The Application of Fungi and Their Secondary Metabolites in Aquaculture
Abstract
1. Introduction
1.1. Objectives
1.2. The Description of Fungi
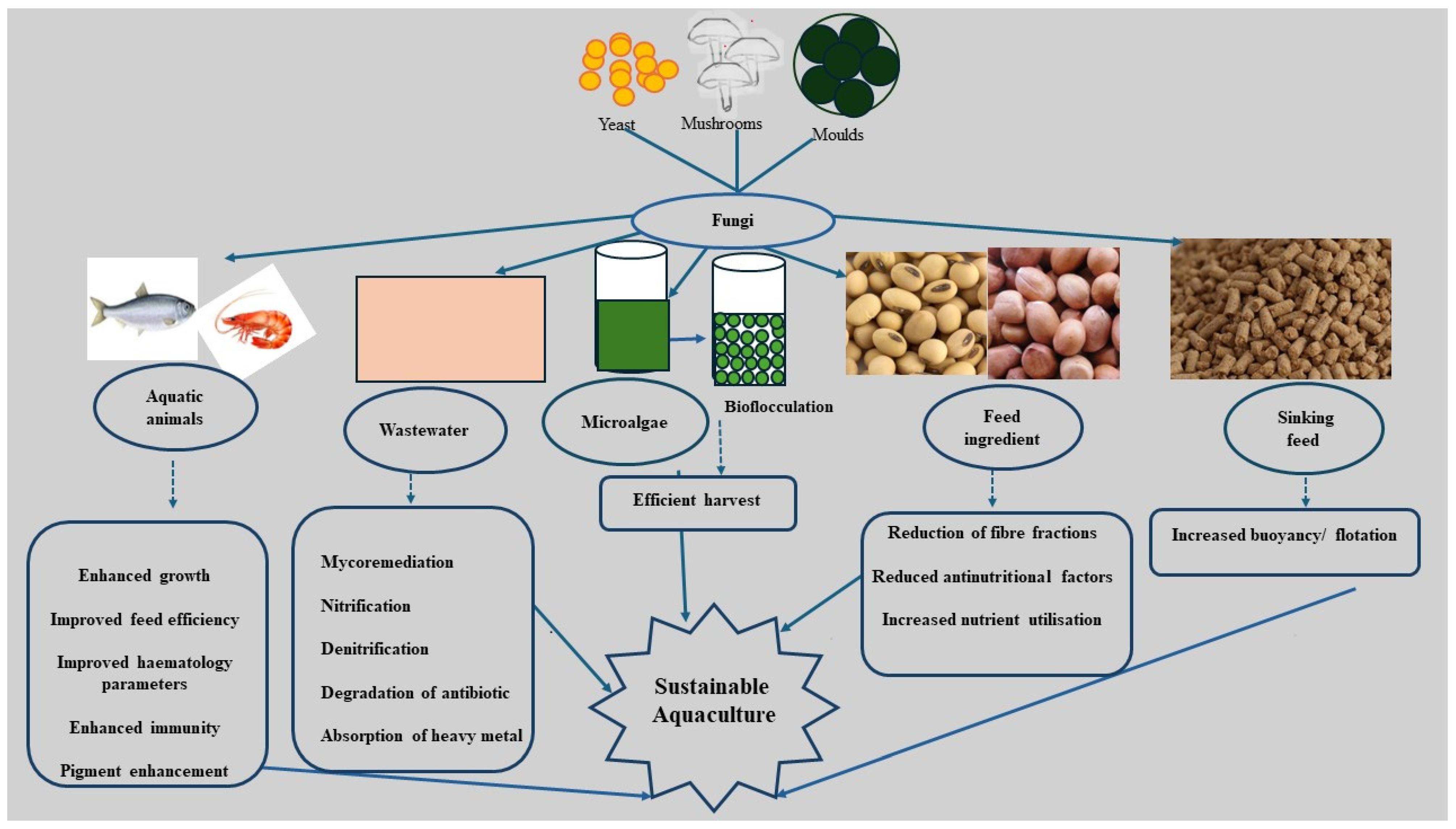
2. Application of Fungi and Its Metabolites in Aquaculture
2.1. Source of Antibiotics, Probiotics and Prebiotics
| Fungi | Fungi Species | Species | Concentration and Duration | Effect on Growth and Survival | Haematological Parameters | Disease Resistance | Reference |
|---|---|---|---|---|---|---|---|
| Mushroom (powder) | Pleurotus eryngii | Koi carp fingerlings (Cyprinus carpio koi) | 0, 0.5, 1, 1.5 and 2% for 61 days | FW, WG, SGR, (↑) (1.5%). FCR (↓) (0.5, 1, 1.5 and 2%) SR (↑) (0, 0.5, 1, 1.5 and 2%) | WBC, Hb, Ht, MCV, MCH, monocyte, MCHC (↑) (1.5 and 2%) Digestive enzymes (Trypsin and lipase) (↑) (2%) α-amylase (↑) (1, 1.5, 2%) | NA | [52] |
| Ganoderma lucidum | Nile Tilapia (Oreochromis niloticus) | 0, 0.5, 1, and 2% for 90 days | FW, WG (%), SGR (↑) (1%) FCR (↓) (10%) SR (↔) (0, 0.5, 1, and 2%) | NA | NA | [53] | |
| Mushroom (fermented by product) | Pleurotus ostreatus | Amur catfish (Silurus asotus) | 0, 0.1, 0.2, 0.4, 0.8% for 56 days. | WG, SGR (%) (↑) (0.1 and 0.2%) FE, PER (↑) (0.2) | Haematocrit (↑) (0.2%) Lysozyme activity (↑) (0.1%) PCV (↑) (0.2, 0.4 and 0.8%) | NA | [45] |
| Spent mushroom substrate | Cordyceps militaris | Labeo rohita | 0, 1, 2, and 3% for 60 days. | NA | NBT, antiprotease and lysozyme activity (↑) (1, 2 and 3%) | SR (↑) after challenged with A. hydrophila (1, 2, and 3%). | [49] |
| Mould | Aspergillus niger | Penaeus vannamei | 0, 0.15 and 0.3% for 28 days | WG (%), SGR (%) and length gain (%) (↑) (1.5%) | ACP and AKP (↑) (1.5%) SOD and catalase activity (↑) (1.5 and 3%) Lysozyme activity (↓) (1 and 3%) | NA | [47] |
| Aspergillus niger | Common Carp (Cyprinus carpio) | 0, 1 × 103 and 1 × 106 for 60 days | FW, WG, FL, PER, LER (↑) (103 and 106). FCR (↓) (103 and 106). | Lysozyme activity, Ig, RBC, Hb (↑) (103 and 106) Body protein and dry matter composition (↑) (103 and 106). Body lipid composition (↓) (103 and 106). Amylase and lipase digestive enzymes (↑) (103 and 106). | N/A | [46] | |
| Aspergillus niger | White shrimp (Penaeus vannamei) | 0, 0.5, 1 and 1.5 g (kg−1 diet) for 56 days | FW and WG (↑) (0.5, 1 and 1.5 g kg−1 diet) FCR (↔) (0.5, 1 and 1.5 g kg−1 diet) | Lysozyme, phygocytic and phenoloxidase activity (↑) 0.5, 1 and 1.5 g kg−1 diet) Reduced vibrio-like count in the gut of the fish | Mortality (↓) (0.5, 1 and 1.5 g kg−1 diet) | [50] | |
| Yeast | Saccharomyces cerevisiae | Nile Tilapia Oreochromis niloticus | 0, 1, 2 and 4 g kg−1 for 60 days. | SGR, PER (↑) (4 g kg−1) | gut villus wall thickness, villus length, width, and area (↑) (4 g kg−1) | N/A | [54] |
| Saccharomyces cerevisiae | Nile Tilapia Oreochromis niloticus | 0, 3, 5 and 7% for 84 days | WG (↔) PER (↑) (3, 5 and 7%) FCR (↓) (3, 5 and 7%) | Mortality (↓) (7%) | [55] | ||
| Saccharomyces cerevisiae | Snakehead Channa punctatus | 0 and 2.5 g kg−1 for 56 days. | WG, PER, FCR, (↔) SGR (↑) | Intestinal protease, lipase, and amylase enzyme activities (↑) | Mortality (↓) | [56] |
2.2. Reduction of Anti-Nutritional Factors and Fibre Fractions in Plant Ingredients
2.3. Increased Nutrient Availability in Feed
2.4. Increased Mineral Utilization and Reduced Nutrients in Effluents
2.5. Fungi as a Buoyancy/Flotation Agent in Aquafeed
2.6. Pigment
2.7. Bioremediation
2.8. Bioflocculation
3. Limitations on the Use of Fungi
4. Conclusions and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving Sustainable Aquaculture: Historical and Current Perspectives and Future Needs and Challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Onomu, A.J.; Okuthe, G.E. The Role of Functional Feed Additives in Enhancing Aquaculture Sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Ahmad, A. Aquaculture Industry: Supply and Demand, Best Practices, Effluent and Its Current Issues and Treatment Technology. J. Environ. Manag. 2021, 14, 112271. [Google Scholar] [CrossRef] [PubMed]
- Gyan, W.R.; Ayiku, S.; Yang, Q. Effects of Replacing Fishmeal with Soybean Products in Fish and Crustaceans Performance. J. Aquac. Res. Dev. 2019, 10, 573. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Sun, Y.; Chen, Y.; Chen, S.; Han, T.; Wang, J. Excessive Substitution of Fish Meal with Fermented Soybean Meal Induces Oxidative Stress by Impairing Glutathione Metabolism in Largemouth Bass (Micropterus salmoides). Antioxidants 2023, 12, 2096. [Google Scholar] [CrossRef]
- He, M.; Yu, Y.; Li, X.; Poolsawat, L.; Yang, P.; Bian, Y.; Guo, Z.; Leng, X. An Evaluation of Replacing Fish Meal with Fermented Soybean Meal in the Diets of Largemouth Bass (Micropterus salmoides): Growth, Nutrition Utilization and Intestinal Histology. Aquac. Res. 2020, 51, 4302–4314. [Google Scholar] [CrossRef]
- Jannathulla, R.; Dayal, J.S.; Ambasankar, K.; Eugine, A.C.; Muralidhar, M. Fungus, Aspergillus niger, Fermented Groundnut Oil Cake as a Fishmeal Alternative in the Diet of Penaeus vannamei. Aquac. Res. 2018, 49, 2891–2902. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Lemos, D.; Tacon, A.G.J. Use of Phytases in Fish and Shrimp Feeds: A Review. Rev. Aquac. 2017, 9, 266–282. [Google Scholar] [CrossRef]
- Baruah, K.; Sahu, N.P.; Pal, A.K.; Debnath, D. Dietary Phytase: An Ideal Approach for a Cost Effective and Low-Polluting Aquafeed. Naga 2004, 27, 15–19. [Google Scholar]
- Onomu, A.; Slater, M.; Vine, N. Feeding Indicators and Bioremediation Ability of Warty Sea Cucumber Neostichopus Grammatus Fed Potential Wastes from Abalone Haliotis Midae Farming. Aquacult. Environ. Interact. 2023, 15, 45–57. [Google Scholar] [CrossRef]
- Cocker, L.M. Strategic Review on African Aquaculture Feeds; Partnership for African Fisheries (PAF) Aquaculture Working Group, NEPAD: Lusaka, Zambia, 2014; pp. 1–85. [Google Scholar]
- Walker, G.M.; White, N.A. Introduction to Fungal Physiology. In Fungi: Biology and Applications; Kavanagh, K., Ed.; Wiley-Blackwell: Chichester, UK, 2017; pp. 1–35. ISBN 978-1-119-37432-9. [Google Scholar]
- Jia, D.; Tang, Y.; Qin, F.; Liu, B.; Hu, T.; Chen, W. Ganoderma Lucidum Polysaccharide Alleviates Cd Toxicity in Common Carp (Cyprinus carpio): Neuropeptide, Growth Performance and Lipid Accumulation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109663. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Mao, X.; Zheng, P.; Huang, Z.; Chen, D. Physicochemical Properties Analysis and Secretome of Aspergillus niger in Fermented Rapeseed Meal. PLoS ONE 2016, 11, e0153230. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.M.; Ayub, M.Y.; Salam, B.A.; Hadijah, H.; Azahan, E.A.E.; Tarmizi, S.A. Protein Quality of Aspergillus niger-Fermented Palm Kernel Cake. J. Trop. Agric. Food Sci. 2008, 36, 1–11. [Google Scholar]
- Tanemura, N.; Akiyoshi, Y.; Okano, K.; Sugiura, S. Effects of Culturing Rapeseed Meal, Soybean Meal, Macrophyte Meal, and Algal Meal with Three Species of White-Rot Fungi on Their in Vitro and in Vivo Digestibilities Evaluated Using Rainbow Trout. Aquaculture 2016, 453, 130–134. [Google Scholar] [CrossRef]
- Naiel, M.A.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The Risk Assessment of High-fat Diet in Farmed Fish and Its Mitigation Approaches: A Review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 948–969. [Google Scholar] [CrossRef]
- Akthar, M.N.; Mohan, P.M. Bioremediation of Toxic Metal Ions from Polluted Lake Waters and Industrial Effluents by Fungal Biosorbent. Curr. Sci. 1995, 69, 1028–1030. [Google Scholar]
- Hariyono, C.M.; Yunianta; Harijono; Sriherwanto, C.; Suja’i, I.; Nadaviana, A.; Junaedi, H.; Ma’hadah, R. Komarudin Physico-Chemical Characteristics of Rhizopus sp.-Fermented Fish Feed Pellets Containing Black Soldier Fly Larvae (Hermetia illucens) Meal. IOP Conf. Ser. Earth Environ. Sci. 2021, 744, 012024. [Google Scholar] [CrossRef]
- Leiskayanti, Y.; Sriherwanto, C.; Suja’i, I. FERMENTASI MENGGUNAKAN RAGI TEMPE SEBAGAI CARA BIOLOGIS PENGAPUNGAN PAKAN IKAN. J. Bioteknol. Biosains Indones 2017, 4, 1. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Wang, X.; Huang, B. Effects of Stocking Density on Stress Response, Innate Immune Parameters, and Welfare of Turbot (Scophthalmus maximus). Aquac. Int. 2019, 27, 1599–1612. [Google Scholar] [CrossRef]
- Oliveira, M.; Vasconcelos, V. Occurrence of Mycotoxins in Fish Feed and Its Effects: A Review. Toxins 2020, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 Million Species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Dijkstherhuis, J. Fungal Spores: Highly Variable and Stress-Resistant Vehicles for Distribution and Spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef]
- De Lucca, A.J. Harmful Fungi in Both Agriculture and Medicine. Rev. Iberoam. Micol. 2007, 24, 3–13. [Google Scholar] [CrossRef]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.; Van Dijck, P.W. On the Safety of Aspergillus Niger—A Review. Appl. Microbiol. Biotechnol. 2002, 59, 426–435. [Google Scholar] [CrossRef]
- Shurson, G.C. Yeast and Yeast Derivatives in Feed Additives and Ingredients: Sources, Characteristics, Animal Responses, and Quantification Methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Tomé, D. Yeast Extracts: Nutritional and Flavoring Food Ingredients. ACS Food Sci. Technol. 2021, 1, 487–494. [Google Scholar] [CrossRef]
- Stone, C.W. Yeast Products in the Feed Industry: A Practical Guide for Feed Professionals. 2006. Available online: https://en.engormix.com/feed-machinery/articles/yeast-products-infeed-industry-t33489.htm (accessed on 1 September 2024).
- Bennett, J.W. Mycotechnology: The Role of Fungi in Biotechnology. J. Biotechnol. 1998, 66, 101–107. [Google Scholar] [CrossRef]
- Buerth, C.; Tielker, D.; Ernst, J.F. Candida Utilis and Cyberlindnera (Pichia) Jadinii: Yeast Relatives with Expanding Applications. Appl. Microbiol. Biotechnol. 2016, 100, 6891–6990. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 31. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, U.; Stephenson, S. Wild Edible Mushrooms from High Elevations in the Garhwal Himalaya-I. Curr. Res. Environ. Appl. Mycol. 2016, 6, 118–131. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Karthick Rajan, D.; Muralisankar, T.; Ramu Ganesan, A.; Marimuthu, K.; Sathishkumar, P. The Potential Role of Medicinal Mushrooms as Prebiotics in Aquaculture: A Review. Rev. Aquac. 2022, 14, 1300–1332. [Google Scholar] [CrossRef]
- Sandrin, T.R.; Dowd, S.E.; Herman, D.C.; Maier, R.M. Aquatic Environments. In Environmental Micrbiology; Maier, R.M., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: Burlington, MA, USA, 2009; pp. 103–122. [Google Scholar]
- Tarman, K.; Lindequist, U.; Wende, K.; Porzel, A.; Arnold, N.; Wessjohann, L.A. Isolation of a New Natural Product and Cytotoxic and Antimicrobial Activities of Extracts from Fungi of Indonesian Marine Habitats. Mar. Drugs 2011, 9, 294–306. [Google Scholar] [CrossRef]
- Al-Fakih, A.A.; Almaqtri, W.Q.A. Overview on Antibacterial Metabolites from Terrestrial Aspergillus spp. Mycology 2019, 10, 191–209. [Google Scholar] [CrossRef]
- Sibero, M.T.; Herdikiawan, D.; Radjasa, O.K.; Sabdono, A.; Trianto, A.; Triningsih, D.W. Antibacterial Activity of Sponge Associated Fungi against Vibriosis Agents in Shrimp and Its Toxicity to Litopenaeus Vannamei. AACL Bioflux 2018, 11, 10–18. [Google Scholar]
- Jannathulla, R.; Dayal, J.S. Beneficial Effects, Challenges and Opportunities of the Filamentous Fungus, Aspergillus niger with Special Reference to the Shrimp Feed Industry—A Review. Rev. Aquac. 2023, 15, 1311–1334. [Google Scholar] [CrossRef]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Wahjuningrum, D.; Tarman, K.; Effendi, I. Feeding Duration of Dietary Nodulisporium sp. KT29 to Prevent the Infection of Vibrio Harveyi on Pacific White Shrimp Litopenaeus vannamei. Aquac. Aquar. Conserv. Legis. 2016, 9, 1265–1277. [Google Scholar]
- Katya, K.; Yun, Y.H.; Yun, H.; Lee, J.Y.; Bai, S.C. Effects of Dietary Fermented By-product of Mushroom, Pleurotus ostreatus, as an Additive on Growth, Serological Characteristics and Nonspecific Immune Responses in Juvenile Amur Catfish, Silurus asotus. Aquac. Res. 2016, 47, 1622–1630. [Google Scholar] [CrossRef]
- Jasim, S.A.; Abdelbasset, W.K.; Shichiyakh, R.A.; Al-Shawi, S.G.; Yasin, G.; Jalil, A.T.; Karim, Y.S.; Mustafa, Y.F.; Norbakhsh, M. Probiotic Effects of the Fungi, Aspergillus niger on Growth, Immunity, Haematology, Intestine Fungal Load and Digestive Enzymes of the Common Carp, Cyprinus carpio. Aquac. Res. 2022, 53, 3828–3840. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, H.; Lei, Y.; Zhang, Y.; Tan, Z.; Chen, W.; Li, S.; Peng, X.; Tran, N.T. Aspergillus Niger Confers Health Benefits and Modulates the Gut Microbiota of Juvenile Pacific White Shrimp (Penaeus vannamei) under Farming Conditions. Front. Mar. Sci. 2023, 10, 1211993. [Google Scholar] [CrossRef]
- Onomu, A.J. Growth and Haematological Response of Clarias gariepinus to Garlic (Allium sativum) Supplemented Diet. SAR 2018, 8, 67. [Google Scholar] [CrossRef]
- Devi, W.M.; Saha, H.; Irungbam, S.; Saha, R.K. A Novel Approach in Valorization of Spent Mushroom Substrate of Cordyceps Militaris as In-Feed Antibiotics in Labeo rohita against Aeromonas Hydrophila Infection. Environ. Sci. Pollut. Res. 2023, 1–10. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Peng, X.; Chen, W.; Tseng, D.-Y.; Tan, Z.; Liu, H.; Qin, Z.; Ballantyne, R.; Liu, C.-H. Dietary Probiotic Aspergillus niger Preparation Improves the Growth Performance, Health Status, and Gut Microbiota of White Shrimp, Penaeus vannamei. Aquaculture 2023, 577, 739988. [Google Scholar] [CrossRef]
- Sarlin, P.J.; Philip, R. Efficacy of Marine Yeasts and Baker’s Yeast as Immunostimulants in Fenneropenaeus indicus: A Comparative Study. Aquaculture 2011, 321, 173–178. [Google Scholar] [CrossRef]
- Safari, O.; Sarkheil, M. Dietary Administration of Eryngii Mushroom (Pleurotus eryngii) Powder on Haemato-Immunological Responses, Bactericidal Activity of Skin Mucus and Growth Performance of Koi Carp Fingerlings (Cyprinus carpio Koi). Fish Shellfish Immunol. 2018, 80, 505–513. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ergün, S.; Şahin, T.; Çelik, E.Ş.; Abdel-Latif, H.M.R. Effects of Dietary Reishi Mushroom (Ganoderma lucidum) on the Growth Performance of Nile Tilapia, Oreochromis niloticus Juveniles. Aquaculture 2023, 564, 739057. [Google Scholar] [CrossRef]
- Islam, S.M.; Rohani, M.F.; Shahjahan, M. Probiotic Yeast Enhances Growth Performance of Nile Tilapia (Oreochromis niloticus) through Morphological Modifications of Intestine. Aquac. Rep. 2021, 21, 10800. [Google Scholar] [CrossRef]
- Abass, D.A.; Obirikorang, K.A.; Champion, B.B.; Edziyie, R.E.; Skov, P.V. Dietary Supplementation of Yeast (Saccharomyces cerevisiae) Improves Growth, Stress Tolerance, and Disease Resistance in Juvenile Nile Tilapia (Oreochromis niloticus). Aquac. Int. 2018, 26, 843–855. [Google Scholar] [CrossRef]
- Devi, G.; Harikrishnan, R.; Paray, B.A.; Al-Sadoon, M.K.; Hoseinifar, S.H.; Balasundaram, C. Comparative Immunostimulatory Effect of Probiotics and Prebiotics in Channa Punctatus against Aphanomyces invadans. Fish Shellfish Immunol. 2019, 86, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Antinutritional Factors in Foods for Live Stock. In Animal Production in Developing Countries; Gill, M., Owen, E., Pollot, G.E., Lawrence, T.L.J., Eds.; Occasional Publication No. 16; British Society of Animal Production: Cambridge, UK, 1993; pp. 69–85. [Google Scholar] [CrossRef]
- Krogdahl, A. Alternative Protein Sources from Plants Contain Anti Nutrients Affecting Digestion in Salmonids. In Proceedings of the Third International Symposium on Feeding and Nutrition in Fish; Takeda, M., Watanabe, T., Eds.; Tokyo University of Fisheries: Tokyo, Japan, 1989; pp. 253–261. [Google Scholar]
- Jannathulla, R.; Dayal, J.S.; Vasanthakumar, D.; Ambasankar, K.; Muralidhar, M. Effect of Fermentation Methods on Amino Acids, Fiber Fractions and Anti Nutritional Factors in Different Plant Protein Sources and Essential Amino Acid Index for Penaeus (Litopenaeus) vannamei. Indian J. Fish. 2017, 64, 40–47. [Google Scholar] [CrossRef]
- Shi, C.; Dong, S.; Pei, S.; Wang, F.; Tian, X.; Gao, Q. Effects of Diatom Concentration in Prepared Feeds on Growth and Energy Budget of the Sea Cucumber Apostichopus japonicus (Selenka). Aquac. Res. 2015, 46, 609–617. [Google Scholar] [CrossRef]
- Maciel, G.M.; Haminiuk, I.; Fendrich, R.C.; Bianca, B.E.D. Xylanase Production by Aspergillus niger LPB 326 in Solid-State Fermentation Using Statistical Experimental Designs. Food Technol. Biotechnol. 2008, 46, 183–189. [Google Scholar]
- Reddy, G.P.K.; Narasimha, G.; Kumar, K.D.; Ramanjaneyulu, G.; Ramya, A.; Kumari, B.S.; Reddy, B.R. Cellulase Production by Aspergillus Niger on Different Natural Lignocellulosic Substrates. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 835–845. [Google Scholar]
- Solis-Pereyra, S.; Favela-Torres, E.; Gutibrrez-Rojas, M.; Roussos, S.; Saucedo-CastaAeda, G.; Gunasekaran, P.; Viniegra-GonzAlez, G. Production of Pectinases by Aspergihs Niger in Solid State Fermentation at High Initial Glucose Concentrations. World J. Microbiol. Biotechnol. 1996, 12, 257–260. [Google Scholar] [CrossRef]
- Putra, A.N.; Rohayati, D.; Syamsunarno, M.B.; Putra, A.N.; Rohayati, D.; Syamsunarno, M.B. Effect of Fermented Rice Bran Used as Feed Ingredient on Apparent Digestibility Coefficient of Tilapia (Oreochromis niloticus) Feed. In IOP Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 934, p. 012009. [Google Scholar]
- Putra, A.N.; Indayah, U.; Nokiyah, N.; Syamsunarno, M.B. Improving Quality of Cassava Peel Meal as Raw Material for Tilapia Feed. Depik 2022, 11, 319–326. [Google Scholar] [CrossRef]
- Cui, Y.; Li, J.; Deng, D.; Lu, H.; Tian, Z.; Liu, Z.; Ma, X. Solid-State Fermentation by Aspergillus niger and Trichoderma koningii Improves the Quality of Tea Dregs for Use as Feed Additives. PLoS ONE 2021, 16, e0260045. [Google Scholar] [CrossRef]
- Altop, A.; Güngör, E.; Erener, G. Improvement of Nutritional Quality of Some Oilseed Meals through Solid-State Fermentation Using Aspergillus niger. Tukish J. Agric. Food Sci. Technol. 2019, 7, 1411–1414. [Google Scholar] [CrossRef]
- Afeni, M.; Adeyemi, M.; Ogundeji, S.; Akinfala, E. Effect of Aspergillus Niger Treated with Fibre Feedstuffs on Growth Performance and Carcass Cholesterol of Growing Pigs. Ife J. Agric. 2024, 36, 1–9. [Google Scholar]
- Altop, A.; Güngör, E.; Özlü, S.; Erener, G. Solid-State Fermentation of Wheat Bran by Aspergillus Niger Strains: Effect on the Nutritional Composition and in Vitro Digestibility. Anadolu Tarım Bilim. Derg. 2024, 39, 239–246. [Google Scholar] [CrossRef]
- Demirgül, K.; Ozturk, E. Changes in Nutrients, Energy, Antioxidant and Carotenoid Levels of Dried Tomato (Lycopersicon esculentum) Pomage Treated with Aspergillus Niger Solid-State Fermentation. Tukish J. Agric. Food Sci. Technol. 2021, 9, 701–708. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, A.A.; Rabbani, M.; Ashraf, K.; Awais, M.M.; Nawaz, M.; Ahmad, N.; Asif, A.; Sana, S. Effect of Fermented Rice Bran on Growth Performance and Bioavailability of Phosphorus in Broiler Chickens. Indian J. Anim. Res. 2019, 53, 361–365. [Google Scholar] [CrossRef]
- Lücke, F.K.; Fritz, V.; Tannhäuser, K.; Arya, A. Controlled Fermentation of Rapeseed Presscake by Rhizopus, and Its Effect on Some Components with Relevance to Human Nutrition. Food Res. Int. 2019, 120, 726–732. [Google Scholar] [CrossRef]
- Croat, J.R.; Berhow, M.; Karki, B.; Muthukumarappan, K.; Gibbons, W.R. Conversion of Canola Meal into a High-Protein Feed Additive via Solid-State Fungal Incubation Process. J. Am. Oil Chem. Soc. 2016, 93, 499–507. [Google Scholar] [CrossRef]
- Ardiansyah, A.; Dahlia, D.; Hartinah, H.; Wahidah, W. Improvement of the Nutritive Quality of Sargassum Powder through Aspergillus Niger, Saccharomyces Cerevisiae, and Lactobacillus Spp. Fermentations. AACL Bioflux 2018, 11, 753–764. [Google Scholar]
- Storebakken, T.; Shearer, K.D.; Roem, A.J. Availability of Protein, Phosphorus and Other Elements in Fish Meal, Soy-Protein Concentrate and Phytase-Treated Soy-Protein-Concentrate-Based Diets to Atlantic Salmon, Salmo Salar. Aquaculture 1998, 161, 365–379. [Google Scholar] [CrossRef]
- Vielma, J.; Mäkinen, T.; Ekholm, P.; Koskela, J. Influence of Dietary Soy and Phytase Levels on Performance and Body Composition of Large Rainbow Trout (Oncorhynchus mykiss) and Algal Availability of Phosphorus Load. Aquaculture 2000, 183, 349–362. [Google Scholar] [CrossRef]
- Hughes, K.P.; Soares, J.H. Efficacy of Phytase on Phosphorus Utilization in Practical Diets Fed to Striped Bass Morone saxatilis. Aquac. Nutr. 1998, 4, 133–140. [Google Scholar] [CrossRef]
- Papatryphon, E.; Howell, R.A.; Soares, J.H. Growth and Mineral Absorption by Striped Bass Morone Saxatilis Fed a Plant Feedstuff Based Diet Supplemented with Phytase. World Aquac. Soc. 1999, 30, 161–173. [Google Scholar] [CrossRef]
- Yan, W.; Reigh, R.C.; Xu, Z. Effects of Fungal Phytase on Utilization of Dietary Protein and Minerals, and Dephosphorylation of Phytic Acid in the Alimentary Tract of Channel Catfish Ictalurus punctatus Fed an All-Plant-Protein Diet. J. World Aquac. Soc. 2002, 33, 10–22. [Google Scholar] [CrossRef]
- Baeverfjord; Åsgård; Shearer. Development and Detection of Phosphorus Deficiency in Atlantic Salmon, Salmo salar L., Parr and Post-Smolts. Aquac. Nutr. 1998, 4, 1–11. [Google Scholar] [CrossRef]
- Ravindran, V.; Ravindran, G.; Sivalogan, S. Total and Phytate Phosphorus Contents of Various Foods and Feedstuffs of Plant Origin. Food Chem. 1994, 50, 133–136. [Google Scholar] [CrossRef]
- Ellestad, L.E.; Angel, R.; Soares, J.H. Intestinal Phytase I: Detection and Preliminary Characterization of Activity in the Intestinal Brush Border Membrane of Hybrid Striped Bass. Fish Physiol. Bichemistry 2002, 26, 249–258. [Google Scholar] [CrossRef]
- Pallauf, J.; Rimbach, G. Nutritional Significance of Phytic Acid and Phytases. Arch. Anim. Nutr. 1997, 50, 301–319. [Google Scholar] [CrossRef]
- Roy, P.K.; Lall, S.P. Dietary Phosphorus Requirement of Juvenile Haddock (Melanogrammus aeglefinus L.). Aquaculture 2003, 221, 451–468. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, H.; Maulu, S.; Ge, X.; Ren, M.; Xie, J.; Xi, B. Dietary Phosphorus Affects Growth, Glucolipid Metabolism, Antioxidant Activity and Immune Status of Juvenile Blunt Snout Bream (Megalobrama amblycephala). Anim. Feed Sci. Technol. 2021, 274, 114896. [Google Scholar] [CrossRef]
- Runge-Metzger, A. Closing the Cycle: Obstacles to Efficient P Management for Improved Global Security. In Phosphorus in the Global Environment; Tiessen, H., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 1995; pp. 27–42. [Google Scholar]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus Acquisition and Use: Critical Adaptations by Plants for Securing a Nonrenewable Resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Philips, H.F. Dietary Calcium, Phytate and Zinc Interactions in Channel Catfish. Aquacultture 1989, 79, 259–266. [Google Scholar] [CrossRef]
- Satoh, S.; Poe, W.; Wilson, R.P. Effect of Supplemental Phytate and/or Tricalcium Phosphate on Weight Gain, Feed Efficiency and Zinc Content in Vertebrae of Channel Catfish. Aquacultture 1989, 80, 155–161. [Google Scholar] [CrossRef]
- Rizwanuddin, S.; Kumar, V.; Naik, B.; Singh, P.; Mishra, S.; Rustagi, S.; Kumar, V. Microbial Phytase: Their Sources, Production, and Role in the Enhancement of Nutritional Aspects of Food and Feed Additives. J. Agric. Food Res. 2023, 12, 100559. [Google Scholar] [CrossRef]
- Song, H.Y.; El Sheikha, A.F.; Hu, D.M. The Positive Impacts of Microbial Phytase on Its Nutritional Applications. Trends Food Sci. Technol. 2019, 86, 553–562. [Google Scholar] [CrossRef]
- Hung, L.T.; Thanh, N.T.; Pham, M.A.; Browdy, C.L. A Comparison of the Effect of Dietary Fungal Phytase and Dicalcium Phosphate Supplementation on Growth Performances, Feed and Phosphorus Utilization of Tra Catfish Juveniles (Pangasianodon hypophthalmus Sauvage, 1878). Aquacult. Nutr. 2015, 21, 10–17. [Google Scholar] [CrossRef]
- Robinson, E.H.; Li, M.H.; Manning, B.B. Comparison of Microbial Phytase and Dicalcium Phosphate for Growth and Bone Mineralization of Pond-Raised Channel Catfish, Ictalurus punctatus. J. Appl. Aquac. 2002, 12, 81–88. [Google Scholar] [CrossRef]
- Lall, S.P.; Tibbetts, S.M. Nutrition, Feeding, and Behavior of Fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 361–372. [Google Scholar] [CrossRef]
- Falayi, B.A.; Sadiku, O.E. Biotechnology of Floating Feed Development. Int. J. Innov. Res. Dev. 2013, 2, 387–1407. [Google Scholar]
- Onomu, A.J.; Slater, M.J.; Vine, N.G. Coculture of Abalone (Haliotis midae) and Sea Cucumber (Neostichopus grammatus) to Reduce Tank Cleaning Frequency in Abalone Farming. Aquac. Int. 2024, 32, 7173–7199. [Google Scholar] [CrossRef]
- Onomu, A.J.; Slater, M.J.; Vine, N.G. Sea cucumber (Neostichopus grammatus) density and tank cleaning frequency affect abalone (Haliotis midae) growth in integrated multi-trophic aquaculture. J. World Aquac. 2024. in press. [Google Scholar]
- Amirkolaie, A.K. Reduction in the Environmental Impact of Waste Discharged by Fish Farms through Feed and Feeding: Aquaculture and the Environment. Rev. Aquac. 2011, 3, 19–26. [Google Scholar] [CrossRef]
- Sriherwanto, C. Recent Potential Biotechnological Applications of the Tempeh Mould Rhizopus. A Short Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 759, 012066. [Google Scholar] [CrossRef]
- Adekunle, H.L.; Sadiku, S.O.; Orire, A.M. Development of Farm Made Floating Feed for Aquaculture Species. Int. J. Adv. Biol. Res. 2012, 2, 579–583. [Google Scholar]
- Orire, A.M.; Sadiku, S.; Fache, P.O. The Effects of Varying Inclusion Levels of Saccharomyces Cerevisae and Incubation Periods on Aqua-Feed Buoyancy. Niger. J. Fish. 2015, 12. [Google Scholar]
- Joshi, K.; Meher, M.K.; Poluri, K.M. Fabrication and Characterization of Bioblocks from Agricultural Waste Using Fungal Mycelium for Renewable and Sustainable Applications. ACS Appl. Bio Mater. 2020, 3, 1884–1892. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; De Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a Circular Economy with Fungal Biotechnology: A White Paper. Fungal Biology and Biotechnology. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Ebeneezar, S.; Prabu, D.L.; Chandrasekar, S.; Tejpal, C.S.; Madhu, K.; Sayooj, P.; Vijayagopal, P. Evaluation of Dietary Oleoresins on the Enhancement of Skin Coloration and Growth in the Marine Ornamental Clown Fish, Amphiprion Ocellaris (Cuvier, 1830). Aquacultture 2020, 529, 735728. [Google Scholar] [CrossRef]
- Saxena, A. Health Colouration of Fish. In International Symposium on Aquatic Animal Health: Program and Abstracts; University of California, School of Veterinary Medicine: Davis, CA, USA, 1994; p. 94. [Google Scholar]
- Rekha, R.; Nimsi, K.A.; Manjusha, K.; Sirajudheen, T.K. Marine Yeast Rhodotorula Paludigena VA 242 a Pigment Enhancing Feed Additive for the Ornamental Fish Koi Carp. Aquac. Fish. 2024, 9, 66–70. [Google Scholar] [CrossRef]
- Sanchez, S.; Ruiz, B.; Rodríguez-Sanoja, R.; Flores-Cotera, L.B.; McNeil, B.; Archer, D.; Giavasis, I.; Harvey, L. Microbial Production of Carotenoids. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2013; pp. 194–233. [Google Scholar]
- Velmurugan, P.; Sralathan, K.; Vellingiri, B.; Perumalsamy, L.; Jong-Chan, C.; Byung-Taek, O. Natural Pigment Extraction from Five Filamentous Fungi for Industrial Applications and Dyeing of Leather. Carbohydr. Polym. 2010, 79, 262–268. [Google Scholar] [CrossRef]
- Gusdinar, T.; Singgih, M.; Priatni, S.; Sukmawati, A.E.; Suciati, T. Enkapsulasi Dan Stabilitas Pigmen Karotenoid Dari Neurospora Intermedia N-1 (Encapsulation and the Stability of Carotenoids from Neurospora Intermedia n-1). J. Mns. Dan Lingkung. 2014, 18, 206–211. [Google Scholar]
- Pagano, M.C.; Dhar, P.P. Fungal pigments: An overview. In Fungal Biomolecules: Sources, Applications and Recent Developments, 1st ed.; Gupta, V.K., Mach, R.L., Sreenivasaprasad, S., Eds.; Wiley: London, UK, 2015; ISBN 978-1-118-95829-2. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Deng, D.F.; Dominy, W.G.; Forster, I.P. Pigmentation of Pacific White Shrimp, Litopenaeus vannamei, by Dietary Astaxanthin Extracted from Haematococcus pluvialis. J. World Aquac. Soc. 2011, 42, 633–644. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Song, X.; Liu, W.; Zhang, L.; Wang, X.; Lv, C. Astaxanthin Ameliorates Lung Fibrosis in Vivo and in Vitro by Preventing Transdifferentiation, Inhibiting Proliferation, and Promoting Apoptosis of Activated Cells. Food Chem. Toxicol. 2013, 56, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.J.; Tian, L.X.; Yang, H.J.; Liang, G.Y.; Yue, Y.R.; Xu, D.H. Effects of Dietary Astaxanthin on Growth, Antioxidant Capacity and Gene Expression in Pacific White Shrimp Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 917–927. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.-H. Astaxanthin from Microbial Sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Stachowiak, B.; Piotr, S. Astaxanthin for the Food Industry M. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Meruvu, H.; Dos Santos, J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021, 41, 1153–1177. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a Potential Source of Pigments: Harnessing Filamentous Fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and Some Other Pigments from Fungi and Yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and Cultural Stimulants in the Production of Carotenoids from Microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef]
- Hailei, W.; Ping, L.; Yufeng, L.; Zhifang, R.; Gang, W. Overproduction of a Potential Red Pigment by a Specific Self-Immobilization Biomembrane Surface Liquid Culture of Penicillium Novae-Zeelandiae. Bioprocess Biosyst. Eng. 2012, 35, 1407–1416. [Google Scholar] [CrossRef]
- Pandit, A.; Maheshwari, R. Life-History of Neurospora Intermedia in a Sugar Cane Field. J. Biosci. 1996, 21, 57–79. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Pereira, J.F.B.; Dufossé, L.; Raghavan, V.; Santos-Ebinuma, V.C.; Pessoa, A. Advances and Trends in Biotechnological Production of Natural Astaxanthin by Phaffia rhodozyma Yeast. Crit. Rev. Food Sci. Nutr. 2023, 63, 1862–1876. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Chang, J.-J.; Huang, H.-T.; Lee, C.-P.; Hu, Y.-F.; Wu, M.-L.; Huang, C.-Y.; Nan, F.-H. Improving Red-Color Performance, Immune Response and Resistance to Vibrio Parahaemolyticus on White Shrimp Penaeus vannamei by an Engineered Astaxanthin Yeast. Sci. Rep. 2023, 13, 2248. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Luo, K.; Zhang, S.; Wei, C.; Wang, L.; Qiu, Y.; Tian, X. ‘Biotic’ Potential of the Red Yeast Rhodotorula mucilaginosa Strain JM-01 on the Growth, Shell Pigmentation, and Immune Defense Attributes of the Shrimp, Penaeus vannamei. Aquaculture 2023, 572, 739543. [Google Scholar] [CrossRef]
- Montes De Oca, S.; Téllez-Téllez, M.; Arce, E.; García-Rodríguez, J.; Burciaga, L.M.; De Lourdes Acosta-Urdapilleta, M. Effect of Mushroom Powder (Pleurotus ostreatus and Pleurotus djamor) on Skin Coloration and Inflammation in Siamese Fighting Fish (Betta splendens). J. Appl. Aquac. 2024, 1–16. [Google Scholar] [CrossRef]
- Mapari, S.A.; Meyer, A.S.; Thrane, U. Photostability of Natural Orange–Red and Yellow Fungal Pigments in Liquid Food Model Systems. J. Agric. Food Chem. 2009, 57, 6253–6261. [Google Scholar] [CrossRef]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste Production in Aquaculture: Sources, Components and Managements in Different Culture Systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Tomasini, A.; León-Santiesteban, H.H. The Role of the Filamentous Fungi in Bioremediation. In Fungal Bioremediation: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Molla, A.H.; Fakhru’l-Razi, A.; Alam, M.Z. Evaluation of Solid-State Bioconversion of Domestic Wastewater Sludge as a Promising Environmental-Friendly Disposal Technique. Water Res. 2004, 38, 4143–4152. [Google Scholar] [CrossRef]
- Guest, R.K.; Smith, D.W. A Potential New Role for Fungi in a Wastewater MBR Biological Nitrogen Reduction System. J. Environ. Eng. Sci. 2002, 6, 433–437. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of Selected Pharmaceutical and Personal Care Products (PPCPs) by White-Rot Fungi. World J. Microbiol. Biotechnol. 2011, 27, 1839–1846. [Google Scholar] [CrossRef]
- Salandez, V.; Emami, S.; Taha, A.Y.; Saponara, V.L. Use of Ganoderma lucidum Grown on Agricultural Waste to Remove Antibiotics from Water. bioRxiv 2022, 1–39. [Google Scholar] [CrossRef]
- Lalitha, N.; Patil, P.K.; Rajesh, R.; Muralidhar, M. Usage of Pleurotus Ostreatus for Degradation of Oxytetracycline in Varying Water Salinities in Brackishwater Aquaculture System. J. Coast. Res. 2019, 86, 138. [Google Scholar] [CrossRef]
- Prieto, A.; Möder, M.; Rodil, R.; Adrian, L.; Marco-Urrea, E. Degradation of the Antibiotics Norfloxacin and Ciprofloxacin by a White-Rot Fungus and Identification of Degradation Products. Bioresour. Technol. 2011, 102, 10987–10995. [Google Scholar] [CrossRef] [PubMed]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive Use of Filamentous Fungi to Remove Pharmaceutical Substances from Wastewater. J. Water Process Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Jureczko, M.; Przystaś, W.; Krawczyk, T.; Gonciarz, W.; Rudnicka, K. White-Rot Fungi-Mediated Biodegradation of Cytostatic Drugs-Bleomycin and Vincristine. J. Hazard. Mater. 2021, 407, 124632. [Google Scholar] [CrossRef]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, T.; Marco-Urrea, E. Hospital Wastewater Treatment by Fungal Bioreactor: Removal Efficiency for Pharmaceuticals and Endocrine Disruptor Compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef]
- Chandra, D.; Mishra, A.; Trakroo, M.D.; Chauhan, R.S.; Mishra, S.K. Impact of Mycofiltration on Water Quality. Environ. Qual. Mgmt 2022, 31, 253–266. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravi Kumar, R.; Ganesan, V.; Anbazhagan, C. Microalgae: A Sustainable Feed Source for Aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Onomu, A.J.; Vine, N.G.; Cyrus, M.D.; Macey, B.M.; Bolton, J.J. The Effect of Fresh Seaweed and a Formulated Diet Supplemented with Seaweed on the Growth and Gonad Quality of the Collector Sea Urchin, Tripneustes gratilla, under Farm Conditions. Aquac. Res. 2020, 51, 4087–4102. [Google Scholar] [CrossRef]
- Qin, S.; Wang, K.; Gao, F.; Ge, B.; Cui, H.; Li, W. Biotechnologies for Bulk Production of Microalgal Biomass: From Mass Cultivation to Dried Biomass Acquisition. Biotechnol. Biofuels 2023, 16, 131. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Bhattacharya, A.; Kumar, P.; Malik, A.; Vijay, V.K. A Method for Simultaneous Bioflocculation and Pretreatment of Algal Biomass Targeting Improved. Green Chem. 2016, 8, 5230–5238. [Google Scholar] [CrossRef]
- Wan, C.; Alam, A.; Zhao, X.; Guo, S.; Ho, S.; Bai, F. Current Progress and Future Prospect of Microalgal Biomass Harvest Using Various Flocculation Technologies. Bioresour. Technol. 2015, 184, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Patidar, S.K. Microalgae Harvesting Techniques: A Review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of Coagulation/Flocculation in Oily Wastewater Treatment: A Review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of Microalgal Biomass: Efficient Method for Flocculation through pH Modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef]
- Li, Y.; Nie, H.; Zhang, H.; Niu, W.; Li, S.; Wang, H. Promotion Effect of Bacteria in Phycosphere on Flocculation Activity of Aspergillus Niger on Synechocystis Biomass. Aquaculture 2022, 550, 737833. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a Low-Cost Method for Harvesting Microalgae for Bulk Biomass Production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Zhu, T.; Song, J.; Zhou, X.; Liu, Y. Preparation, Characterization and Application of a Composite Bioflocculant. Environ. Technol. 2024, 1–9. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Bassin, I.D.; Cammarota, M.C. Bioflocculation of Cyanobacteria with Pellets of Aspergillus Niger: Effects of Carbon Supplementation, Pellet Diameter, and Other Factors in Biomass Densification. Bioresour. Technol. 2019, 294, 122167. [Google Scholar] [CrossRef]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A Comparative Study between Fungal Pellet- and Spore-Assisted Microalgae Harvesting Methods for Algae Bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef]
- Madkour, A.; Ibrahim, H.; El-Sayed, W.; El-Moselhy, K. Bioflocculation Technique for Microalgal Harvesting and Wastewater Nutrient Recovery. Iran. J. Fish. Sci. 2018, 19, 1780–1794. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Larsen, T.O.; Thrane, U.; Meijer, M.; Varga, J.; Samson, R.A.; Nielsen, K.F. Fumonisin and Ochratoxin Production in Industrial Aspergillus niger Strains. PLoS ONE 2011, 6, e23496. [Google Scholar] [CrossRef] [PubMed]
- Månsson, M.; Klejnstrup, M.L.; Phipps, R.K.; Nielsen, K.F.; Frisvad, J.C.; Gotfredsen, C.H.; Larsen, T.O. Isolation and NMR Characterization of Fumonisin B2 and a New Fumonisin B6 from Aspergillus Niger. J. Agric. Food Chem. 2010, 58, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; El-Samawaty, A.E.R.M.; Dawoud, T.M.; Abd-Elkader, O.H.; Al Maary, K.S.; Hatamleh, A.A.; Elgorban, A.M. Characterization and Anti-Aspergillus Flavus Impact of Nanoparticles Synthesized by Penicillium citrinum. Saudi J. Biol. Sci. 2017, 24, 1243–1248. [Google Scholar] [CrossRef]
- Chang, P.K.; Ehrlich, K.C.; Fujii, I. Cyclopiazonic Acid Biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins 2009, 1, 74–99. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Közégi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
| Fibre Fractions | Feed Ingredient | Fungi Species | Raw Ingredient | Fermented | Variation (%) | References |
|---|---|---|---|---|---|---|
| Crude fibre | Rice bran | Aspergillus niger | 10.66 | 4.60 | 56.85 | [65] |
| Tea dregs | Trichoderma koningii Aspergillus niger | 18.2 18.2 | 22.3 19.7 | −22.53 −8.24 | [66] | |
| Palm kernel cake | Aspergillus niger | 16.8 | 14.5 | 13.69 | [16] | |
| Cotton seed meal | Aspergillus niger | 30.67 | 30.57 | 0.33 | [67] | |
| Sunflower meal | Aspergillus niger | 20.43 | 23.05 | −12.83 | [67] | |
| Hazelnut meal | Aspergillus niger | 13.78 | 8.60 | 37.59 | [67] | |
| Wheat Bran | Aspergillus niger | 8.97 | 5.20% | 42.02 | [68] | |
| Brewer’s spent grain | Aspergillus niger | 13.31% | 9.20 | 30.88 | [68] | |
| Palmkernel cake | Aspergillus niger | 15.59 | 14.91% | 4.36 | [68] | |
| Wheat bran | Aspergillus niger ATCC 200345 | 10.95 | 8.97% | 18.08 | [69] | |
| Tomato pomace | Aspergillus niger | 21.71 | 23.0 | −5.94 | [70] | |
| Rice bran | Aspergillus flavus | 12.05 | 9.35 | 22.41 | [71] | |
| Rapeseed meal | Rhizopus microsporus | 10.8 | 11.9 | −10.19 | [72] | |
| ADF | Wheat bran | Aspergillus niger | 13.46 | 11.87 | 11.81 | [69] |
| Canola meal | Aspergillus pullulans (58522) Aspergillus pullulans (42023) Aspergillus pullulans (Y-2311-1) Pichia kudriavzevii Tricoderma reesei Fusarium venenatum Mucor circinelloides | 19.9 19.9 19.9 19.9 19.9 19.9 19.9 | 17.7 18.0 22.4 18.7 19.1 22.5 20.1 | 11.06 9.56 −12.56 6.03 4.02 13.07 −1 | [73] | |
| Rapeseed meal | Aspergillus niger | 33.70 | 30.76 | 8.72 | [15] | |
| Tomato pomace | Aspergillus niger | 25.22 | 26.84 | −6.42 | [70] | |
| Wheat bran | Aspergillus niger ATCC 52172 | 13.46 | 11.87 | 11.81 | [69] | |
| Hazelnut meal | Aspergillus niger ATCC 52172 | 22.44 | 19.36 | 13.73 | [67] | |
| Sunflower meal | Aspergillus niger ATCC 52172 | 23.82 | 26.15 | −9.78 | [67] | |
| Cottonseed meal | Aspergillus niger ATCC 52172 | 39.70 | 43.13 | −8.64 | [67] | |
| Palm kernel cake | Aspergillus niger | 46.8 | 35.8 | 23.50 | [16] | |
| Tea dregs | Aspergillus niger | 24.2 | 26.8 | −10.74 | [66] | |
| Tea dregs | Trichoderma koningii | 24.2 | 29.5 | −21.9 | [66] | |
| Rice bran | Aspergillus niger | 17.28 | 12.43 | 28.07 | [65] | |
| Rapeseed meal | Rhizopus microsporus | 10.8 | 11.9 | −10.1 | [72] | |
| NDF | Rapeseed | Aspergillus niger | 47.08 | 40.74 | 13.47 | [15] |
| Rice bran | Aspergillus flavus | 12.05 | 9.35 | 22.41 | [71] | |
| Tomato pomace | Aspergillus niger | 21.71 | 23.00 | −5.94 | [70] | |
| Wheat bran | Aspergillus niger ATCC 52172 | 10.95 | 9.79 | 10.59 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onomu, A.J.; Okuthe, G.E. The Application of Fungi and Their Secondary Metabolites in Aquaculture. J. Fungi 2024, 10, 711. https://doi.org/10.3390/jof10100711
Onomu AJ, Okuthe GE. The Application of Fungi and Their Secondary Metabolites in Aquaculture. Journal of Fungi. 2024; 10(10):711. https://doi.org/10.3390/jof10100711
Chicago/Turabian StyleOnomu, Abigail John, and Grace Emily Okuthe. 2024. "The Application of Fungi and Their Secondary Metabolites in Aquaculture" Journal of Fungi 10, no. 10: 711. https://doi.org/10.3390/jof10100711
APA StyleOnomu, A. J., & Okuthe, G. E. (2024). The Application of Fungi and Their Secondary Metabolites in Aquaculture. Journal of Fungi, 10(10), 711. https://doi.org/10.3390/jof10100711






