Abstract
Plant diseases caused by pathogenic fungi or oomycetes seriously affect crop growth and the quality and yield of products. A series of novel 1,2,4-triazole derivatives containing carboxamide fragments based on amide fragments widely used in fungicides and the commercialized mefentrifluconazole were designed and synthesized. Their antifungal activities were evaluated against seven kinds of phytopathogenic fungi/oomycete. Results showed that most compounds had similar or better antifungal activities compared to mefentrifluconazole’s inhibitory activity against Physalospora piricola, especially compound 6h (92%), which possessed outstanding activity. Compound 6h (EC50 = 13.095 μg/mL) showed a better effect than that of mefentrifluconazole (EC50 = 39.516 μg/mL). Compound 5j (90%) displayed outstanding anti-oomycete activity against Phytophthora capsici, with an EC50 value of 17.362 μg/mL, far superior to that of mefentrifluconazole (EC50 = 75.433 μg/mL). The result of molecular docking showed that compounds 5j and 6h possessed a stronger affinity for 14α-demethylase (CYP51). This study provides a new approach to expanding the fungicidal spectrum of 1,2,4-triazole derivatives.
1. Introduction
Phytopathogenic fungi are responsible for about two-thirds of infectious plant diseases and pose a great threat to crop production [1,2,3]. They not only significantly affect the production and quality of crops but also endangers the health of humans and animals through the creation and enrichment of toxins [4,5]. In particular, Physalospora piricola (P. piricola), which infects apple branches, fruit, and leaves, has a serious impact on the growth and yield of fruit trees and is the main causal agent of apple ring rot [6]. It is widely distributed in most apple-growing areas in the country but is most severe in eastern China (Liaoning, Shandong, Henan, and Hebei provinces), where summer temperatures and rainfall are high. The earliest record of a severe loss caused by this disease in China was in Chengdu of Sichuan Province in 1942, when the disease caused approximately 20% fruit loss of Yuxia (Mapi) apples before harvest and another 79% loss in storage [7]. Since the 1980s, the importance of apple ring rot in eastern China has increased with the widespread planting of the cultivar Fuji. The emergence of pathogenic fungi like P. piricola, showing resistance to current market fungicides, underscores the need for new compounds [8]. These new antifungals are crucial not only because they can overcome the resistance developed by pathogens but also to expand the arsenal of available treatments. Therefore, there is an urgent and continuous need to seek novel and effective approaches to control pathogenic fungi and mitigate losses caused by P. piricola. Diversified modification from novel leading compounds is an important and widely applied strategy in the continuous innovation and development of chemical fungicides [9,10,11]. Thus, the design and synthesis of new active molecules is one of the most important recent milestones in fungicide research.
Triazole fungicides mainly belong to sterol demethylation inhibitor (DMI) fungicides, which occupy an important position in the field of fungicides, such as mefentrifluconazole, tetraconazole, hexaconzole, cyproconaole, etc. (Figure 1) [12]. Mefentrifluconazole, [(2RS)-2-(4-(4-chlorophenoxy)-α,α,α-trifluoro-o-tolyl)-1-(1H-1,2,4-triazol-1-yl)propan-2-ol], is the first isopropanol triazole broad-spectrum fungicide developed by the company Badische Anilin-und-Soda-Fabrik (BASF) and it has become widely used to control a range of fungal crop diseases [13]. Similar to traditional triazole fungicides, the nitrogen atom on its heterocyclic ring has a lone pair of electrons, which can be combined with the iron atom in the heme-iron active center of 14α-demethylase (CYP51) in pathogenic fungi by ligand bonding to form an iron porphyrin-centered ligand complex, resulting in the reduction in the combination of oxygen atoms with CYP51 and the inhibition of the catalytic capacity of CYP51 oxidation, which results in the blocking of the 14α-demethylation reaction, thus interfering with the synthesis of fungal ergosterol [14,15]. On the one hand, ergosterol deficiency can lead to abnormal cell membrane structure and function, resulting in changes in membrane bonding and enzyme activity, making fungal cells vulnerable to damage and even causing dysfunction in the activation of ergosterol hormones, which affects cell growth and proliferation [16,17]; on the other hand, the blockage of the 14α-demethylation reaction can lead to the accumulation of large quantities of 24-methylenedihydrolanosterol, which inhibits the flow of fungal cell membranes [18]. In brief, mefentrifluconazole impedes the biosynthesis of ergosterol in the cell membrane of pathogens, causing changes in its biological structure, inhibiting cell growth, and finally achieving the effects of being bacteriostatic and bactericidal [19,20,21]. Unlike the existing triazole fungicides, the unique isopropanol moiety of mefentrifluconazole can be easily transformed into a “hook” that binds tightly with the target protein, resulting in potent fungicidal activity with little cross-resistance [19,22,23,24]. Mefentrifluconazole can be used to control many fungal diseases in more than 60 species of crop plants, such as potatoes, pome fruits, and soybeans, and has been registered in many countries [20]. Among them, mefentrifluconazole has been registered for the control of apple brown spot (Marssonina mali) in China, yet no studies have been conducted to determine its inhibitory activity against P. piricola and it is less effective in inhibiting oomycetes.
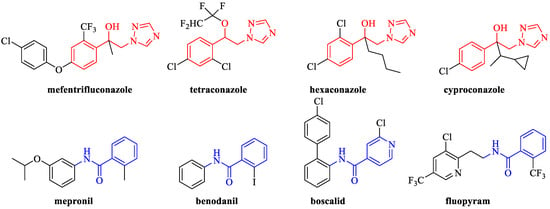
Figure 1.
Some commercialized triazole fungicides and SDHI fungicides contain amide fragments.
Studies have shown that mefentrifluconazole can also be used in combination with succinate dehydrogenase inhibitor (SDHI) fungicides such as florylpicoxamid, fluxapyroxad, boscalid, and bixafen, thereby delaying the development of fungicide resistance. SDHI fungicides disrupt the mitochondrial respiratory chain by inhibiting SDH, which leads to fungal death [25,26]. Their mechanisms of action suggest that these structurally dissimilar substances possess the common pharmacophore carboxamide, which consists of three parts, namely a polar moiety, amide bond, and hydrophobic tail [27,28,29]. Among them, amide bonds are the core feature of SDHIs and the introduction of structurally diverse benzene rings flanking the amide bond enables the compounds to exhibit good broad-spectrum activity [30]. It is desired to enhance the bactericidal activity of triazole derivatives by introducing active groups to give a broader spectrum of bioactivity.
In this study, the commercial fungicide mefentrifluconazole was used as the lead compound, retained its isopropanol triazole part, and then introduced amide fragments by using active substructure splicing. A total of 34 novel 1,2,4-triazole derivatives containing carboxamide fragments were designed and synthesized (Figure 2). Their fungicidal activities against seven kinds of phytopathogenic fungi/oomycete at 50 μg/mL were evaluated and the mechanism of action of highly effective compounds 5j and 6h were preliminarily evaluated.
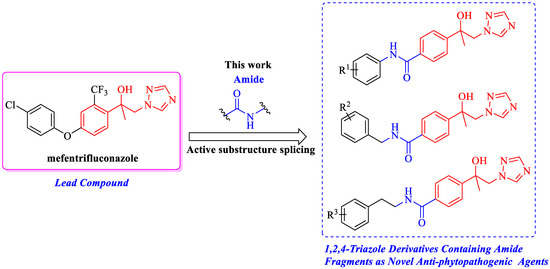
Figure 2.
Design of 1,2,4-triazole derivatives containing carboxamide fragments.
2. Materials and Methods
2.1. Chemicals and Instruments
All reagents used were analytical reagent (AR) grade or chemically pure (CR), which were purchased from commercial sources (Tianjin Guangda Chemical Reagents Ltd., Tianjin, China). The melting point of the target compounds were measured on an X-4 binocular microscope (Beijing Zhongke Instrument Co., Ltd., Beijing, China). Nuclear magnetic resonance (NMR) spectra were acquired with a 400 MHz (100 MHz for 13C) instrument (Bruker, Billerica, MA, USA) at room temperature. Chemical shifts were measured relative to residual solvent peaks of CDCl3 (1H: δ = 7.26 ppm; 13C: δ = 77.0 ppm) and DMSO-d6 (1H: δ = 2.5 and 3.3 ppm; 13C: δ = 39.9 ppm) as internal standards. The following abbreviations are used to designate chemical shift multiplicities: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, m = multiplet, and brs = broad singlet. High-resolution mass spectrometry (HRMS) data were recorded with a quadrupol Fourier transform-electrospray ionization (QFT-ESI) instrument (Varian, Palo Alto, CA, USA).
2.2. Synthetic Procedures
2.2.1. Synthesis of Compounds 5a–5n, 6a–6m, and 7a–7g
To a stirred solution of NaH (60% in mineral oil, 1.840 g, 46.0 mmol) in dry tetrahydrofuran (THF) (30 mL), trimethylsulfonium iodide (Me3S+I−) (8.163 g, 40.0 mmol) in dry dimethyl sulfoxide (DMSO) (50 mL) was added at room temperature under nitrogen. The reaction mixture was left stirring for 1 h at room temperature. A solution of compound 1 (3.564 g, 20.0 mmol) in dry DMSO (20 mL) was added dropwise and the reaction mixture was left stirring for 14 h. The reaction mixture was quenched with a saturated solution of ammonium chloride, followed by extractions with ethyl acetate (EtOAc) (3 × 20 mL). The combined organic layers were dried and the solvent was evaporated. The crude product was purified by column chromatography to obtain compound 2. Light yellow solid, 75% yield, m.p. 46–48 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.93 (d, J = 8.1 Hz, 2H), 7.49 (d, J = 8.0 Hz, 2H), 3.84 (s, 3H), 3.04 (d, J = 6.0 Hz, 1H), 2.79 (d, J = 5.2 Hz, 1H), 1.67 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 147.0, 129.7, 129.2, 126.2, 57.0, 56.7, 52.6, 21.5.
Compound 2 (1.922 g, 10.0 mmol), 1,2,4-Triazole (2.763 g, 40.0 mmol), and NaOH (0.800 g, 20.0 mmol.) were added to N,N-dimethylformamide (DMF) (50 mL) and stirred at 110 °C for 4 h, cooled to room temperature, saturated NH4Cl solution was added, and the aqueous phase was extracted with EtOAc (3 × 15 mL). The combined organic phases were washed with saturated brine, dried over anhydrous magnesium sulfate, filtered with suction, spin-dried, and recrystallized from dichloromethane ether to obtain compound 3. White solid, 80% yield, m.p. 98–100 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.23 (s, 1H), 7.89 (d, J = 8.2 Hz, 2H), 7.83 (s, 1H), 7.57 (d, J = 8.0 Hz, 2H), 5.73 (s, 1H), 4.41 (s, 2H), 3.84 (s, 3H), 1.46 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.6, 151.8, 150.9, 145.4, 129.3, 128.6, 126.2, 73.2, 59.7, 52.6, 27.5.
To a stirred solution of 3 (1.306 g, 5.0 mmol) in THF (30 mL), NaOH (0.600 g, 15.0 mmol.) in H2O (30 mL) was added and the reaction mixture was stirred at room temperature for 6 h. After completion of the reaction, the solvent was removed in vacuo. Then, dilute HCl (10 mL, 1 M) was added dropwise to the reaction mixture to adjust the pH to 3–5, the solid was filtered and dried, and compound 4 was obtained. White solid, 74% yield, m.p. 199–202 °C; 1H NMR (400 MHz, DMSO-d6) δ 12.86 (s, 1H), 8.23 (s, 1H), 7.90–7.82 (m, 3H), 7.55 (d, J = 8.2 Hz, 2H), 5.70 (s, 1H), 4.41 (s, 2H), 1.45 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 167.7, 151.3, 150.8, 145.4, 129.8, 129.5, 126.0, 73.2, 59.7, 27.6.
To a stirred solution of 4 (0.247 g, 1.0 mmol) in CH2Cl2 (3 mL), Et3N (0.202 g, 2.0 mmol), 1-hydroxybenzotriazole (HOBt) (0.203 g, 1.5 mmol), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (0.288 g, 1.5 mmol) were added and the reaction mixture was stirred at room temperature for 30 min. Subsequently, various substituted aromatic amines (1.5 mmol) were added and the resulting solution was stirred at room temperature for 12 h. The reaction mixture was diluted with H2O (10 mL), followed by extractions with EtOAc (3 × 15 mL). The combined organic phase was dried with anhydrous sodium sulfate and the solvent was evaporated. The crude product was purified by column chromatography to obtain compounds 5a–5n, 6a–6m, and 7a–7g.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-phenylbenzamide (5a). White solid, 85% yield, m.p. 181–183 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.21 (s, 1H), 8.27 (s, 1H), 7.93–7.84 (m, 3H), 7.77 (d, J = 7.9 Hz, 2H), 7.59 (d, J = 8.0 Hz, 2H), 7.35 (t, J = 8.1 Hz, 2H), 7.09 (t, J = 7.7 Hz, 1H), 5.71 (s, 1H), 4.45 (s, 2H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.8, 150.9, 150.0, 145.4, 139.7, 133.9, 129.1, 127.8, 125.8, 124.1, 120.8, 73.2, 59.8, 27.8; HR-MS (ESI): calcd for C18H18N4O2 [M+H]+ 323.1503, found (ESI+) 323.1509.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(o-tolyl)benzamide (5b). White solid, 84% yield, m.p. 145–147 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.83 (s, 1H), 8.27 (s, 1H), 7.94–7.84 (m, 3H), 7.58 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 7.8 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.19 (m, 2H), 5.70 (s, 1H), 4.44 (s, 2H), 2.23 (s, 3H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.6, 151.0, 149.9, 145.4, 137.0, 134.3, 133.4, 130.8, 127.8, 127.1, 126.5, 125.8, 73.2, 59.8, 27.8, 18.4; HR-MS (ESI): calcd for C19H20N4O2 [M+H]+ 337.1660, found (ESI+) 337.1651.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(p-tolyl)benzamide (5c). White solid, 79% yield, m.p. 167–169 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 8.26 (s, 1H), 7.91–7.84 (m, 3H), 7.65 (d, J = 8.0 Hz, 2H), 7.58 (d, J = 8.1 Hz, 2H), 7.15 (d, J = 8.0 Hz, 2H), 5.70 (s, 1H), 4.44 (s, 2H), 2.28 (s, 3H), 1.46 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.6, 151.0, 149.9, 145.4, 137.2, 134.0, 133.1, 129.5, 127.8, 125.7, 120.9, 73.2, 59.8, 27.7, 21.0; HR-MS (ESI): calcd for C19H20N4O2 [M+H]+ 337.1660, found (ESI+) 337.1653.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(2-methoxyphenyl)benzamide (5d). Brown solid, 80% yield, m.p. 156–158 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.38 (s, 1H), 8.26 (s, 1H), 7.91–7.86 (t, 3H), 7.77 (d, J = 7.8 Hz, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.18 (t, J = 7.8 Hz, 1H), 7.09 (d, J = 8.2 Hz, 1H), 6.97 (t, J = 7.6 Hz, 1H), 5.71 (s, 1H), 4.44 (s, 2H), 3.83 (s, 3H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 167.7, 165.3, 152.0, 151.0, 150.0, 145.4, 133.4, 129.5, 127.6, 125.9, 124.8, 120.7, 111.9, 73.2, 59.8, 56.2, 27.7; HR-MS (ESI): calcd for C19H20N4O3 [M+H]+ 353.1609, found (ESI+) 353.1617.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(3-methoxyphenyl)benzamide (5e). Yellow crystals, 67% yield, m.p. 116–118 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H), 8.27 (s, 1H), 7.91–7.83 (m, 3H), 7.59 (d, J = 8.0 Hz, 2H), 7.48 (s, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.24 (t, J = 8.1 Hz, 1H), 6.68 (d, J = 8.2 Hz, 1H), 5.71 (s, 1H), 4.44 (s, 2H), 3.75 (s, 3H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.9, 159.9, 150.9, 150.0, 145.4, 140.9, 133.9, 129.9, 127.8, 125.8, 113.0, 109.6, 106.5, 73.2, 59.8, 55.5, 27.7; HR-MS (ESI): calcd for C19H20N4O3 [M+H]+ 353.1609, found (ESI+) 353.1619.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(4-methoxyphenyl)benzamide (5f). White solid, 77% yield, m.p. 175–178 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H), 8.26 (s, 1H), 7.90–7.84 (m, 3H), 7.67 (d, J = 8.6 Hz, 2H), 7.57 (d, J = 8.1 Hz, 2H), 6.92 (d, J = 8.6 Hz, 2H), 5.70 (s, 1H), 4.44 (s, 2H), 3.74 (s, 3H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.4, 156.0, 151.0, 149.8, 145.4, 133.9, 132.7, 127.7, 125.7, 122.5, 114.2, 73.2, 59.8, 55.7, 27.7; HR-MS (ESI): calcd for C19H20N4O3 [M+H]+ 353.1609, found (ESI+) 353.1615.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(3-(trifluoromethyl)phenyl)benzamide (5g). White solid, 51% yield, m.p. 65–67 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.52 (s, 1H), 8.26 (d, J = 6.0 Hz, 2H), 8.06 (d, J = 8.2 Hz, 1H), 7.92 (d, J = 8.2 Hz, 2H), 7.85 (s, 1H), 7.61 (d, J = 8.4 Hz, 3H), 7.45 (d, J = 8.1 Hz, 1H), 5.73 (s, 1H), 4.45 (s, 2H), 1.48 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.2, 150.9, 150.4, 145.4, 140.5, 133.3, 130.4, 129.9 (d, J = 31.7 Hz), 127.9, 125.9, 124.3, 123.3, 120.4, 116.8 (d, J = 4.3 Hz), 73.2, 59.8, 27.7; HR-MS (ESI): calcd for C19H17F3N4O2 [M+H]+ 391.1377, found (ESI+) 391.1370.
N-(3-Fluorophenyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (5h). White solid, 44% yield, m.p. 132–135 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.39 (s, 1H), 8.27 (s, 1H), 7.92–7.83 (m, 3H), 7.76 (d, J = 12.0 Hz, 1H), 7.63–7.53 (m, 3H), 7.38 (q, J = 7.8 Hz, 1H), 6.92 (t, J = 8.8 Hz, 1H), 5.72 (s, 1H), 4.45 (s, 2H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.1, 162.6 (d, J = 240.9 Hz), 150.9, 150.2, 145.4, 141.5 (d, J = 10.9 Hz), 133.5, 130.7 (d, J = 9.5 Hz), 127.9, 125.8, 116.5, 110.6 (d, J = 21.1 Hz), 107.4 (d, J = 26.2 Hz), 73.2, 59.8, 27.8; HR-MS (ESI): calcd for C18H17FN4O2 [M+H]+ 341.1409, found (ESI+) 341.1407.
N-(4-Fluorophenyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (5i). White solid, 74% yield, m.p. 154–157 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.26 (s, 1H), 8.26 (s, 1H), 7.91–7.84 (m, 3H), 7.82–7.75 (m, 2H), 7.59 (d, J = 8.0 Hz, 2H), 7.19 (t, J = 8.9 Hz, 2H), 5.71 (s, 1H), 4.44 (s, 2H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.8, 158.8 (d, J = 240.4 Hz), 150.9, 150.0, 145.4, 136.0, 133.7, 127.8, 125.8, 122. 7 (d, J = 7.8 Hz), 115.7 (d, J = 22.2 Hz), 73.2, 59.8, 27.7; HR-MS (ESI): calcd for C18H17FN4O2 [M+H]+ 341.1409, found (ESI+) 341.1405.
N-(3-Chlorophenyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (5j). Yellow solid, 77% yield, m.p. 115–118 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.36 (s, 1H), 8.26 (s, 1H), 7.96 (s, 1H), 7.90–7.83 (m, 3H), 7.71 (d, J = 8.3 Hz, 1H), 7.59 (d, J = 8.0 Hz, 2H), 7.37 (t, J = 8.1 Hz, 1H), 7.15 (d, J = 8.1 Hz, 1H), 5.72 (s, 1H), 4.44 (s, 2H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 171.0, 166.1, 151.0, 150.3, 145.4, 141.2, 133.4, 130.8, 127.9, 125.9, 123.8, 120.2, 119.1, 73.2, 59.8, 27.8; HR-MS (ESI): calcd for C18H17ClN4O2 [M+H]+ 357.1113, found (ESI+) 357.1105.
N-(4-Chlorophenyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (5k). Light yellow solid, 54% yield, m.p. 215–217 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 8.27 (s, 1H), 7.92–7.77 (m, 5H), 7.59 (d, J = 8.1 Hz, 2H), 7.41 (d, J = 8.5 Hz, 2H), 5.72 (s, 1H), 4.44 (s, 2H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.0, 150.9, 150.1, 145.4, 138.7, 133.6, 129.0, 127.9, 127.7, 125.8, 122.4, 73.2, 59.8, 27.8; HR-MS (ESI): calcd for C18H17ClN4O2 [M+H]+ 357.1113, found (ESI+) 357.1104.
N-(3-Bromophenyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (5l). White solid, 50% yield, m.p. 76–79 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.35 (s, 1H), 8.27 (s, 1H), 8.11 (s, 1H), 7.91–7.84 (m, 3H), 7.76 (d, J = 7.6 Hz, 1H), 7.60 (d, J = 8.1 Hz, 2H), 7.31 (q, J = 8.8, 8.4 Hz, 2H), 5.72 (s, 1H), 4.45 (s, 2H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.1, 150.9, 150.3, 145.4, 141.3, 133.4, 131.1, 127.9, 126.7, 125.8, 123.0, 121.9, 119.5, 73.2, 59.8, 27.8; HR-MS (ESI): calcd for C18H17BrN4O2 [M+H]+ 401.0608, found (ESI+) 401.0616.
N-(4-Bromophenyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (5m). White solid, 75% yield, m.p. 219–222 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.33 (s, 1H), 8.27 (s, 1H), 7.91–7.83 (m, 3H), 7.77 (d, J = 8.6 Hz, 2H), 7.59 (d, J = 8.1 Hz, 2H), 7.53 (d, J = 8.6 Hz, 2H), 5.71 (s, 1H), 4.44 (s, 2H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.0, 150.9, 150.2, 145.4, 139.1, 133.6, 131.9, 127.9, 125.8, 122.7, 115.8, 73.2, 59.8, 27.8; HR-MS (ESI): calcd for C18H17BrN4O2 [M+H]+ 401.0608, found (ESI+) 401.0614.
N-(3-Chloro-4-methoxyphenyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (5n). White solid, 71% yield, m.p. 175–177 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.20 (s, 1H), 8.26 (s, 1H), 7.93 (s, 1H), 7.90–7.84 (m, 3H), 7.67 (d, J = 8.9 Hz, 1H), 7.58 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 8.9 Hz, 1H), 5.71 (s, 1H), 4.44 (s, 2H), 3.84 (s, 3H), 1.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.6, 151.3, 150.9, 150.0, 145.4, 133.6, 133.4, 127.8, 125.8, 122.4, 120.9, 120.7, 113.3, 73.2, 59.8, 56.7, 27.7; HR-MS (ESI): calcd for C19H19ClN4O3 [M+H]+ 387.1219, found (ESI+) 387.1213.
N-Benzyl-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6a). White solid, 81% yield, m.p. 131–134 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.05–8.98 (m, 1H), 8.24 (s, 1H), 7.84 (d, J = 7.4 Hz, 3H), 7.53 (d, J = 8.0 Hz, 2H), 7.32 (s, 5H), 5.67 (s, 1H), 4.48 (d, J = 5.9 Hz, 2H), 4.41 (s, 2H), 1.45 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 150.9, 149.6, 145.4, 140.2, 133.2, 128.8, 127.7, 127.4, 127.2, 125.7, 73.2, 59.8, 43.1, 27.7; HR-MS (ESI): calcd for C19H20N4O2 [M+H]+ 337.1660, found (ESI+) 337.1652.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(2-methylbenzyl)benzamide (6b). White solid, 83% yield, m.p. 118–121 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.87 (m, 1H), 8.25 (s, 1H), 7.84 (d, J = 5.7 Hz, 3H), 7.53 (d, J = 7.9 Hz, 2H), 7.23 (d, J = 5.8 Hz, 1H), 7.16 (s, 3H), 5.67 (s, 1H), 4.45 (d, J = 5.5 Hz, 2H), 4.42 (s, 2H), 2.32 (s, 3H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 150.9, 149.6, 145.4, 137.7, 136.0, 133.3, 130.4, 127.8, 127.4, 127.2, 126.2, 125.7, 73.2, 59.8, 41.1, 27.7, 19.3; HR-MS (ESI): calcd for C20H22N4O2 [M+H]+ 351.1816, found (ESI+) 351.1808.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(3-methylbenzyl)benzamide (6c). White solid, 85% yield, m.p. 55–57 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.97 (t, J = 6.3 Hz, 1H), 8.24 (s, 1H), 7.83 (d, J = 8.0 Hz, 3H), 7.52 (d, J = 8.0 Hz, 2H), 7.20 (t, J = 7.6 Hz, 1H), 7.14–7.08 (m, 2H), 7.05 (d, J = 7.5 Hz, 1H), 5.66 (s, 1H), 4.42 (dd, J = 10.8, 5.2 Hz, 1H), 2.28 (s, 3H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.4, 150.9, 149.6, 145.4, 140.1, 137.8, 133.3, 128.7, 128.3, 127.9, 127.4, 125.7, 124.8, 73.2, 59.8, 43.0, 27.7, 21.6; HR-MS (ESI): calcd for C20H22N4O2 [M+H]+ 351.1816, found (ESI+) 351.1821.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(4-methylbenzyl)benzamide (6d). White solid, 75% yield, m.p. 141–144 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.96 (t, J = 5.1 Hz, 1H), 8.24 (s, 1H), 7.82 (d, J = 9.4 Hz, 3H), 7.52 (d, J = 7.8 Hz, 2H), 7.20 (d, J = 7.8 Hz, 2H), 7.12 (d, J = 7.4 Hz, 2H), 5.66 (s, 1H), 4.42 (m, 4H), 2.27 (s, 3H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.4, 150.9, 149.6, 145.4, 137.2, 136.2, 133.3, 129.3, 127.7, 127.4, 125.7, 73.2, 59.8, 42.8, 27.7, 21.2; HR-MS (ESI): calcd for C20H22N4O2 [M+H]+ 351.1816, found (ESI+) 351.1809.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(4-methoxybenzyl)benzamide (6e). White solid, 85% yield, m.p. 115–118 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.96–8.89 (m, 1H), 8.24 (s, 1H), 7.84–7.79 (m, 3H), 7.51 (d, J = 8.0 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 6.88 (d, J = 8.0 Hz, 2H), 5.66 (s, 1H), 4.40 (d, J = 6.3 Hz, 4H), 3.72 (s, 3H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.4, 158.7, 150.9, 149.5, 145.3, 133.3, 132.2, 129.1, 127.4, 125.7, 114.2, 73.1, 59.8, 55.6, 42.5, 27.7; HR-MS (ESI): calcd for C20H22N4O3 [M+H]+ 367.1765, found (ESI+) 367.1761.
N-(2,3-Dimethoxybenzyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6f). White solid, 58% yield, m.p. 50–52 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.90–8.83 (m, 1H), 8.24 (s, 1H), 7.83 (d, J = 7.6 Hz, 3H), 7.52 (d, J = 8.0 Hz, 2H), 7.01 (t, J = 8.1 Hz, 1H), 6.94 (d, J = 8.1 Hz, 1H), 6.84 (d, J = 7.6 Hz, 1H), 5.66 (s, 1H), 4.48 (d, J = 5.9 Hz, 2H), 4.41 (s, 2H), 3.80 (s, 3H), 3.77 (s, 3H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 152.8, 150.9, 149.7, 146.6, 145.4, 133.4, 133.3, 127.4, 125.7, 124.3, 120.3, 112.0, 73.2, 60.5, 59.8, 56.2, 37.8, 27.7; HR-MS (ESI): calcd for C21H24N4O4 [M+H]+ 397.1871, found (ESI+) 397.1867.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(4-(trifluoromethyl)benzyl)benzamide (6g). White solid, 80% yield, m.p. 63–65 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.17–9.07 (m, 1H), 8.25 (s, 1H), 7.84 (d, J = 6.0 Hz, 3H), 7.69 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 8.0 Hz, 4H), 5.68 (s, 1H), 4.55 (d, J = 5.8 Hz, 2H), 4.42 (s, 2H), 1.45 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.7, 150.9, 149.8, 145.4, 145.1, 133.0, 128.4, 128.0 (d, J = 31.8 Hz), 127.4, 126.2, 125.8, 125.7 (d, J = 4.0 Hz), 123.5, 73.2, 59.8, 42.8, 27.7; HR-MS (ESI): calcd for C20H19F3N4O2 [M+H]+ 405.1533, found (ESI+) 405.1528.
N-(2-Fluorobenzyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6h). White solid, 57% yield, m.p. 165–167 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.03–8.97 (m, 1H), 8.25 (s, 1H), 7.84 (d, J = 7.4 Hz, 3H), 7.53 (d, J = 8.1 Hz, 2H), 7.33 (dt, J = 24.6, 7.2 Hz, 2H), 7.20–7.15 (m, 2H), 5.67 (s, 1H), 4.51 (d, J = 5.7 Hz, 2H), 4.41 (s, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.6, 160.5 (d, J = 244.0 Hz), 150.9, 149.7, 145.4, 133.0, 129.9 (d, J = 4.4 Hz), 129.3 (d, J = 8.2 Hz), 127.4, 125.7, 124.8 (d, J = 3.4 Hz), 115.6 (d, J = 21.3 Hz), 73.2, 59.8, 36.9 (d, J = 4.7 Hz), 27.7; HR-MS (ESI): calcd for C19H19FN4O2 [M+H]+ 355.1565, found (ESI+) 355.1572.
N-(4-Fluorobenzyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6i). White solid, 65% yield, m.p. 149–151 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.05–8.99 (m, 1H), 8.24 (s, 1H), 7.83 (d, J = 9.3 Hz, 3H), 7.52 (d, J = 8.0 Hz, 2H), 7.35 (t, J = 7.0 Hz, 2H), 7.14 (t, J = 8.8 Hz, 2H), 5.67 (s, 1H), 4.45 (d, J = 5.7 Hz, 2H), 4.41 (s, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 161.7 (d, J = 242.0 Hz), 150.9, 149.6, 145.4, 136.4 (d, J = 3.1 Hz), 133.2, 129.7 (d, J = 8.0 Hz), 127.4, 125.7, 115.5 (d, J = 21.3 Hz), 73.2, 59.8, 42.4, 27.7; HR-MS (ESI): calcd for C19H19FN4O2 [M+H]+ 355.1565, found (ESI+) 355.1569.
N-(2-Chlorobenzyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6j). White solid, 66% yield, m.p. 150–153 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.06–8.98 (m, 1H), 8.26 (s, 1H), 7.86 (d, J = 8.2 Hz, 3H), 7.55 (d, J = 8.1 Hz, 2H), 7.46 (d, J = 7.3 Hz, 1H), 7.32 (dd, J = 13.5, 7.0 Hz, 3H), 5.68 (s, 1H), 4.54 (d, J = 5.7 Hz, 2H), 4.42 (s, 2H), 1.45 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.8, 150.9, 149.8, 145.4, 136.9, 133.0, 132.4, 129.6, 129.1, 127.7, 127.5, 125.8, 73.2, 59.8, 41.0, 27.7; HR-MS (ESI): calcd for C19H19ClN4O2 [M+H]+ 371.1270, found (ESI+) 371.1267.
N-(3-Chlorobenzyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6k). White solid, 82% yield, m.p. 132–135 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.09–9.02 (m, 1H), 8.25 (s, 1H), 7.83 (d, J = 8.3 Hz, 3H), 7.53 (d, J = 8.2 Hz, 2H), 7.35 (t, J = 7.7 Hz, 2H), 7.29 (t, J = 8.5 Hz, 2H), 5.67 (s, 1H), 4.47 (d, J = 5.9 Hz, 2H), 4.41 (d, J = 3.7 Hz, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.6, 150.9, 149.7, 145.4, 142.8, 133.5, 133.0, 130.7, 127.4, 127.2, 126.4, 125.8, 73.2, 59.8, 42.6, 27.7; HR-MS (ESI): calcd for C19H19ClN4O2 [M+H]+ 371.1270, found (ESI+) 371.1265.
N-(4-Chlorobenzyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6l). White solid, 60% yield, m.p. 148–151 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.02 (m, 1H), 8.24 (s, 1H), 7.82 (d, J = 9.4 Hz, 3H), 7.52 (d, J = 8.0 Hz, 2H), 7.36 (q, J = 9.6, 8.9 Hz, 4H), 5.66 (s, 1H), 4.45 (d, J = 5.7 Hz, 2H), 4.41 (s, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 150.9, 149.7, 145.4, 139.3, 133.1, 131.8, 129.6, 128.7, 127.4, 125.7, 73.2, 59.8, 42.5, 27.7; HR-MS (ESI): calcd for C19H19ClN4O2 [M+H]+ 371.1270, found (ESI+) 371.1273.
N-(4-Bromobenzyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (6m). White solid, 82% yield, m.p. 74–77 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.05 (m, 1H), 8.23 (s, 1H), 7.85–7.78 (m, 3H), 7.51 (d, J = 8.1 Hz, 4H), 7.27 (d, J = 8.1 Hz, 2H), 5.72 (s, 1H), 4.42 (d, J = 8.3 Hz, 4H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.7, 150.9, 149.6, 145.3, 139.6, 133.0, 131.6, 130.0, 127.4, 125.7, 120.2, 73.2, 59.8, 42.5, 27.7; HR-MS (ESI): calcd for C19H19BrN4O2 [M+H]+ 415.0765, found (ESI+) 415.0763.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-phenethylbenzamide (7a). White solid, 74% yield, m.p. 155–158 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.52 (m, 1H), 8.24 (s, 1H), 7.85 (s, 1H), 7.76 (d, J = 8.1 Hz, 2H), 7.51 (d, J = 8.0 Hz, 2H), 7.25 (m, 5H), 5.65 (s, 1H), 4.40 (s, 2H), 3.48 (q, J = 8.0, 7.4 Hz, 2H), 2.84 (t, J = 7.5 Hz, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 150.9, 149.4, 145.4, 140.1, 133.5, 129.2, 128.9, 127.3, 126.6, 125.6, 73.1, 59.8, 41.4, 35.6, 27.6; HR-MS (ESI): calcd for C20H22N4O2 [M+H]+ 351.1816, found (ESI+) 351.1821.
4-(2-Hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)-N-(4-methoxyphenethyl)benzamide (7b). White solid, 62% yield, m.p. 138–141 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.53–8.46 (m, 1H), 8.24 (s, 1H), 7.85 (s, 1H), 7.76 (d, J = 8.1 Hz, 2H), 7.51 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 8.1 Hz, 2H), 6.86 (d, J = 8.2 Hz, 2H), 5.66 (s, 1H), 4.41 (s, 2H), 3.71 (s, 3H), 3.43 (q, J = 6.8 Hz, 2H), 2.77 (t, J = 7.6 Hz, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 158.2, 150.9, 149.4, 145.4, 133.5, 131.9, 130.1, 127.3, 125.6, 114.3, 73.1, 59.8, 55.5, 41.6, 34.7, 27.6; HR-MS (ESI): calcd for C21H24N4O3 [M+H]+ 381.1922, found (ESI+) 381.1929.
N-(2-Fluorophenethyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (7c). Light pink solid, 73% yield, m.p. 149–151 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.55 (m, 1H), 8.24 (s, 1H), 7.85 (s, 1H), 7.74 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.28 (m, 2H), 7.14 (q, J = 8.8, 7.4 Hz, 2H), 5.65 (s, 1H), 4.40 (s, 2H), 3.48 (q, J = 6.7 Hz, 2H), 2.88 (t, J = 7.5 Hz, 2H), 1.43 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 161.3 (d, J = 243.3 Hz), 150.9, 149.4, 145.3, 133.5, 131.7 (d, J = 4.9 Hz), 128.8 (d, J = 8.0 Hz), 127.2, 126.6 (d, J = 16.0 Hz), 125.6, 124.9 (d, J = 3.2 Hz), 115.6 (d, J = 21.9 Hz), 73.1, 59.8, 41.2, 29.1, 27.6; HR-MS (ESI): calcd for C20H21FN4O2 [M+H]+ 369.1722, found (ESI+) 369.1731.
N-(4-Fluorophenethyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (7d). White solid, 74% yield, m.p. 160–162 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.50 (m, 1H), 8.24 (s, 1H), 7.84 (s, 1H), 7.74 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.27 (t, J = 7.0 Hz, 2H), 7.11 (t, J = 8.7 Hz, 2H), 5.65 (s, 1H), 4.40 (s, 2H), 3.46 (q, J = 6.8 Hz, 2H), 2.83 (t, J = 7.5 Hz, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 161.3 (d, J = 241.3 Hz), 150.9, 149.4, 145.3, 136.2 (d, J = 3.0 Hz), 133.5, 130.9 (d, J = 7.9 Hz), 127.3, 125.6, 115.5 (d, J = 21.0 Hz), 73.1, 59.8, 41.3, 34.7, 27.6; HR-MS (ESI): calcd for C20H21FN4O2 [M+H]+ 369.1722, found (ESI+) 369.1719.
N-(4-Chlorophenethyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (7e). White solid, 66% yield, m.p. 185–188 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.50 (m, 1H), 8.24 (s, 1H), 7.84 (s, 1H), 7.74 (d, J = 8.1 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.1 Hz, 2H), 7.26 (d, J = 8.1 Hz, 2H), 5.65 (s, 1H), 4.40 (s, 2H), 3.47 (q, J = 6.8 Hz, 2H), 2.83 (t, J = 7.3 Hz, 2H), 1.43 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 150.9, 149.4, 145.3, 139.1, 133.5, 131.1, 128.7, 127.2, 125.6, 73.1, 59.8, 41.1, 34.8, 27.6; HR-MS (ESI): calcd for C20H21ClN4O2 [M+H]+ 385.1426, found (ESI+) 385.1422.
N-(2-Bromophenethyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (7f). White solid, 77% yield, m.p. 57–59 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.55 (m, 1H), 8.24 (s, 1H), 7.84 (s, 1H), 7.75 (d, J = 8.1 Hz, 2H), 7.60 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 6.8 Hz, 2H), 7.16 (t, J = 8.0 Hz, 1H), 5.65 (s, 1H), 4.40 (s, 2H), 3.50 (q, J = 6.8 Hz, 2H), 2.98 (t, J = 7.3 Hz, 2H), 1.44 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.6, 150.9, 149.4, 145.4, 139.1, 133.5, 133.0, 131.6, 129.0, 128.3, 127.3, 125.6, 124.5, 73.1, 59.8, 41.1, 35.8, 27.6; HR-MS (ESI): calcd for C20H21BrN4O2 [M+H]+ 429.0921, found (ESI+) 429.0927.
N-(4-Bromophenethyl)-4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)benzamide (7g). White solid, 75% yield, m.p. 188–190 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.53–8.47 (m, 1H), 8.24 (s, 1H), 7.84 (s, 1H), 7.74 (d, J = 8.2 Hz, 2H), 7.53–7.45 (m, 4H), 7.20 (d, J = 8.1 Hz, 2H), 5.65 (s, 1H), 4.40 (s, 2H), 3.47 (q, J = 6.9 Hz, 2H), 2.82 (t, J = 7.2 Hz, 2H), 1.43 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.5, 150.9, 149.4, 145.4, 139.5, 133.5, 131.7, 131.5, 127.3, 125.7, 119.7, 73.1, 59.8, 41.0, 34.9, 27.6; HR-MS (ESI): calcd for C20H21BrN4O2 [M+H]+ 429.0921, found (ESI+) 429.0917.
2.2.2. Synthesis of Mefentrifluconazole
Mefentrifluconazole was synthesized according to the reported methods [31,32,33]. It was smoothly prepared from 1-(4-fluoro-2-(trifluoromethyl)phenyl)ethenone as a raw material via etherification, epoxidation, and ring-opening reactions.
2.3. In Vitro Target Compounds against Seven Phytopathogenic Fungi
We selected seven common and representative phytopathogenic fungi/oomycete that are harmful to crops (cereals, fruits, and vegetables) and have a range of impacts to test the newly synthesized compounds, including Pyricularia oryzae (P. oryzae), Sclerotinia sclerotiorum (S. sclerotiorum), Fusarium oxysporium f. sp. Cucumeris (F. oxysporium f. sp. Cucumeris), Cercospora arachidicola Hori (C. arachidicola Hori), P. piricola, Rhizoctonia cerealis (R. cerealis), and Phytophthora capsici (P. capsici), with the agricultural fungicide mefentrifluconazole as the control [34,35,36,37,38]. The in vitro antifungal/anti-oomycete activity test was performed using the mycelial growth rate method to evaluate the activity of the compounds. The literature was provided in the Supporting Information.
2.4. Calculation Procedures for Molecular Docking Research
The 3D crystal structure of C-14α demethylase (PDB code: 3L4D) was downloaded from the protein data bank (PDB). Detailed procedures are provided in the support information.
3. Results
3.1. Chemicals
The synthesis of intermediates and target compounds was performed as shown in Scheme 1. The intermediate 2, 3, and 4 were prepared with a similar method described by Theodorou and Gebhardt [32,39]. Intermediate 4 was reacted with various substituted amines in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) and 1-hydroxybenzotriazole (HOBt) to obtain the target compounds 5–7 [40]. The chemical structures of all target compounds were confirmed by 1H and 13C NMR and HRMS and characterization data are provided in the Supplementary Materials.
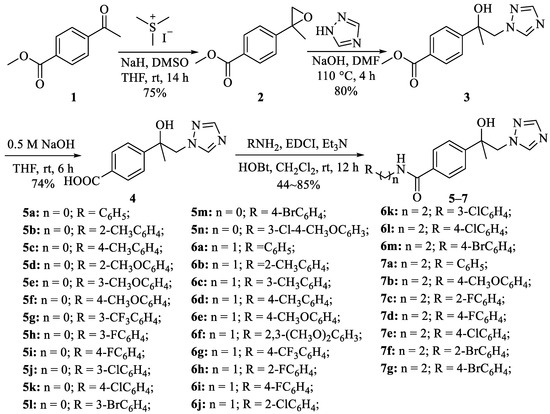
Scheme 1.
Synthesis of the compounds 5a–5n, 6a–6m, and 7a–7g.
3.2. In Vitro Antifungal/Anti-Oomycete Activities of Target Compounds 5a–5n, 6a–6m, and 7a–7g
A series of novel 1,2,4-triazole derivatives were tested for in vitro antifungal/anti-oomycete activity against seven phytopathogenic fungi at 50 μg/mL. The results were compared to the commercialized fungicide mefentrifluconazole, as indicated in Table 1. According to the results in Table 1, compounds 5k, 6h, and 7d showed higher antifungal activities compared to mefentrifluconazole against P. piricola (80% versus 54%, 92% versus 54%, and 66% versus 54% inhibition, respectively). For P. capsici, most of the compounds especially compounds 5j, 6k, and 6m, showed much better anti-oomycete activity (90%, 73%, and 73%, respectively) than that of mefentrifluconazole (32%).

Table 1.
In vitro antifungal/anti-oomycete activities of the compounds 5a–5n, 6a–6m, 7a–7m, and mefentrifluconazole at 50 μg/mL 1.
To understand the antifungal/anti-oomycete activities of compounds 5j, 6h, 6k, and 6m more clearly and intuitively, the half maximal effective concentration (EC50) value of compounds 5j, 6h, 6k, and 6m were determined and the results are shown in Table 2. Compound 6h (EC50 = 13.095 μg/mL) showed about three times higher potency than mefentrifluconazole (EC50 = 39.516 μg/mL). Compounds 5j, 6k, and 6m exhibited excellent in vitro activity effects against P. capsici, with EC50 values of 17.362, 29.970, and 33.152 μg/mL, superior to the intrinsic activity of mefentrifluconazole (EC50 = 75.433 μg/mL).

Table 2.
In vitro EC50 value (μg/mL) of selected compounds against P. piricola and P. capsici 1.
3.3. Molecular Docking Research
To further elucidate the possible mechanism of the interaction between designed 1,2,4-triazole derivatives and CPY51, AutoDock Vina 1.1.2 was used for molecular docking [41]. It can be proven that there are some H-bond interactions and strong binding affinity between 1,2,4-triazole derivatives 5j and 6h containing amide fragments and CPY51.
4. Discussion
4.1. Synthesis
According to a similar approach reported, the 4-position acetyl of methyl 4-acetylbenzoate was epoxidized with trimethylsulfonium iodide in the presence of NaH to obtain compound 2 and then through a substitution reaction with 1,2,4-triazole in DMF to obtain compound 3. The key intermediate 4 was obtained from 3 through hydrolysis in the presence of sodium hydroxide, which was amidated with aniline, benzylamine, and phenethylamine derivatives in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) and 1-hydroxybenzotriazole (HOBt) to obtain the target compounds 5–7 (Scheme 1) [40]. The agricultural fungicide mefentrifluconazole was synthesized according to the procedure reported [32]. With 1-(4-Fluoro-2-(trifluoromethyl)phenyl)ethanone as the starting material, a series of reactions by substitution, epoxidation, and triazole substitution were performed and mefentrifluconazole was finally synthesized. Aniline, benzylamine, and phenethylamine derivative fragments were mainly introduced into the isopropanol triazole skeleton by the active structure splicing strategy to achieve structural diversity in the derivation of isopropanol triazole compounds.
The structures of all compounds were characterized and confirmed by 1H NMR, 13C NMR, and HRMS. Mefentrifluconazole is consistent with the literature report. In the 1H NMR spectra of target compounds, the following can be said: (i) for compounds 5a–5n, the characteristic amide group proton signal can be found around 10.0 ppm as a single peak; (ii) for compounds 6a–6m, the characteristic amide group proton signal can be found around 9.0 ppm as multiple peaks and the double peak at around 4.0 ppm was assigned as the CH2 on the benzyl; and (iii) for compounds 7a–7g, the characteristic amide group proton signal can be found around 8.5 ppm as a multiple peak and the triple peak at around 2.8 ppm and the quadruple peak at around 3.5 ppm were assigned as the CH2 on the phenylethyl. In the 13C NMR spectra, the single peak around 42 ppm was assigned as the CH2 on the benzyl and the single peak around 34 ppm and 41 ppm were assigned as the CH2 on the phenylethyl. These spectroscopic features confirmed that aniline, benzylamine, and phenethylamine derivative fragments were successfully introduced into the isopropanol triazole skeleton.
4.2. Structure–Activity Relationship (SAR) Analysis for the Antifungal/Anti-Oomycete Activity
The agricultural fungicide mefentrifluconazole was chosen as one positive control. The preliminary in vitro antifungal activities of target compounds 5a–5n, 6a–6m, and 7a–7g are shown in Table 1 and the EC50 values of the selected compounds are shown in Table 2.
As shown in Table 1, most target compounds showed poor antifungal activities against P. oryzae, F. oxysporium f. sp. Cucumeris, C. arachidicola Hori, and R. cerealis at the concentration of 50 μg/mL. For S. sclerotiorum, only two compounds 6b and 6j displayed good fungicidal activities (more than 70% inhibition rate), which was much lower than mefentrifluconazole (96%). To our excitement, most of the compounds had similar or better antifungal activities compared to mefentrifluconazole against P. piricola inhibitory activity, especially compounds 5k, 6h, and 7d, which showed higher antifungal activities compared to mefentrifluconazole (80% versus 54%, 92% versus 54%, and 66% versus 54% inhibition, respectively). Most compounds displayed good anti oomycete activities against P. capsici, with compounds 5g, 5j, 6c, 6g, 6k, and 6m showing greatly superior inhibition rates (71%, 90%, 71%, 71%, 73%, and 73%, respectively) with mefentrifluconazole (32%).
For the antifungal effect of different substituted aniline derivatives against P. oryzae, S. sclerotiorum, and P. capsici, the meta substitution on the benzene ring was better than that of the para substitution. For example, compound 5e, bearing a methoxy at the meta position of the phenyl ring, showed much higher antifungal activity than compound 5f containing a para substitution. This effect could also be verified by the results that compounds 5h, 5j, and 5l were more active than compounds 5i, 5k, and 5m, respectively.
In terms of anti-oomycete activities against P. capsici, substituents on the benzene ring of benzylamine derivatives, whether electron-withdrawing groups [compounds 6b (2-CH3C6H4), 6c (3-CH3C6H4), and 6d (4-CH3C6H4)] or electron-rich groups [compounds 6j (2-ClC6H4), 6k (3-ClC6H4), and 6l (4-ClC6H4)], the meta substitution was better than that of the para substitution, which was superior to that of the ortho substitution. For aniline derivatives, position 3 of the benzene ring was substituted with –Cl and the corresponding compound 5j exhibited optimal anti-oomycete activity. In terms of antifungal activities against S. sclerotiorum, substituents on the benzene ring of benzylamine derivatives, whether electron-withdrawing groups [compounds 6b (2-CH3C6H4), 6c (3-CH3C6H4), and 6d (4-CH3C6H4)] or electron-rich groups [compounds 6j (2-ClC6H4), 6k (3-ClC6H4), and 6l (4-ClC6H4)], the ortho substitution was better than that of the meta substitution, which was superior to that of the para substitution. For P. piricola, position 2 of the benzyl ring was substituted with –F and the corresponding compound 6h exhibited optimal antifungal activity. Therefore, it is further shown that the ortho substitution on the benzyl ring is beneficial for antifungal activity.
As shown in Table 2, compound 6h (EC50 = 13.095 μg/mL) showed about three times higher potency than mefentrifluconazole (EC50 = 39.516 μg/mL). It is further shown that compound 6h has an excellent antifungal effect against P. piricola. For P. capsici, even though compounds 5j, 6k, and 6m only exhibited moderate anti oomycete activity, with EC50 values of 17.362, 29.970, and 33.152 μg/mL, respectively, they were still much better than those of mefentrifluconazole (EC50 = 75.433 μg/mL). Remarkably, the substitution of –Cl in the meta position of the benzene ring may be an important moiety to enhance the anti-oomycete activity of the compounds.
4.3. Molecular Docking
Since the newly synthesized 1,2,4-triazole derivatives were derived from mefentrifluconazole, which can be combined with the iron atom in the heme-iron active center of 14α-demethylase (CYP51), the interaction between the selected compounds and CYP51 was further studied. To elucidate the possible mechanism of designed compounds, compounds 5i, 5j, 6h, and mefentrifluconazole were selected for the docking simulation and the model was generated based on the reported crystal complex (PDB code: 3L4D) [42]. Major hydrogen bonds between the compounds and the amino acid residues are shown in Figure 3. In the binding modes of 5j, the oxygen of the hydroxyl group formed a hydrogen bond (2.6 Å) with the NH of amino acid residue ARG-227, the H of the hydroxyl group formed a hydrogen bond (2.0 Å) with the oxygen of amino acid residue ALA-201, and the H of the amide formed a hydrogen bond (2.1 Å) with the oxygen of amino acid residue GLU-204 (Figure 3B). In the docking models with 6h, the oxygen of the hydroxyl group formed a hydrogen bond with the NH of amino acid residue LYS-361 and the oxygen of the amide and the NH of amino acid residue TRP-216 formed a hydrogen bond with corresponding binding distances of 2.4 and 2.2, respectively (Figure 3C). In the binding modes of mefentrifluconazole, the H of the hydroxyl group formed a hydrogen bond (2.8 Å) with the oxygen of amino acid residue GLU-204 and the oxygen on the ether group formed a hydrogen bond (2.6 Å) with the OH of amino acid residue THR-458 (Figure 3D). Compounds 5j, 6h, and mefentrifluconazole are similar in that both have active sites on the hydroxyl group. However, compound 5i only formed a hydrogen bond (2.8 Å) between the oxygen of the amide and the NH of amino acid residue HIS-293 (Figure 3A). These results may partly explain the differences in the aforementioned antifungal phenotypic profiles, with the introduction of carboxamide group increasing its antifungal activity but the hydroxyl group remaining the main active group interacting with CPY51.
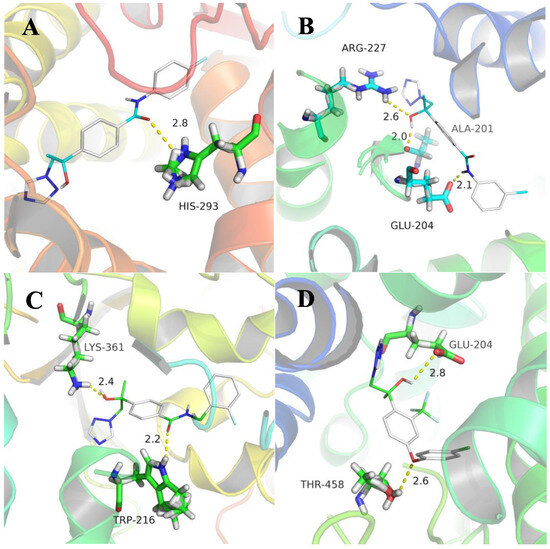
Figure 3.
Molecule docking results of 5i (A), 5j (B), 6h (C), and mefentrifluconazole (D) with CYP51 (PDB code: 3L4D).
In summary, a series of novel 1,2,4-triazole derivatives containing carboxamide fragments were designed and synthesized. Their fungicidal activities against seven kinds of phytopathogenic fungi at 50 μg/mL were evaluated. The bioassay results showed that most compounds had better inhibitory effects against P. piricola, especially compounds 5k, 6h, and 7d, which showed higher antifungal activities compared to commercial DMI fungicide mefentrifluconazole (80 versus 54%, 92 versus 54%, and 66 versus 54% inhibition, respectively). Compound 6h (EC50 = 13.095 μg/mL) showed about three times higher potency than mefentrifluconazole (EC50 = 39.516 μg/mL). For P. capsici, most of the compounds showed good anti-oomycete activity, especially compounds 5j, 6k, and 6m (90, 73, and 73%, respectively), which showed much better inhibition than mefentrifluconazole (32%). Of these, compound 5j (EC50 = 17.362 μg/mL) showed about four times higher potency than mefentrifluconazole (EC50 = 75.433 μg/mL). Molecular docking analysis revealed that compounds 5j and 6h possessed a stronger affinity to CYP51. All of these results showed that compound 6h was a novel and promising candidate as a fungicide for the control of apple ring rot and compound 5j was a candidate for the control of oomycetes. Remarkably, the chemical structure of mefentrifluconazole contains a chiral center with a pair of enantiomers and further study of its stereoselective differences from the chiral level will be a key direction for future research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jof10020160/s1. Section S1: Detailed bioassay procedures for the in vitro antifungal/anti-oomycete activities; Section S2: Calculation procedures for molecular docking research; Section S3: Copies of NMR spectra (Figures S1–S74). References [43,44] were cited in Supplementary Materials.
Author Contributions
Project administration, supervision, A.L.; writing—original draft, J.W.; chemical methodology, J.W.; biological methodology, J.W. and H.S.; docking studies, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the S&T Program of Hebei (21326504D) and the Hebei Natural Science Foundation (B2020202028).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors also acknowledge the State Key Laboratory of Elemento-Organic Chemistry (Nankai University) for the biological activity test.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wen, L.; Jian, W.L.; Shang, J.B.; He, D.H. Synthesis and antifungal activities of novel thiophene-based stilbene derivatives bearing an 1,3,4-oxadiazole unit. Pest Manag. Sci. 2019, 75, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, S.; Song, D.; Cao, X.; Huang, W.; Ke, S. Discovery of γ-lactam alkaloid derivatives as potential fungicidal agents targeting steroid biosynthesis. J. Agric. Food Chem. 2020, 68, 14438–14451. [Google Scholar] [CrossRef]
- Leadbeater, A. Recent developments and challenges in chemical disease control. Plant Prot. Sci. 2015, 51, 163–169. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Heath, J.J.; Stireman, J.O. Dissecting the association between a gall midge, Asteromyia carbonifera, and its symbiotic fungus, Botryosphaeria dothidea. Entomol. Exp. Appl. 2010, 137, 36–49. [Google Scholar] [CrossRef]
- Tang, W.; Ding, Z.; Zhou, Z.Q.; Wang, Y.Z.; Guo, L.Y. Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea. Plant Dis. 2011, 96, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Fu, L.; Li, X.J.; Zhai, H.; Liu, L.; Qu, J.L. Susceptibility of Botryosphaeria dothiden to tebuconazole and virulence of its resistant mutants. Plant Prot. 2017, 43, 140–147. [Google Scholar] [CrossRef]
- Poester, V.R.; Mattei, A.S.; Mendes, J.F.; Klafke, G.B.; Ramis, I.B.; Sanchotene, K.O.; Xavier, M.O. Antifungal activity of diphenyl diselenide alone and in combination with itraconazole against Sporothrix brasiliensis. Med. Mycol. 2018, 57, 328–331. [Google Scholar] [CrossRef]
- Maksimov, A.Y.; Balandina, S.Y.; Topanov, P.A.; Mashevskaya, I.V.; Chaudhary, S. Organic antifungal drugs and targets of their action. Curr. Top. Med. Chem. 2021, 21, 705–736. [Google Scholar] [CrossRef]
- Donlin, M.J.; Meyers, M.J. Repurposing and optimization of drugs for discovery of novel antifungals. Drug Discov. Today 2022, 27, 2008–2014. [Google Scholar] [CrossRef]
- Song, H.; Wang, S.; Cai, Q.; Chen, J. Research progress of triazole derivatives in the discovery of agricultural chemicals. J Heterocycl. Chem. 2023, 61, 365–386. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, X.N.; He, Q.; Lin, H.D.; Chang, H.; Liu, Y.P.; Sun, H.B.; Song, X.B. Analysis of the fungicidal efficacy, environmental fate, and safety of the application of a mefentrifluconazole and pyraclostrobin mixture to control mango anthracnose. J. Sci. Food Agric. 2023, 103, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Mair, W.J.; Deng, W.; Mullins, J.G.; West, S.; Wang, P.; Besharat, N.; Ellwood, S.R.; Oliver, R.P.; Lopez-Ruiz, F.J. Demethylase inhibitor fungicide resistance in Pyrenophora teres f. sp. teres associated with target site modification and inducible overexpression of CYP51. Front. Microbiol. 2016, 7, 1279. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Corran, A.J.; Baldwin, B.C.; Kelly, D.E. Mode of action and resistance to azole antifungals associated with the formation of 14α-methylergosta-8, 24(28)-dien-3β, 6α-diol. Biochem. Biophys. Res. Commun. 1995, 207, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.Z.; Yin, D.F.; Dawood, D.H.; Liu, X.; Chen, Y.; Ma, Z.H. Functional characterization of FgERG3 and FgERG5 associated with ergosterol biosynthesis, vegetative differentiation and virulence of Fusarium graminearum. Fungal Genet. Biol. 2014, 68, 60–70. [Google Scholar] [CrossRef]
- Sombardier, A.; Dufour, M.C.; Blancard, D.; Corio-Costet, M.F. Sensitivity of Podosphaera aphanis isolates to DMI fungicides: Distribution and reduced cross-sensitivity. Pest Manag. Sci. 2010, 66, 35–43. [Google Scholar] [CrossRef]
- Koller, W.; Wubben, J.P. Variable resistance factors of fungicides acting as sterol demethylation inhibitors. Pestic. Sci. 1989, 26, 133–145. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; He, L.; Zhu, J.; Wu, B.; Liu, F.; Mu, W. Activity of the novel fungicide mefentrifluconazole against Colletotrichum scovillei. Plant Dis. 2021, 105, 1522–1530. [Google Scholar] [CrossRef]
- Li, L.S.; Sun, X.F.; Zhao, X.J.; Xiong, Y.D.; Gao, B.B.; Zhang, J.; Shi, H.Y.; Wang, M.H. Absolute configuration, enantioselective bioactivity, and degradation of the novel chiral triazole fungicide mefentrifluconazole. J. Agric. Food Chem. 2021, 69, 4960–4967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.H.; Li, X.B.; Duan, T.T.; Xu, J.; Dong, F.S.; Liu, X.G.; Guo, L.Y.; Zheng, Y.Q. A fast and sensitive ultra-high-performance liquid chromatography-tandem mass spectrometry method for determining mefentrifluconazole in plant- and animal-derived foods. Food Addit. Contam. Part A 2019, 36, 1348–1357. [Google Scholar] [CrossRef]
- Ishii, H.; Bryson, P.K.; Kayamori, M.; Miyamoto, T.; Yamaoka, Y.; Schnabel, G. Cross-resistance to the new fungicide mefentrifluconazole in DMI-resistant fungal pathogens. Pestic. Biochem. Physiol. 2020, 171, 104737. [Google Scholar] [CrossRef] [PubMed]
- Tesh, S.A.; Tesh, J.M.; Fegert, I.; Buesen, R.; Schneider, S.; Mentzel, T.; van Ravenzwaay, B.; Stinchcombe, S. Innovative selection approach for a new antifungal agent mefentrifluconazole (Revysol®) and the impact upon its toxicity profile. Regul. Toxicol. Pharm. 2019, 106, 152–168. [Google Scholar] [CrossRef] [PubMed]
- An, X.K.; Pan, X.L.; Li, R.N.; Jiang, D.D.; Dong, F.S.; Zhu, W.T.; Xu, J.; Liu, X.G.; Wu, X.H.; Zheng, Y.Q. Enantioselective monitoring chiral fungicide mefentrifluconazole in tomato, cucumber, pepper and its pickled products by supercritical fluid chromatography tandem mass spectrometry. Food Chem. 2022, 376, 131883. [Google Scholar] [CrossRef]
- Rich, P.R.; Maréchal, A. The mitochondrial respiratory chain. Essays Biochem. 2010, 47, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Xiong, L.; Li, H.; Song, X.Y.; Liu, J.J.; Yang, G.F. Computational and experimental insight into the molecular mechanism of carboxamide inhibitors of succinate-ubquinone oxidoreductase. ChemMedChem 2014, 9, 1512–1521. [Google Scholar] [CrossRef]
- Luo, B.; Ning, Y.L. Comprehensive overview of carboxamide derivatives as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2022, 70, 957–975. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, T.; Wang, J.; Cheng, W.; Lu, T.; Yan, Y.; Tang, X. Design, synthesis, inhibitory activity, and molecular modeling of novel pyrazole-furan/thiophene carboxamide hybrids as potential pungicides targeting succinate dehydrogenase. J. Agric. Food Chem. 2023, 71, 729–738. [Google Scholar] [CrossRef]
- Wei, G.; Gao, M.Q.; Zhu, X.L.; Yang, G.F. Research progress on carboxamide fungicides targeting succinate dehydrogenase. Chin. J. Pestic. Sci. 2019, 21, 673–680. [Google Scholar] [CrossRef]
- Wu, Z.; Park, H.Y.; Xie, D.; Yang, J.; Hou, S.; Shahzad, N.; Kim, C.K.; Yang, S. Synthesis, biological evaluation, and 3D-QSAR studies of N-(Substituted pyridine-4-yl)-1-(substituted phenyl)-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives as potential succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2021, 69, 1214–1223. [Google Scholar] [CrossRef]
- Dietz, J.; Riggs, R.; Boudet, N.; Lohmann, J.K.; Craig, I.R.; Haden, E.; Lauterwasser, E.M.W. Fungicidal Substituted 2-[2-halogenalkyl-4-(phenoxy)-phenyl]-1-[1,2,4] triazol-1-yl-ethanol Compounds. Patent EP2731935(A1), 21 May 2014. [Google Scholar]
- Gebhardt, J.; Saelinger, D.; Ehresmann, M. Method for Producing 2-[4-(4-chlorophenoxy)-2-(trif-luoromethyl) phenyl]-1-(1,2,4-triazol-1-yl)propan-2-ol. Patent WO2017102905, 22 June 2017. [Google Scholar]
- Lohmann, J.K. Composition Comprising a Triazole Compound and Their Use in Controlling Phytopathogenic fungi Known to Cause Plant Diseases. Patent WO2014095994, 26 June 2014. [Google Scholar]
- Nyongesa, B.O.; Bigirimana, J.; Were, B.A.; Murori, R. Virulence spectrum of populations of Pyricularia oryzae in irrigated rice ecosystems in Kenya. Eur. J. Plant Pathol. 2016, 146, 911–922. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, W.; Wang, Y.; Xia, D.; Yin, F.; Liu, Q.; Luo, H.; Li, M.; Zhang, C.; Cao, H.; et al. Novel pyrazolo[3,4-d]pyrimidin-4-one derivatives as potential antifungal agents: Design, synthesis, and biological evaluation. J. Agric. Food Chem. 2021, 69, 11395–11405. [Google Scholar] [CrossRef]
- Tian, J.; Chen, C.; Sun, H.; Wang, Z.; Steinkellner, S.; Feng, J.; Liang, Y. Proteomic analysis reveals the importance of exudates on sclerotial development in Sclerotinia sclerotiorum. J. Agric. Food Chem. 2021, 69, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Lemańczyk, G.; Kwaśna, H. Effects of sharp eyespot (Rhizoctonia cerealis) on yield and grain quality of winter wheat. Eur. J. Plant Pathol. 2012, 135, 187–200. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, S.H.; Hoffmeister, R.A.; Yoon, M.Y.; Kim, S.K. Novel peptide-based inhibitors for microtubule polymerization in Phytophthora capsici. Int. J. Mol. Sci. 2019, 20, 2641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Wang, L.; Mao, M.Z.; Wang, W.; Ning, B.K. A review of the synthesis of mefentrifluconazole. Agrochemicals 2019, 58, 457–477. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, Y.; Chu, K.; Gao, T.; Smith, Z.J. High-resolution low-power hyperspectral line-scan imaging of fast cellular dynamics using azo-enhanced Raman scattering probes. J. Am. Chem. Soc. 2022, 144, 15314–15323. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Wawrzak, Z.; Liu, J.; Nes, W.D.; Waterman, M.R.; Lepesheva, G.I. Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14α-demethylase (CYP51) from Leishmania infantum. J. Biol. Chem. 2011, 286, 26838–26848. [Google Scholar] [CrossRef]
- Zhao, H.P.; Liu, Y.X.; Cui, Z.P.; Beattie, D.; Gu, Y.C.; Wang, Q.M. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shukri, M.; Hassandarvish, P.; Oo, A.; Muthu, S.E.; Abubakar, S.; Zandi, K. Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci. Rep. 2016, 6, 24027. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).