Comparative Analysis of Freeze-Dried Pleurotus ostreatus Mushroom Powders on Probiotic and Harmful Bacteria and Its Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
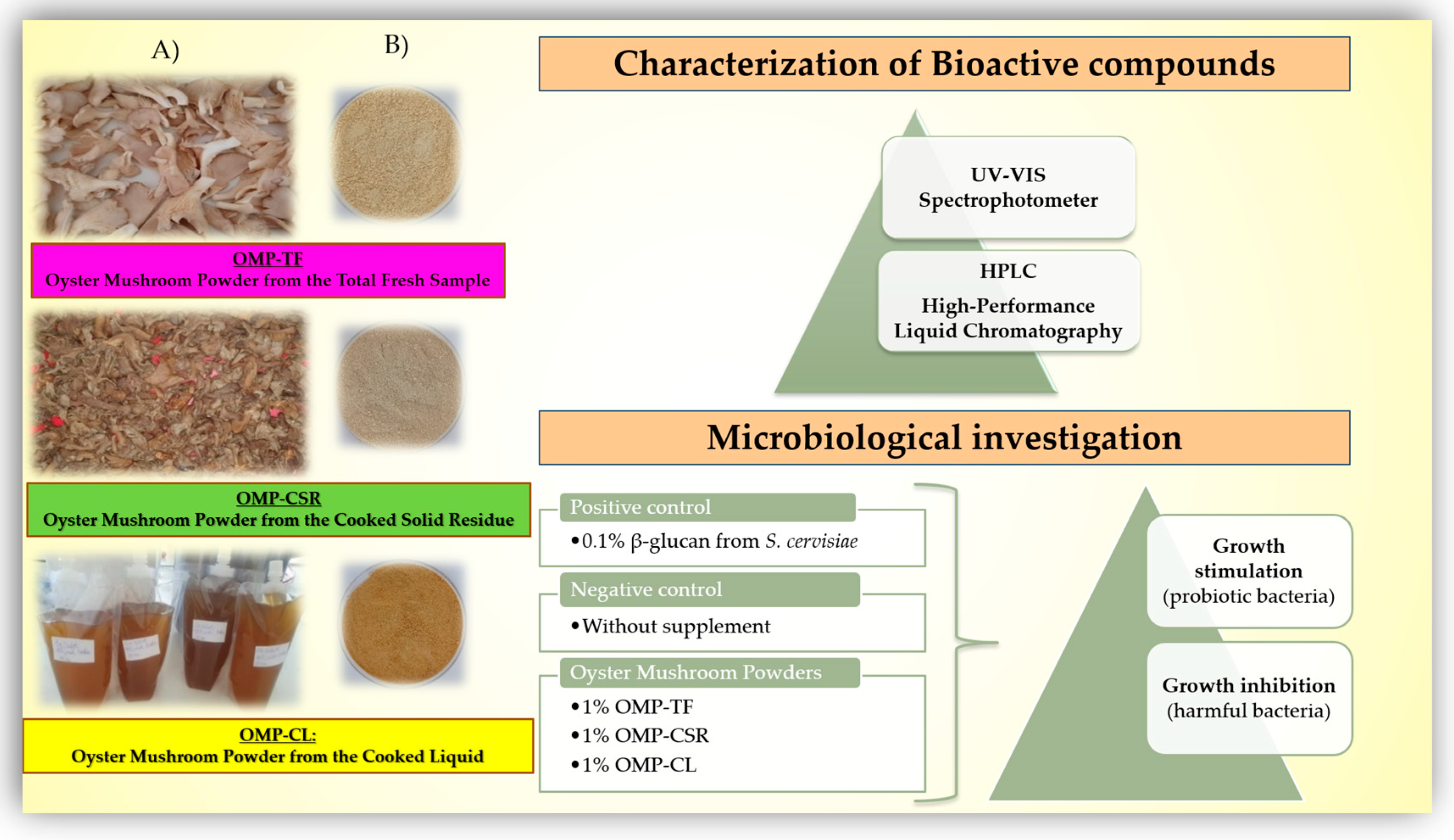
2.1. Experimental Design for the Production and Characterization of Freeze-Dried P. ostreatus Mushroom
- (1)
- We utilized the liquid portion to create a dry powder; however, its viscous consistency initially complicated the freeze-drying process. To overcome this challenge, we strategically added a gelling agent, 2% (m/v) agar-agar (VWR International Hungary Ltd.). We heated it to 100 °C for 5 min, transforming it into a gel contained in a stainless steel tray. Once stabilized, the gel was perfectly prepared for freeze-drying.
- (2)
- The cooked and stabilized liquid (filtrate), the cooked and centrifugated fruiting body (residue on the filter), and the raw mushroom samples (total fruiting body) were pre-frozen at −20 °C for 4 h without cooking. All pre-frozen samples were freeze-dried (Bionanoferm Ltd., Debrecen, Hungary). The freeze-drying process took 24 h at 40 °C. After freeze-drying, the samples were ground into fine powders.
2.2. Bioactive Compounds Presented in P. ostreatus Mushroom Powder
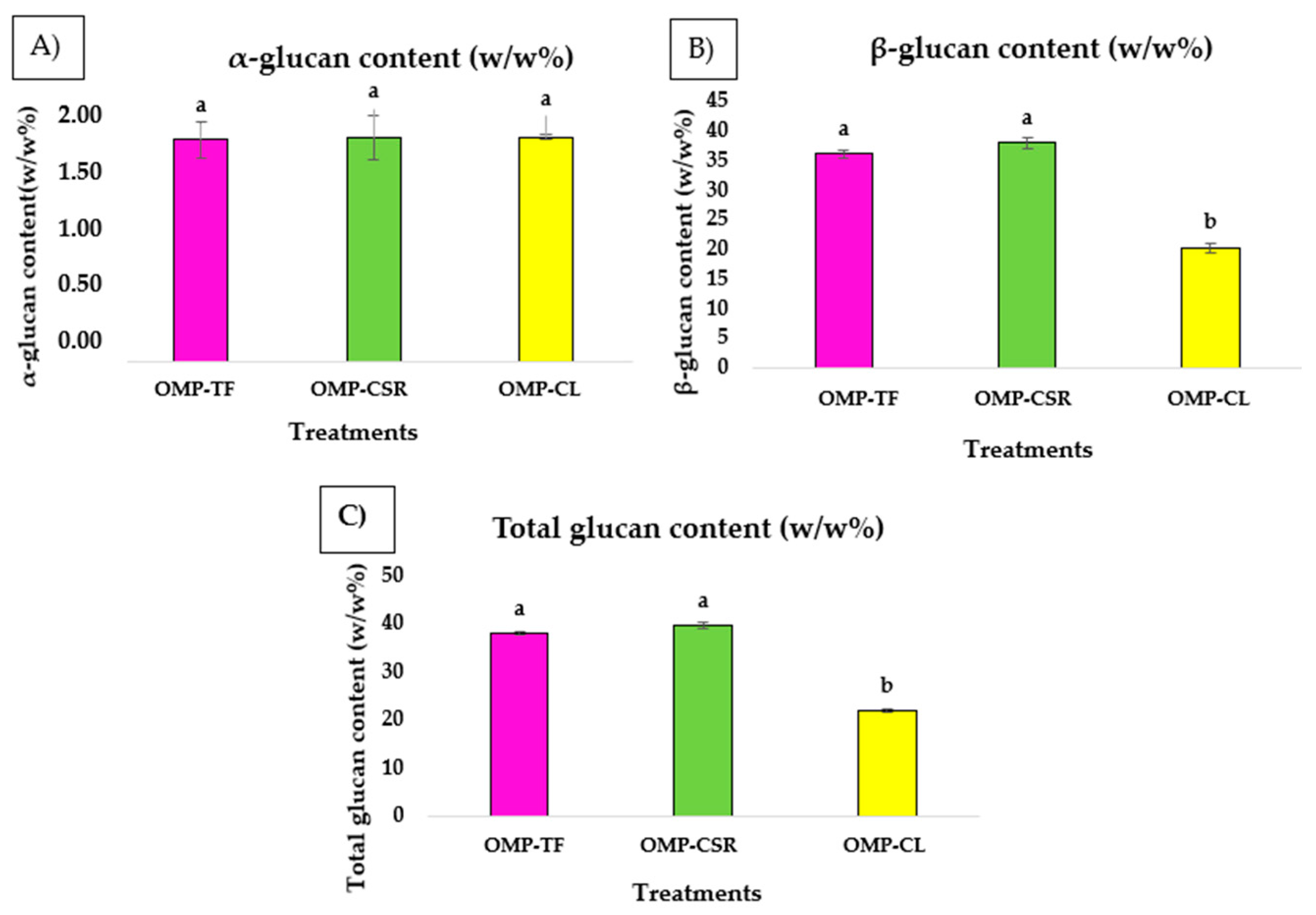
2.2.1. Investigation of Glucan Content
2.2.2. Content of Phenolic and Flavonoid Compounds and Radical Scavenging Activity
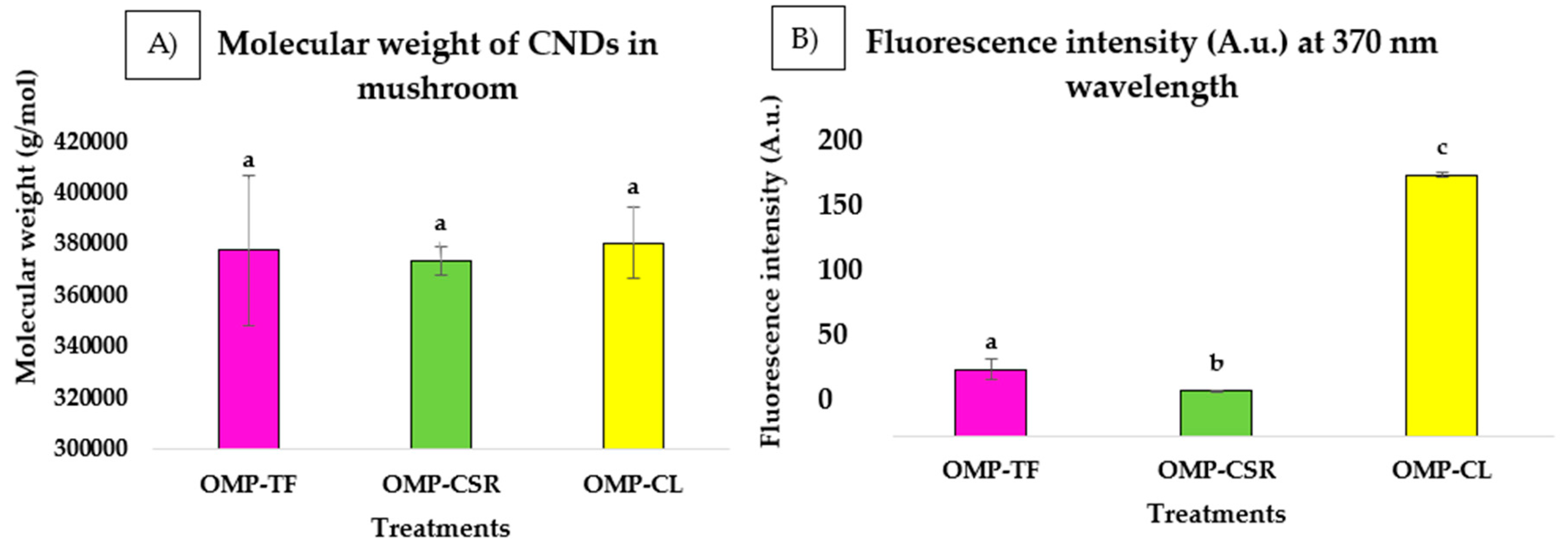
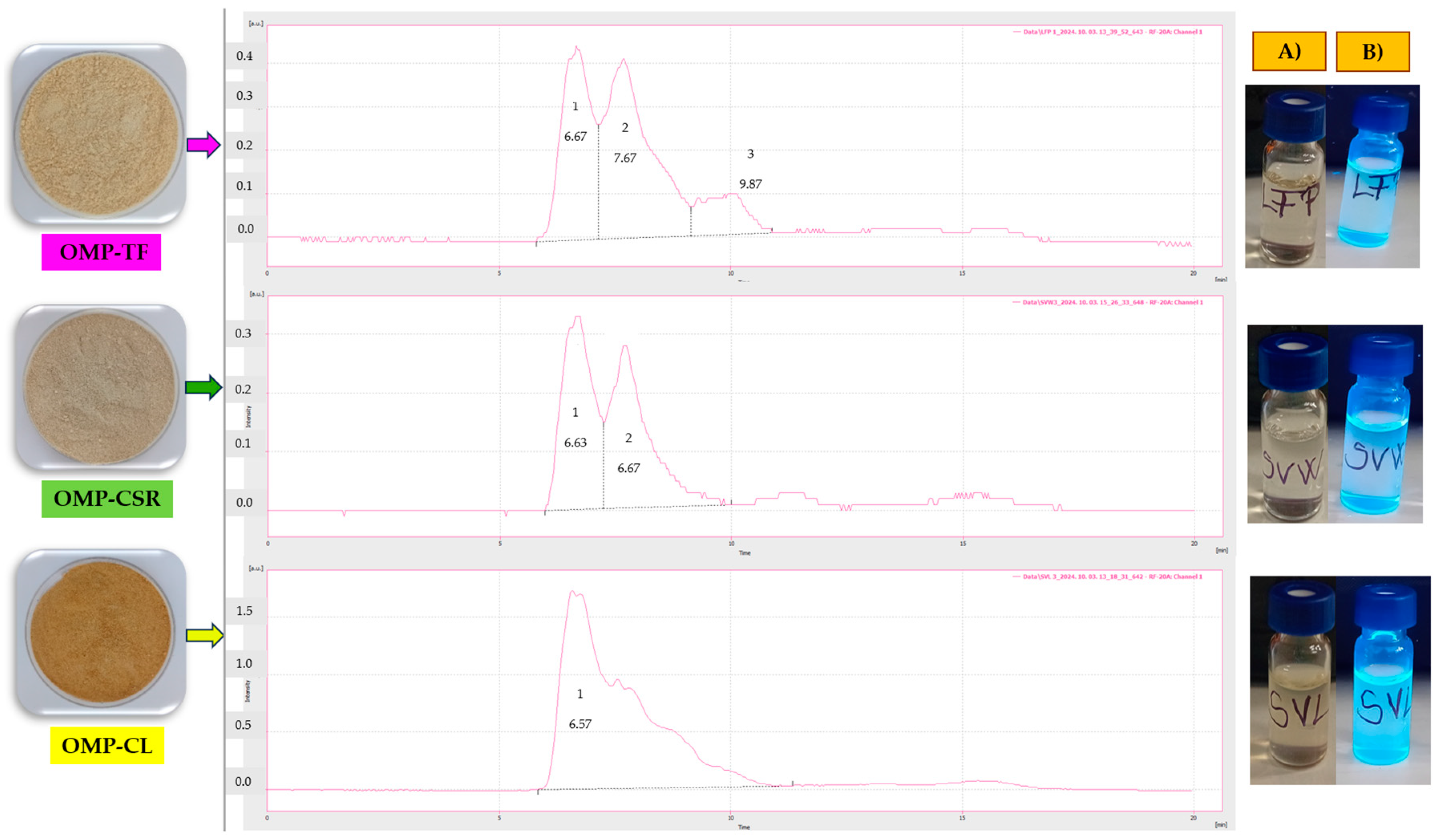
2.2.3. Size, Molecular Mass, and Final Concentrations of CNDs Presented in Freeze-Dried P. ostreatus Mushroom Powders (OMPs)
2.3. In Vitro Study of the Effectiveness of OMPs for the Beneficial Modulation of Gut Microbiota
2.3.1. Test Microorganisms
2.3.2. Microbiological Assay Setup and Preparation of Starter Cultures
2.3.3. Treatment and Experimental Design
- (1)
- 1% OMP-TF → 1% (w/v) of oyster mushroom powder from the total fresh sample.
- (2)
- 1% OMP-CSR → 1% (w/v) of oyster mushroom powder from the cooked solid residue.
- (3)
- 1% OMP-CL → 1% (w/v) of oyster mushroom powder from the cooked liquid.
- (4)
- 0.1% PC → 0.1% (w/v) commercially available β-glucans extracted from Saccharomyces cerevisiae (Medinvest Hungary Ltd., Kecskemét, Hungary), positive control.
- (5)
- NC → Without added supplementation, a negative control means no treatment.
2.3.4. Serial Dilution, Plating, and Incubation
2.4. Statistical Analysis
3. Results
3.1. Polysaccharide Fractions (Glucans)
3.2. Production of CNDs and Their Properties (Molecular Mass and Size) at Different Fractions
3.3. Investigation of Antioxidants and 11,1-Diphenyl-2-Picryl-Hydrazyl (DPPH)
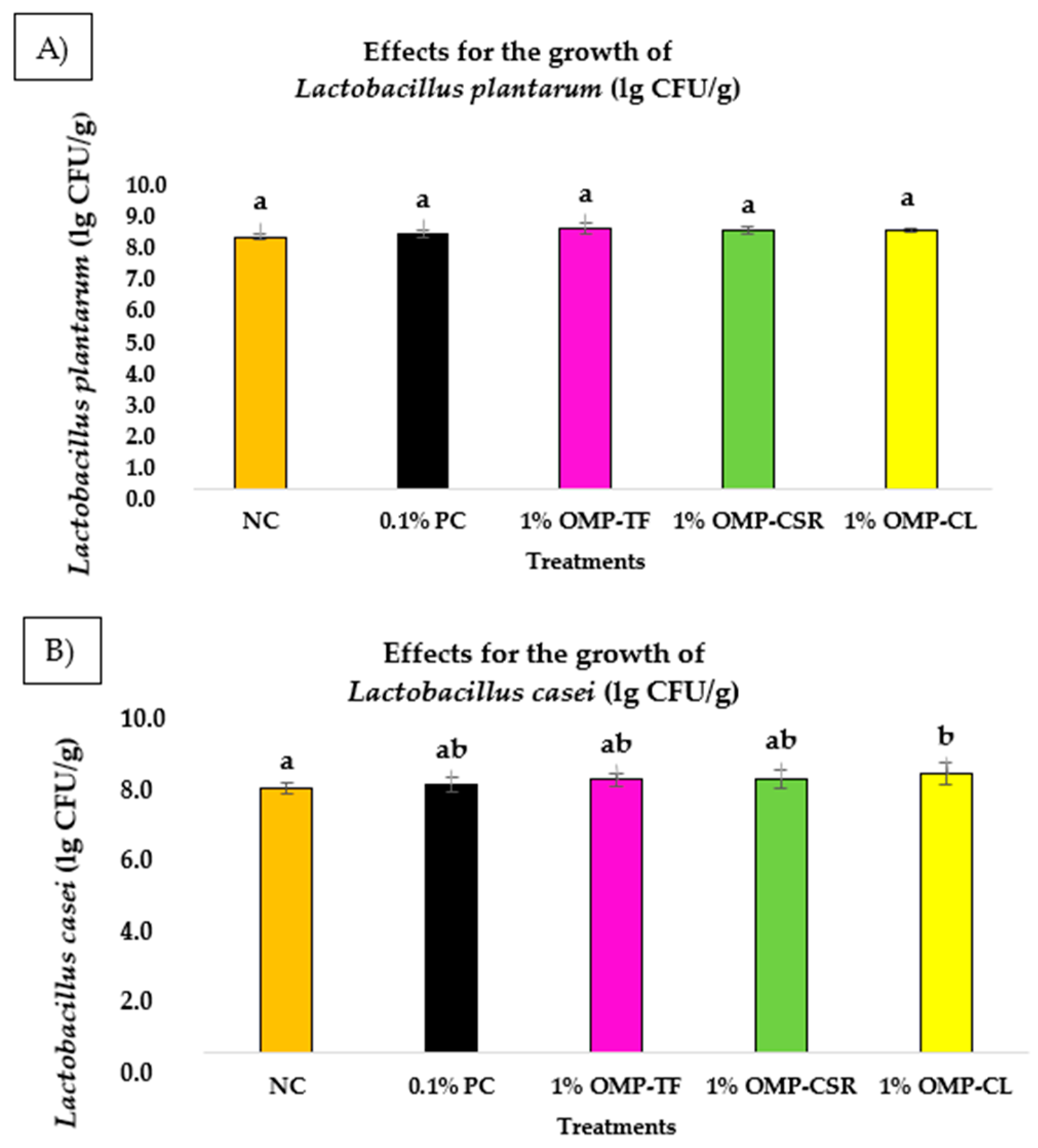
3.4. Probiotic Growth Stimulation of OMPs
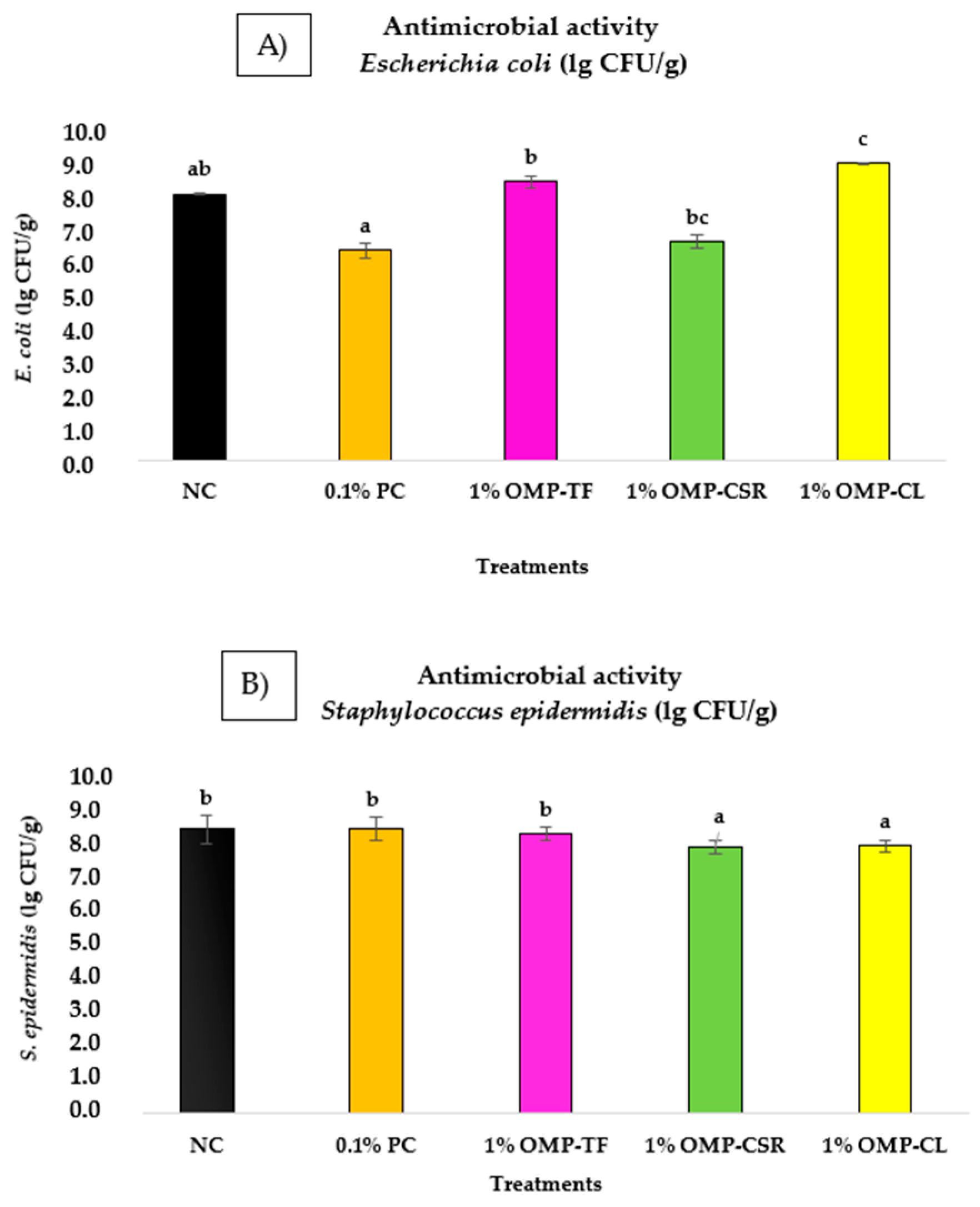
3.5. Antimicrobial Activity of OMPs
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, W.; Tang, X.; Cui, S.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H. Recent Advance in Quality Preservation of Non-Thermal Preservation Technology of Fresh Mushroom: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 7878–7894. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Ferreira, R.C.; Antonio, A.L.; Queiroz, M.-J.R.P.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Bioactive Compounds and Antioxidant Properties of Three Edible Mushrooms from Poland. Food Biosci. 2015, 11, 48–55. [Google Scholar] [CrossRef]
- Silva, M.; Ramos, A.C.; Lidon, F.J.; Reboredo, F.H.; Gonçalves, E.M. Pre- and Postharvest Strategies for Pleurotus Ostreatus Mushroom in a Circular Economy Approach. Foods 2024, 13, 1464. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.V.; Islam, J.; Narzary, P.; Sharma, D.; Sultana, F. Bioactive Compounds, Nutraceutical Values and Its Application in Food Product Development of Oyster Mushroom. J. Future Foods 2024, 4, 335–342. [Google Scholar] [CrossRef]
- Chang, R.; Chen, L.; Qamar, M.; Wen, Y.; Li, L.; Zhang, J.; Li, X.; Assadpour, E.; Esatbeyoglu, T.; Kharazmi, M.S.; et al. The Bioavailability, Metabolism and Microbial Modulation of Curcumin-Loaded Nanodelivery Systems. Adv. Colloid Interface Sci. 2023, 318, 102933. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the Human Gut Microbiota by Phenolics and Phenolic Fiber-rich Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef]
- Mihai, R.A.; Melo Heras, E.J.; Florescu, L.I.; Catana, R.D. The Edible Gray Oyster Fungi Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm a Potent Waste Consumer, a Biofriendly Species with Antioxidant Activity Depending on the Growth Substrate. J. Fungi 2022, 8, 274. [Google Scholar] [CrossRef]
- Assemie, A.; Abaya, G. The Effect of Edible Mushroom on Health and Their Biochemistry. Int. J. Microbiol. 2022, 2022, 8744787. [Google Scholar] [CrossRef]
- Kılıç, C.; Gürgen, A.; Yıldız, S.; Can, Z.; Değirmenci, A. Total Phenolics, Tannin Contents, Antioxidant Properties, Protein and Sensory Analysis of Pleurotus ostreatus, Pleurotus citrinopileatus and Pleurotus djamor Cultivated on Different Sawdusts. Maderas-Cienc. Tecnol. 2024, 26. [Google Scholar] [CrossRef]
- Ayser, M.; Tonny, W.; Hernandez, I.S.; Kuriakose, R.; Smith, J.D.; Wallaert, S.J.; Karim, A.; Robertson, M.L.; Balan, V. Fractionating Chitin-Glucan Complex and Coproducts from Pleurotus Ostreatus Mushrooms. Waste Biomass Valor. 2023, 15, 2897–2910. [Google Scholar] [CrossRef]
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the Key Aroma and Phenolic Compounds of Champignon (Agaricus bisporus) and Oyster (Pleurotus ostreatus) Mushrooms after Two Cooking Treatments as Elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lv, F.; Tian, J.; Ye, X.Q.; Chen, J.; Sun, P. Domestic Cooking Methods Affect Nutrient, Phytochemicals, and Flavor Content in Mushroom Soup. Food Sci. Nutr. 2019, 7, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Browning and Pigmentation in Food through the Maillard Reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef]
- Ahmed, K.J.A.J.; Bornare, D.; Jaiswal, S. Review on Extraction of Melanoidins from Coffee Waste and Value Addition in Food. J. Curr. Res. Food Sci. 2024, 5, 157–161. [Google Scholar] [CrossRef]
- Prajapati, B.G.; Pandey, V.; Sharma, S.; Patel, S.; Shah, D.P.; Kapoor, D.U. Carbon Nanodots: An Illuminating Paradigm in Production, Characterization, and Oncological Targeting Methodologies—A Review. BioNanoScience 2024, 14, 4322–4341. [Google Scholar] [CrossRef]
- Tejwan, N.; Kundu, M.; Ghosh, N.; Chatterjee, S.; Sharma, A.; Abhishek Singh, T.; Das, J.; Sil, P.C. Synthesis of Green Carbon Dots as Bioimaging Agent and Drug Delivery System for Enhanced Antioxidant and Antibacterial Efficacy. Inorg. Chem. Commun. 2022, 139, 109317. [Google Scholar] [CrossRef]
- Lysenko, V.; Kuznietsova, H.; Dziubenko, N.; Byelinska, I.; Zaderko, A.; Lysenko, T.; Skryshevsky, V. Application of Carbon Dots as Antibacterial Agents: A Mini Review. BioNanoScience 2024, 14, 1819–1831. [Google Scholar] [CrossRef]
- Izham, I.; Avin, F.; Raseetha, S. Systematic Review: Heat Treatments on Phenolic Content, Antioxidant Activity, and Sensory Quality of Malaysian Mushroom: Oyster (Pleurotus spp.) and Black Jelly (Auricularia spp.). Front. Sustain. Food Syst. 2022, 6, 882939. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Tu, J.; Brennan, M.; Brennan, C. An Insight into the Mechanism of Interactions between Mushroom Polysaccharides and Starch. Curr. Opin. Food Sci. 2021, 37, 17–25. [Google Scholar] [CrossRef]
- Törős, G.; Béni, Á.; Peles, F.; Rai, M.; Elramady, H.; Prokisch, J. Enhancing Biomass and β-Glucan Yield from Oyster Mushroom Pleurotus ostreatus (Agaricomycetes) Mycelia through Extract Valorization. Int. J. Med. Mushrooms 2024, 26, 77–87. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Kumari, M.; Karn, S.K.; Bhambri, A.; Mahale, V.G.; Mahale, S. Submerged Cultivation and Phytochemical Analysis of Medicinal Mushrooms (Trametes sp.). Front. Fungal Biol. 2024, 5, 1414349. [Google Scholar] [CrossRef]
- Wickramasinghe, M.A.; Nadeeshani, H.; Sewwandi, S.M.; Rathnayake, I.; Kananke, T.C.; Liyanage, R. Comparison of Nutritional Composition, Bioactivities, and FTIR—ATR Microstructural Properties of Commercially Grown Four Mushroom Species in Sri Lanka; Agaricus Bisporus, Pleurotus Ostreatus, Calocybe sp. (MK-White), Ganoderma Lucidum. Food Prod. Process Nutr. 2023, 5, 43. [Google Scholar] [CrossRef]
- Ahmad, N.; Mahmood, F.; Khalil, S.A.; Zamir, R.; Fazal, H.; Abbasi, B.H. Antioxidant Activity via DPPH, Gram-Positive and Gram-Negative Antimicrobial Potential in Edible Mushrooms. Toxicol. Ind. Health 2014, 30, 826–834. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; Muthu, A.; El-Ramady, H.; Daróczi, L.; Nagy, L.; Kéki, S.; Béni, Á.; Csarnovics, I.; Prokisch, J. Optimization of Extraction Conditions to Synthesize Green Carbon Nanodots Using the Maillard Reaction. Mater. Adv. 2024, 5, 3499–3505. [Google Scholar] [CrossRef]
- Hassan, R.A.; Shafi, M.E.; Attia, K.M.; Assar, M.H. Influence of Oyster Mushroom Waste on Growth Performance, Immunity and Intestinal Morphology Compared with Antibiotics in Broiler Chickens. Front. Vet. Sci. 2020, 7, 333. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S. Sustainable Development of Carbon Nanodots Technology: Natural Products as a Carbon Source and Applications to Food Safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.-W.; Jeong, S.W.; Kim, C.H.; Lee, Y.-C.; Huh, Y.S.; Lee, J. Photoluminescent Green Carbon Nanodots from Food-Waste-Derived Sources: Large-Scale Synthesis, Properties, and Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M. Fungus-Derived Photoluminescent Carbon Nanodots for Ultrasensitive Detection of Hg2+ Ions and Photoinduced Bactericidal Activity. Sens. Actuators B Chem. 2018, 258, 172–183. [Google Scholar] [CrossRef]
- Banger, A.; Gautam, S.; Jadoun, S.; Jangid, N.K.; Srivastava, A.; Pulidindi, I.N.; Dwivedi, J.; Srivastava, M. Synthetic Methods and Applications of Carbon Nanodots. Catalysts 2023, 13, 858. [Google Scholar] [CrossRef]
- Zong, Q.; Chen, H.; Zhao, Y.; Wang, J.; Wu, J. Bioactive Carbon Dots for Tissue Engineering Applications. Smart Mater. Med. 2024, 5, 1–14. [Google Scholar] [CrossRef]
- Kerezoudi, E.N.; Mitsou, E.K.; Gioti, K.; Terzi, E.; Avgousti, I.; Panagiotou, A.; Koutrotsios, G.; Zervakis, G.I.; Mountzouris, K.C.; Tenta, R.; et al. Fermentation of Pleurotus ostreatus and Ganoderma lucidum Mushrooms and Their Extracts by the Gut Microbiota of Healthy and Osteopenic Women: Potential Prebiotic Effect and Impact of Mushroom Fermentation Products on Human Osteoblasts. Food Funct. 2021, 12, 1529–1546. [Google Scholar] [CrossRef]
- Alves, M.; Ferreira, I.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Zhao, W.-B.; Wang, R.-T.; Liu, K.-K.; Du, M.-R.; Wang, Y.; Wang, Y.-Q.; Zhou, R.; Liang, Y.-C.; Ma, R.-N.; Sui, L.-Z.; et al. Near-Infrared Carbon Nanodots for Effective Identification and Inactivation of Gram-Positive Bacteria. Nano Res. 2022, 15, 1699–1708. [Google Scholar] [CrossRef]
- Ahmed, T.; Suzauddula, M.; Akter, K.; Hossen, M.; Islam, M.N. Green Technology for Fungal Protein Extraction—A Review. Separations 2024, 11, 186. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, J.-H.; Zhao, H.; Yu, C.; Wu, J. Targeting the Outer Membrane of Gram-Negative Foodborne Pathogens for Food Safety: Compositions, Functions, and Disruption Strategies. Crit. Rev. Food Sci. Nutr. 2024, 1–14. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Hargis, B.M.; Tellez, G. Use of Prebiotics as an Alternative to Antibiotic Growth Promoters in the Poultry Industry. In Prebiotics and Probiotics-Potential Benefits in Nutrition and Health; IntechOpen: London, UK, 2019. [Google Scholar]


 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törős, G.; Béni, Á.; Peles, F.; Gulyás, G.; Prokisch, J. Comparative Analysis of Freeze-Dried Pleurotus ostreatus Mushroom Powders on Probiotic and Harmful Bacteria and Its Bioactive Compounds. J. Fungi 2025, 11, 1. https://doi.org/10.3390/jof11010001
Törős G, Béni Á, Peles F, Gulyás G, Prokisch J. Comparative Analysis of Freeze-Dried Pleurotus ostreatus Mushroom Powders on Probiotic and Harmful Bacteria and Its Bioactive Compounds. Journal of Fungi. 2025; 11(1):1. https://doi.org/10.3390/jof11010001
Chicago/Turabian StyleTörős, Gréta, Áron Béni, Ferenc Peles, Gabriella Gulyás, and József Prokisch. 2025. "Comparative Analysis of Freeze-Dried Pleurotus ostreatus Mushroom Powders on Probiotic and Harmful Bacteria and Its Bioactive Compounds" Journal of Fungi 11, no. 1: 1. https://doi.org/10.3390/jof11010001
APA StyleTörős, G., Béni, Á., Peles, F., Gulyás, G., & Prokisch, J. (2025). Comparative Analysis of Freeze-Dried Pleurotus ostreatus Mushroom Powders on Probiotic and Harmful Bacteria and Its Bioactive Compounds. Journal of Fungi, 11(1), 1. https://doi.org/10.3390/jof11010001










