google Scholar] [CrossRef]
- Zhuoma, L.; Xin, F.H.; Yang, X.L.; Zhao, K.T. Effect of drought stress on growth and physiological indicators of Sabina pingii var.wilsonii seedlings. J. Northwest AF Univ. (Nat. Sci. Ed.) 2015, 43, 51–57+70. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.F. Effects of drought stress on growth and physiological characteristics in Sorbus folgneri seedlings. J. For. Environ. 2011, 31, 330–334. [Google Scholar] [CrossRef]
- Liu, C.Y.; Liang, S.Y.; Wu, J.W.; Gu, J.Y.; Duan, H.J. Response of growth and physiological-biochemical characteristics in Pinus yunnanensis seedlings to drought and rewatering. J. Northwest AF Univ. (Nat. Sci. Ed.) 2026, 54, 99–108. [Google Scholar] [CrossRef]
- Qiao, M.Y.; Hong, C.H.; Jiao, Y.J.; Hou, S.J.; Gao, H.B. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, W.M.; Wang, G.F.; Hu, Y.X.; Zhong, X.B.; Tang, G.X. Physiological Regulation of Photosynthetic-Related Indices, Antioxidant Defense, and Proline Anabolism on Drought Tolerance of Wild Soybean (Glycine soja L.). Plants 2024, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.F.; Zhang, H.C.; Bian, L.M.; Zhou, L.; Ge, Y.F.; Casella, E. Three-Dimensional Quantification and Visualization of Leaf Chlorophyll Content in Poplar Saplings under Drought Using SFM-MVS. Forests 2024, 15, 20. [Google Scholar] [CrossRef]
- Zong, J.W.; Chang, Y.W.; Zhu, Y.Q.; Deng, H.F.; Cai, Y.Y.; Yang, Y.H. Effects of AM fungi on Xanthoceras sorbifolium growth and photosynthetic physiology under drought stress. J. Northwest AF Univ. (Nat. Sci. Ed.) 2025, 53, 73–82+92. [Google Scholar] [CrossRef]
- Dai, W.J.; Ding, Y.F.; Zhao, Y.; Zhou, Y.; Fan, H.Y. Effects of drought stress on physiological and photosyntheticcharacteristics of three species of Corydalis. J. Northwest AF Univ. (Nat. Sci. Ed.) 2025, 1–11. [Google Scholar] [CrossRef]
- Maimaiti, A.; Tuohetimaimaiti, Z.; Jiang, Y.; Qin, Q.; Nur, Y.; Xu, M. Photosynthetic and physiological responses of Aronia Melanocarpa seedlings to soil drought. Non-Wood For. Res. 2024, 42, 131–144. [Google Scholar] [CrossRef]
- Miranda, V.; Silva-Castro, G.A.; Ruiz-Lozano, J.M.; Fracchia, S.; García-Romera, I. Fungal Endophytes Enhance Wheat and Tomato Drought Tolerance in Terms of Plant Growth and Biochemical Parameters. J. Fungi 2023, 9, 384. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Wang, P.T.; Liu, W.C.; Han, C.; Wang, S.T.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Luo, D.L.; Zhai, G.F.; Cao, S.; Zhu, T.; Chen, R.J.; Xiao, Q.; Zhang, D.; Ba, L.J. Effect of preharvest salicylic acid combined with postharvest 1-MCP treatment on quality and antioxidant ability of plum fruit during storage. Sci. Technol. Food Ind. 2022, 43, 327–333. [Google Scholar] [CrossRef]
- Ma, J.J.; Bai, M.H.; Paizilijiang, O.; Tian, R.; Tang, P.L.; Song, Y.N.; Jieken, G.; Cui, K.B. Effects of ozone treatment on AsA-GSH cycle and membrane lipid peroxidation in postharvest storage of prunus fruits. Sci. Technol. Food Ind. 2025, 46, 1–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.S.; Xu, Y.M.; Chen, Y.T.; Xie, B.; Hu, J. Antagonistic mechanisms of phytohormones ABA and GA in plant droughtresponses. Biotic Resour. 2025, 47, 1–15. [Google Scholar] [CrossRef]
- Vidal, A.; Cantabella, D.; Bernal-Vicente, A.; Díaz-Vivancos, P.; Hernández, J.A. Nitrate- and nitric oxide-induced plant growth in pea seedlings is linked to antioxidative metabolism and the ABA/GA balance. J. Plant Physiol. 2018, 230, 13–20. [Google Scholar] [CrossRef]
- Liao, Z.Q.; Chen, B.B.; Boubakri, H.; Farooq, M.; Mur, L.A.J.; Urano, D.; Teo, C.H.; Tan, B.C.; Hasan, M.M.; Aslam, M.M.; et al. The regulatory role of phytohormones in plant drought tolerance. Planta 2025, 261, 98. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Jin, L.; Sun, Y.; Zhan, C.H.; Tang, S.Q.; Qin, T.; Liu, N.; Huang, J.L. OsNAC120 balances plant growth and drought tolerance by integrating GA and ABA signaling in rice. Plant Commun. 2024, 5, 100782. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.P.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Qin, X.F.; He, Z.R.; Zhang, Y.; Li, Z.; Nie, G.; Zhao, J.M.; Feng, G.Y.; Peng, Y. The White Clover TrMYB33-TrSAMS1 Module Contributes to Drought Tolerance by Modulation of Spermidine Biosynthesis via an ABA-Dependent Pathway. Int. J. Mol. Sci. 2024, 25, 6974. [Google Scholar] [CrossRef]
- Li, J.J.; Li, Y.; Yin, Z.G.; Jiang, J.H.; Zhang, M.H.; Guo, X.; Ye, Z.J.; Zhao, Y.; Xiong, H.Y.; Zhang, Z.Y.; et al. OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2O2 signalling in rice. Plant Biotechnol. J. 2017, 15, 183–196. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Sun, R.; Hu, D.; Yang, Y.; Cheng, Z.; Hu, P.; Fei, Y. Serendipita indica Enhances Drought Tolerance in Phoebe sheareri Seedlings by Improving Photosynthetic Efficiency, Stimulating the Antioxidant Defense System, and Modulating Hormone Synthesis. J. Fungi 2025, 11, 717. https://doi.org/10.3390/jof11100717
Chen X, Sun R, Hu D, Yang Y, Cheng Z, Hu P, Fei Y. Serendipita indica Enhances Drought Tolerance in Phoebe sheareri Seedlings by Improving Photosynthetic Efficiency, Stimulating the Antioxidant Defense System, and Modulating Hormone Synthesis. Journal of Fungi. 2025; 11(10):717. https://doi.org/10.3390/jof11100717
Chicago/Turabian StyleChen, Xiaohu, Rui Sun, Die Hu, Yujie Yang, Zihan Cheng, Ping Hu, and Yongjun Fei. 2025. "Serendipita indica Enhances Drought Tolerance in Phoebe sheareri Seedlings by Improving Photosynthetic Efficiency, Stimulating the Antioxidant Defense System, and Modulating Hormone Synthesis" Journal of Fungi 11, no. 10: 717. https://doi.org/10.3390/jof11100717
APA StyleChen, X., Sun, R., Hu, D., Yang, Y., Cheng, Z., Hu, P., & Fei, Y. (2025). Serendipita indica Enhances Drought Tolerance in Phoebe sheareri Seedlings by Improving Photosynthetic Efficiency, Stimulating the Antioxidant Defense System, and Modulating Hormone Synthesis. Journal of Fungi, 11(10), 717. https://doi.org/10.3390/jof11100717





