State of the Field: Cytotoxic Immune Cell Responses in C. neoformans and C. deneoformans Infection
Abstract
1. Introduction
2. CD8+ T-Cells in the Adaptive Immune Response
2.1. Murine Localized Pulmonary Infection
2.2. Murine Cryptococcal Meningitis
2.3. Summary of Murine Studies
2.4. Human PBMCs in Culture
2.5. Human Cryptococcal Meningitis
2.6. Summary of Human Studies
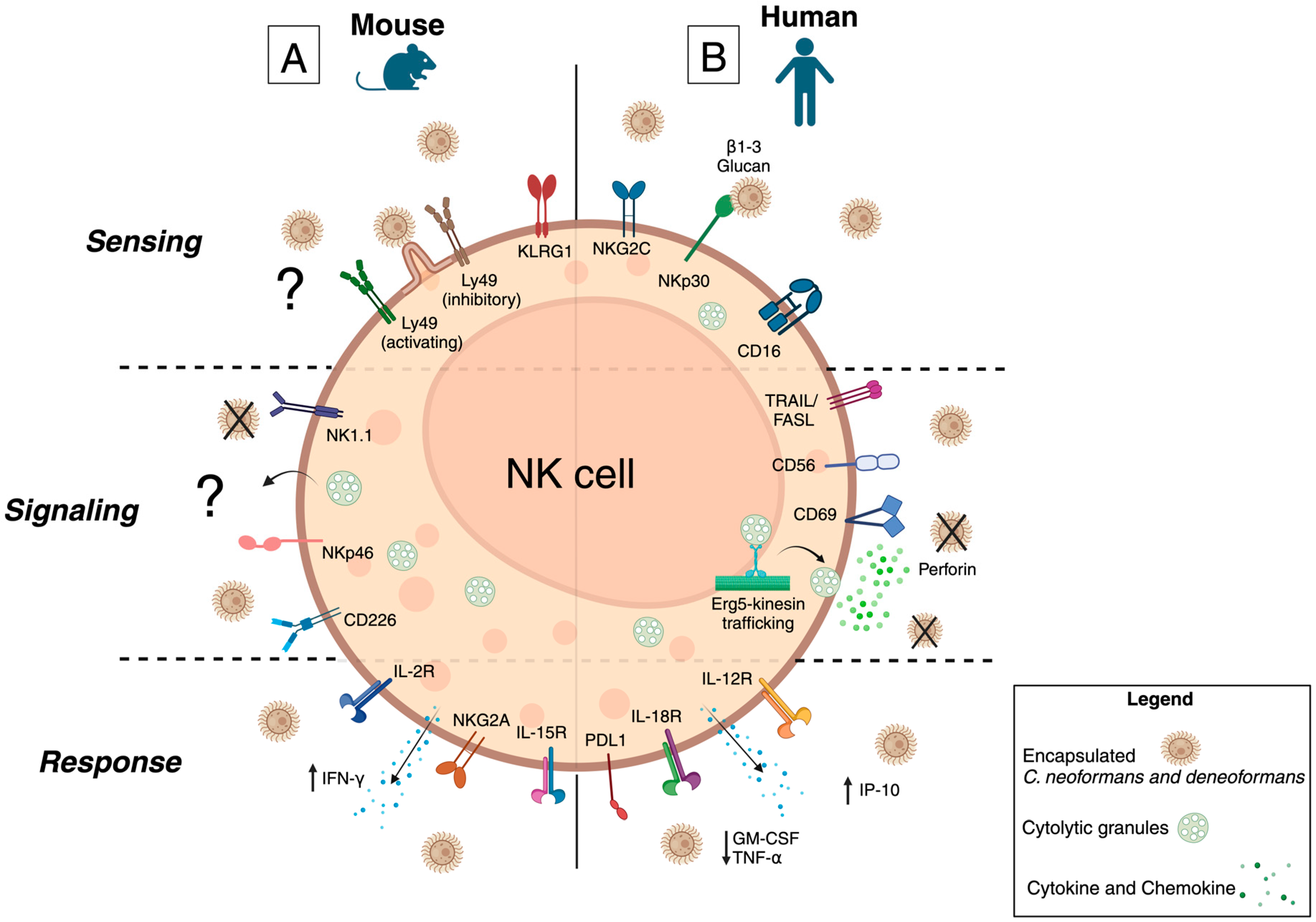
3. NK Cells in the Innate Immune Response
3.1. Murine Localized Pulmonary Infection
3.2. Murine Cryptococcal Meningitis
3.3. Summary of Murine Studies
3.4. Human PBMCs in Culture
3.5. Human Cryptococcal Meningitis
3.6. Summary of Human Studies
4. Lesser Studied Cytotoxic Cell Populations in Cryptococcosis
4.1. γδ T-Cells in Murine Pulmonary Infection
4.2. NK T-Cells in Murine Pulmonary Infection
4.3. Cytotoxic CD4+ T-Cells in Human C. neoformans Infection
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirofski, L.A.; Casadevall, A. The state of latency in microbial pathogenesis. J. Clin. Investig. 2020, 130, 4525–4531. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A. Dormancy in Cryptococcus neoformans: 60 years of accumulating evidence. J. Clin. Investig. 2020, 130, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.L.; Khine, H.; Abadi, J.; Lindenberg, D.J.; Pirofski, L.-A.; Niang, R.; Casadevall, A. Serologic Evidence forCryptococcus neoformansInfection in Early Childhood. Pediatrics 2001, 107, e66. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, D.; Janbon, G.; Dromer, F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 1999, 37, 3204–3209. [Google Scholar] [CrossRef]
- Saha, D.C.; Goldman, D.L.; Shao, X.; Casadevall, A.; Husain, S.; Limaye, A.P.; Lyon, M.; Somani, J.; Pursell, K.; Pruett, T.L.; et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin. Vaccine Immunol. 2007, 14, 1550–1554. [Google Scholar] [CrossRef]
- Singh, N.; Dromer, F.; Perfect, J.R.; Lortholary, O. Immunocompromised Hosts: Cryptococcosis in Solid Organ Transplant Recipients: Current State of the Science. Clin. Infect. Dis. 2008, 47, 1321–1327. [Google Scholar] [CrossRef]
- Domaica, C.I.; Fuertes, M.B.; Uriarte, I.; Girart, M.V.; Sardanons, J.; Comas, D.I.; Di Giovanni, D.; Gaillard, M.I.; Bezrodnik, L.; Zwirner, N.W. Human natural killer cell maturation defect supports in vivo CD56(bright) to CD56(dim) lineage development. PLoS ONE 2012, 7, e51677. [Google Scholar] [CrossRef]
- Brunet, K.; Alanio, A.; Lortholary, O.; Rammaert, B. Reactivation of dormant/latent fungal infection. J. Infect. 2018, 77, 463–468. [Google Scholar] [CrossRef]
- Ding, M.; Smith, K.D.; Wiesner, D.L.; Nielsen, J.N.; Jackson, K.M.; Nielsen, K. Use of Clinical Isolates to Establish Criteria for a Mouse Model of Latent Cryptococcus neoformans Infection. Front. Cell Infect. Microbiol. 2021, 11, 804059. [Google Scholar] [CrossRef]
- Ellis, J.; Bangdiwala, A.S.; Cresswell, F.V.; Rhein, J.; Nuwagira, E.; Ssebambulidde, K.; Tugume, L.; Rajasingham, R.; Bridge, S.C.; Muzoora, C.; et al. The Changing Epidemiology of HIV-Associated Adult Meningitis, Uganda 2015–2017. Open Forum Infect. Dis. 2019, 6, ofz419. [Google Scholar] [CrossRef]
- Hakim, J.G.; Gangaidzo, I.T.; Heyderman, R.S.; Mielke, J.; Mushangi, E.; Taziwa, A.; Robertson, V.J.; Musvaire, P.; Mason, P.R. Impact of HIV infection on meningitis in Harare, Zimbabwe: A prospective study of 406 predominantly adult patients. AIDS 2000, 14, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Meintjes, G.; Williams, A.; Brown, Y.; Crede, T.; Harrison, T.S. Adult meningitis in a setting of high HIV and TB prevalence: Findings from 4961 suspected cases. BMC Infect. Dis. 2010, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.; Rajasingham, R.; Rolfes, M.A.; Birkenkamp, K.E.; Meya, D.B.; Boulware, D.R. Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis. PLoS Med. 2012, 9, e1001316. [Google Scholar] [CrossRef]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2015, 14, 106–117. [Google Scholar] [CrossRef]
- Litvintseva, A.P.; Carbone, I.; Rossouw, J.; Thakur, R.; Govender, N.P.; Mitchell, T.G. Evidence that the Human Pathogenic Fungus Cryptococcus neoformans var. grubii May Have Evolved in Africa. PLoS ONE 2011, 6, e19688. [Google Scholar] [CrossRef]
- Mukaremera, L.; Lee, K.K.; Wagener, J.; Wiesner, D.L.; Gow, N.A.R.; Nielsen, K. Titan cell production in cryptococcus neofromans reshapes the cell wall and capsule composition during infection. Cell Surf. 2018, 1, 15–24. [Google Scholar] [CrossRef]
- Oscar Zaragoza, M.L.R.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. Chapter 4 The Capsule of the Fungal Pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar] [CrossRef]
- Baker, L.G.; Specht, C.A.; Lodge, J.K. Cell Wall Chitosan Is Necessary for Virulence in the Opportunistic Pathogen Cryptococcus neoformans. Eukaryot. Cell 2011, 10, 1264–1268. [Google Scholar] [CrossRef]
- Reese, A.J.; Yoneda, A.; Breger, J.A.; Beauvais, A.; Liu, H.; Griffith, C.L.; Bose, I.; Kim, M.J.; Skau, C.; Yang, S.; et al. Loss of cell wall alpha(1–3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 2006, 63, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Decote-Ricardo, D.; LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nascimento, D.O.; Nunes, M.P.; Morrot, A.; Freire-de-Lima, L.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Immunomodulatory Role of Capsular Polysaccharides Constituents of Cryptococcus neoformans. Front. Med. 2019, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Monari, C.; Paganelli, F.; Bistoni, F.; Kozel, T.R.; Vecchiarelli, A. Capsular polysaccharide induction of apoptosis by intrinsic and extrinsic mechanisms. Cell. Microbiol. 2008, 10, 2129–2137. [Google Scholar] [CrossRef]
- LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nunes, M.P.; Oliveira, P.A.V.; Nascimento, D.d.O.; Freire-de-Lima, L.; Takiya, C.M.; Morrot, A.; Decote-Ricardo, D.; Previato, J.O.; et al. Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci. Rep. 2018, 8, 16378. [Google Scholar] [CrossRef]
- Dong, Z.M.; Jackson, L.; Murphy, J.W. Mechanisms for induction of L-selectin loss from T lymphocytes by a cryptococcal polysaccharide, glucuronoxylomannan. Infect. Immun. 1999, 67, 220–229. [Google Scholar] [CrossRef]
- Doitsh, G.; Greene, W.C. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 2016, 19, 280–291. [Google Scholar] [CrossRef]
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef]
- Schaefer, M.R.; Wonderlich, E.R.; Roeth, J.F.; Leonard, J.A.; Collins, K.L. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PLoS Pathog. 2008, 4, e1000131. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Casazza, J.P.; Stone, H.H.; Meintjes, G.; Lawn, S.D.; Levitz, S.M.; Harrison, T.S.; Koup, R.A. The Phenotype of the Cryptococcus-Specific CD4+ Memory T-Cell Response Is Associated With Disease Severity and Outcome in HIV-Associated Cryptococcal Meningitis. J. Infect. Dis. 2013, 207, 1817–1828. [Google Scholar] [CrossRef]
- Mukaremera, L.; Nielsen, K. Adaptive Immunity to Cryptococcus neoformans Infections. J. Fungi 2017, 3, 64. [Google Scholar] [CrossRef]
- Lindell, D.M.; Moore, T.A.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Distinct Compartmentalization of CD4+ T-Cell Effector Function Versus Proliferative Capacity during Pulmonary Cryptococcosis. Am. J. Pathol. 2006, 168, 847–855. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Yates, J.L.; Lipscomb, M.F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 1991, 173, 793–800. [Google Scholar] [CrossRef]
- Hill, J.O. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J. Exp. Med. 1992, 175, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.L.; Kozel, T.R.; Doyle, H.A. Requirement for CD4+T Lymphocytes in Host Resistance againstCryptococcus neoformansin the Central Nervous System of Immunized Mice. Infect. Immun. 2000, 68, 456–462. [Google Scholar] [CrossRef]
- Lim, T.S.; Murphy, J.W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect. Immun. 1980, 30, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Elsegeiny, W.; Marr, K.A.; Williamson, P.R. Immunology of Cryptococcal Infections: Developing a Rational Approach to Patient Therapy. Front. Immunol. 2018, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Onyishi, C.U.; May, R.C. Human immune polymorphisms associated with the risk of cryptococcal disease. Immunology 2022, 165, 143–157. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Nyazika, T.K.; Ssebambulidde, K.; Lionakis, M.S.; Meya, D.B.; Drummond, R.A. Fungal CNS Infections in Africa: The Neuroimmunology of Cryptococcal Meningitis. Front. Immunol. 2022, 13, 804674. [Google Scholar] [CrossRef]
- Meya, D.B.; Williamson, P.R. Cryptococcal Disease in Diverse Hosts. N. Engl. J. Med. 2024, 390, 1597–1610. [Google Scholar] [CrossRef]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The Natural Cytotoxicity Receptors in Health and Disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bevan, M.J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Shiromizu, C.M.; Jancic, C.C. γδ T Lymphocytes: An Effector Cell in Autoimmunity and Infection. Front. Immunol. 2018, 9, 2389. [Google Scholar] [CrossRef] [PubMed]
- Cenerenti, M.; Saillard, M.; Romero, P.; Jandus, C. The Era of Cytotoxic CD4 T Cells. Front. Immunol. 2022, 13, 867189. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inoges, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017, 95, 347–355. [Google Scholar] [CrossRef]
- Cassioli, C.; Baldari, C.T. The Expanding Arsenal of Cytotoxic T Cells. Front. Immunol. 2022, 13, 883010. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Lindell, D.M.; Moore, T.A.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J. Immunol. 2005, 174, 7920–7928. [Google Scholar] [CrossRef]
- Lindell, D.M.; Ballinger, M.N.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Diversity of the T-Cell Response to PulmonaryCryptococcus neoformansInfection. Infect. Immun. 2006, 74, 4538–4548. [Google Scholar] [CrossRef]
- Murdock, B.J.; Huffnagle, G.B.; Olszewski, M.A.; Osterholzer, J.J. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect. Immun. 2014, 82, 937–948. [Google Scholar] [CrossRef]
- Neal, L.M.; Qiu, Y.; Chung, J.; Xing, E.; Cho, W.; Malachowski, A.N.; Sandy-Sloat, A.R.; Osterholzer, J.J.; Maillard, I.; Olszewski, M.A. T Cell–Restricted Notch Signaling Contributes to Pulmonary Th1 and Th2 Immunity during Cryptococcus neoformans Infection. J. Immunol. 2017, 199, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, F.; Lortholary, O.; Kansau, I.; Neuville, S.; Gray, F.; Dromer, F. Pathogenesis of Cerebral Cryptococcus neofromans Infection after Fungemia. J. Infect. Dis. 2002, 186, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Mody, C.H.; Chen, G.-H.; Jackson, C.; Curtis, J.L.; Toews, G.B. Un vivo depletion of murine CD8 positive T cells impairs survival during infection with a highly virulent strain ofCryptococcus neoformans. Mycopathologia 1994, 125, 7–17. [Google Scholar] [CrossRef]
- Normile, T.G.; Rella, A.; Del Poeta, M. Cryptococcus neoformans Δsgl1 Vaccination Requires Either CD4+ or CD8+ T Cells for Complete Host Protection. Front. Cell. Infect. Microbiol. 2021, 11, 739027. [Google Scholar] [CrossRef]
- Wang, R.; Oliveira, L.V.N.; Lourenco, D.; Gomez, C.L.; Lee, C.K.; Hester, M.M.; Mou, Z.; Ostroff, G.R.; Specht, C.A.; Levitz, S.M. Immunological correlates of protection following vaccination with glucan particles containing Cryptococcus neoformans chitin deacetylases. Npj Vaccines 2023, 8, 6. [Google Scholar] [CrossRef]
- Specht, C.A.; Wang, R.; Oliveira, L.V.N.; Hester, M.M.; Gomez, C.; Mou, Z.; Carlson, D.; Lee, C.K.; Hole, C.R.; Lam, W.C.; et al. Immunological correlates of protection mediated by a whole organism, Cryptococcus neoformans, vaccine deficient in chitosan. mBio 2024, 15, e0174624. [Google Scholar] [CrossRef] [PubMed]
- Syme, R.M.; Wood, C.J.; Wong, H.; Mody, C.H. Both CD4+ and CD8+ human lymphocytes are activated and proliferate in response to Cryptococcus neoformans. Immunology 1997, 92, 194–200. [Google Scholar] [CrossRef]
- Ma, L.L.; Spurrell, J.C.; Wang, J.F.; Neely, G.G.; Epelman, S.; Krensky, A.M.; Mody, C.H. CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J. Immunol. 2002, 169, 5787–5795. [Google Scholar] [CrossRef]
- Meya, D.B.; Okurut, S.; Zziwa, G.; Rolfes, M.A.; Kelsey, M.; Cose, S.; Joloba, M.; Naluyima, P.; Palmer, B.E.; Kambugu, A.; et al. Cellular immune activation in cerebrospinal fluid from ugandans with cryptococcal meningitis and immune reconstitution inflammatory syndrome. J. Infect. Dis. 2015, 211, 1597–1606. [Google Scholar] [CrossRef]
- Chang, C.C.; Omarjee, S.; Lim, A.; Spelman, T.; Gosnell, B.I.; Carr, W.H.; Elliott, J.H.; Moosa, M.-Y.S.; Ndung’u, T.; French, M.A.; et al. Chemokine Levels and Chemokine Receptor Expression in the Blood and the Cerebrospinal Fluid of HIV-Infected Patients With Cryptococcal Meningitis and Cryptococcosis-Associated Immune Reconstitution Inflammatory Syndrome. J. Infect. Dis. 2013, 208, 1604–1612. [Google Scholar] [CrossRef]
- Boulware, D.R.; Meya, D.B.; Muzoora, C.; Rolfes, M.A.; Huppler Hullsiek, K.; Musubire, A.; Taseera, K.; Nabeta, H.W.; Schutz, C.; Williams, D.A.; et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 2014, 370, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease among Adults, Adolescents and Children Living with HIV; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Warren, J.A.; Clutton, G.; Goonetilleke, N. Harnessing CD8+ T Cells Under HIV Antiretroviral Therapy. Front. Immunol. 2019, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Perdomo-Celis, F.; Taborda, N.A.; Rugeles, M.T. CD8+ T-Cell Response to HIV Infection in the Era of Antiretroviral Therapy. Front. Immunol. 2019, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Bayiyana, A.; Okurut, S.; Nabatanzi, R.; Zziwa, G.; Boulware, D.R.; Lutwama, F.; Meya, D. Longitudinal Changes in Cd4(+), Cd8(+) T Cell Phenotype and Activation Marker Expression Following Antiretroviral Therapy Initiation among Patients with Cryptococcal Meningitis. J. Fungi 2019, 5, 63. [Google Scholar] [CrossRef]
- Neal, L.M.; Xing, E.; Xu, J.; Kolbe, J.L.; Osterholzer, J.J.; Segal, B.M.; Williamson, P.R.; Olszewski, M.A. CD4(+) T Cells Orchestrate Lethal Immune Pathology despite Fungal Clearance during Cryptococcus neoformans Meningoencephalitis. mBio 2017, 8, e02063-17. [Google Scholar] [CrossRef]
- Delliere, S.; Guery, R.; Candon, S.; Rammaert, B.; Aguilar, C.; Lanternier, F.; Chatenoud, L.; Lortholary, O. Understanding Pathogenesis and Care Challenges of Immune Reconstitution Inflammatory Syndrome in Fungal Infections. J. Fungi 2018, 4, 139. [Google Scholar] [CrossRef]
- Meya, D.B.; Okurut, S.; Zziwa, G.; Cose, S.; Boulware, D.R.; Janoff, E.N. HIV-Associated Cryptococcal Immune Reconstitution Inflammatory Syndrome Is Associated with Aberrant T Cell Function and Increased Cytokine Responses. J. Fungi 2019, 5, 42. [Google Scholar] [CrossRef]
- Uezu, K.; Kawakami, K.; Miyagi, K.; Kinjo, Y.; Kinjo, T.; Ishikawa, H.; Saito, A. Accumulation of γδ T Cells in the Lungs and Their Regulatory Roles in Th1 Response and Host Defense against Pulmonary Infection with Cryptococcus neoformans. J. Immunol. 2004, 172, 7629–7634. [Google Scholar] [CrossRef]
- Heung, L.J.; Hohl, T.M. DAP12 Inhibits Pulmonary Immune Responses to Cryptococcus neoformans. Infect. Immun. 2016, 84, 1879–1886. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nakamura, Y.; Sato, K.; Takahashi, Y.; Nomura, T.; Miyasaka, T.; Ishii, K.; Hara, H.; Yamamoto, N.; Kanno, E.; et al. Defect of CARD9 leads to impaired accumulation of gamma interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans. Infect. Immun. 2014, 82, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.H.; Zhang, T.; Koguchi, Y.; Nakashima, K.; Okamura, H.; Kurimoto, M.; Kawakami, K. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur. J. Immunol. 1999, 29, 643–649. [Google Scholar] [CrossRef]
- Murphy, J.W.; Hidore, M.R.; Nabavi, N. Binding interactions of murine natural killer cells with the fungal target Cryptococcus neoformans. Infect. Immun. 1991, 59, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Koguchi, Y.; Qureshi, M.H.; Yara, S.; Kinjo, Y.; Uezu, K.; Saito, A. NK Cells Eliminate Cryptococcus neoformans by Potentiating the Fungicidal Activity of Macrophages Rather than by Directly Killing Them upon Stimulation with IL-12 and IL-18. Microbiol. Immunol. 2013, 44, 1043–1050. [Google Scholar] [CrossRef]
- Murphy, J.W.; Hidore, M.R.; Wong, S.C. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J. Clin. Investig. 1993, 91, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- LEVITZ, S.M.; P.DUPONT, M.; SMAIL, E.H. Direct Activity of Human T Lymphocytes and Natural Killer Cells against Cryptococcus neoforman. Infect. Immun. 1994, 61, 194–202. [Google Scholar] [CrossRef]
- Murphy, J.W.; Zhou, A.; Wong, S.C. Direct Interaction of Human Natural Killer Cells with Cryptococcus neofromans inhibits Granulocytes-macrophage colony-stimulating Factor and Tumor Necrosis Factor Alpha Production. Infect. Immun. 1997, 65, 4564–4571. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, C.L.C.; Neely, G.G.; Epelman, S.; Krensky, A.M.; Mody, C.H. NK Cells Use Perforin Rather than Granulysin for Anticryptococcal Activity. J. Immunol. 2004, 173, 3357–3365. [Google Scholar] [CrossRef]
- Wiseman, J.C.D.; Ma, L.L.; Marr, K.J.; Jones, G.J.; Mody, C.H. Perforin-Dependent Cryptococcal Microbicidal Activity in NK Cells Requires PI3K-Dependent ERK1/2 Signaling. J. Immunol. 2007, 178, 6456–6464. [Google Scholar] [CrossRef]
- Li, S.S.; Kyei, S.K.; Timm-McCann, M.; Ogbomo, H.; Jones, G.J.; Shi, M.; Xiang, R.F.; Oykhman, P.; Huston, S.M.; Islam, A.; et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe 2013, 14, 387–397. [Google Scholar] [CrossRef]
- Li, S.S.; Ogbomo, H.; Mansour, M.K.; Xiang, R.F.; Szabo, L.; Munro, F.; Mukherjee, P.; Mariuzza, R.A.; Amrein, M.; Vyas, J.M.; et al. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat. Commun. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Kyei, S.K.; Ogbomo, H.; Li, S.; Timm-McCann, M.; Xiang, R.F.; Huston, S.M.; Ganguly, A.; Colarusso, P.; Gill, M.J.; Mody, C.H. Mechanisms by Which Interleukin-12 Corrects Defective NK Cell Anticryptococcal Activity in HIV-Infected Patients. mBio 2016, 7, e00878-16. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.J.; Jones, G.J.; Zheng, C.; Huston, S.M.; Timm-McCann, M.; Islam, A.; Berenger, B.M.; Ma, L.L.; Wiseman, J.C.; Mody, C.H. Cryptococcus neoformans directly stimulates perforin production and rearms NK cells for enhanced anticryptococcal microbicidal activity. Infect. Immun. 2009, 77, 2436–2446. [Google Scholar] [CrossRef]
- Oykhman, P.; Timm-McCann, M.; Xiang, R.F.; Islam, A.; Li, S.S.; Stack, D.; Huston, S.M.; Ma, L.L.; Mody, C.H. Requirement and redundancy of the Src family kinases Fyn and Lyn in perforin-dependent killing of Cryptococcus neoformans by NK cells. Infect. Immun. 2013, 81, 3912–3922. [Google Scholar] [CrossRef]
- Islam, A.; Li, S.S.; Oykhman, P.; Timm-McCann, M.; Huston, S.M.; Stack, D.; Xiang, R.F.; Kelly, M.M.; Mody, C.H. An acidic microenvironment increases NK cell killing of Cryptococcus neoformans and Cryptococcus gattii by enhancing perforin degranulation. PLoS Pathog. 2013, 9, e1003439. [Google Scholar] [CrossRef]
- Ogbomo, H.; Timm-McCann, M.; Barnes, T.; Xiang, R.F.; Jamil, K.; Ganguly, A.; Stack, D.; Huston, S.M.; Li, S.S.; Colarusso, P.; et al. Granule-Dependent NK Cell Killing of Cryptococcus Requires Kinesin to Reposition the Cytolytic Machinery for Directed Cytotoxicity. Cell Rep. 2018, 24, 3017–3032. [Google Scholar] [CrossRef]
- Naranbhai, V.; Chang, C.C.; Durgiah, R.; Omarjee, S.; Lim, A.; Moosa, M.-Y.S.; Elliot, J.H.; Ndung’u, T.; Lewin, S.R.; French, M.A.; et al. Compartmentalization of innate immune responses in the central nervous system during cryptococcal meningitis/HIV coinfection. Aids 2014, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Louis, I.V.-S.; Chang, C.C.; Shahid, S.; French, M.A.; Bohjanen, P.R. Transcriptomic Predictors of Paradoxical Cryptococcosis-Associated Immune Reconstitution Inflammatory Syndrome. Open Forum Infect. Dis. 2018, 5, ofy157. [Google Scholar] [CrossRef]
- Sato, K.; Yamamoto, H.; Nomura, T.; Kasamatsu, J.; Miyasaka, T.; Tanno, D.; Matsumoto, I.; Kagesawa, T.; Miyahara, A.; Zong, T.; et al. Production of IL-17A at Innate Immune Phase Leads to Decreased Th1 Immune Response and Attenuated Host Defense against Infection with Cryptococcus deneoformans. J. Immunol. 2020, 205, 686–698. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Kolls, J.K.; Wormley, F.L. Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by gamma/delta T cells. BMC Immunol. 2012, 13, 65. [Google Scholar] [CrossRef]
- Normile, T.G.; Chu, T.H.; Sheridan, B.S.; Del Poeta, M. Vaccine protection by Cryptococcus neoformans Deltasgl1 is mediated by gammadelta T cells via TLR2 signaling. Mucosal Immunol. 2022, 15, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Kinjo, Y.; Uezu, K.; Yara, S.; Miyagi, K.; Koguchi, Y.; Nakayama, T.; Taniguchi, M.; Saito, A. Monocyte Chemoattractant Protein-1-Dependent Increase of Vα14 NKT Cells in Lungs and Their Roles in Th1 Response and Host Defense in Cryptococcal Infection. J. Immunol. 2001, 167, 6525–6532. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.F.; Ma, L.L.; Jones, G.J.; Gill, M.J.; Krensky, A.M.; Kubes, P.; Mody, C.H. Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood 2006, 109, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, C.; Duran, G.; Hellings, N.; Broux, B. When Helpers Go Above and Beyond: Development and Characterization of Cytotoxic CD4+ T Cells. Front. Immunol. 2022, 13, 951900. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.A.; Hauser, P.J.; Bandey, I.; Laskowski, T.; Wang, Q.; Najjar, A.M.; Kumaresan, P.R. Glucuronoxylomannan in the Cryptococcus species capsule as a target for Chimeric Antigen Receptor T-cell therapy. Cytotherapy 2021, 23, 119–130. [Google Scholar] [CrossRef]
- Dos Santos, M.H.; Machado, M.P.; Kumaresan, P.R.; da Silva, T.A. Titan Cells and Yeast Forms of Cryptococcus neoformans and Cryptococcus gattii Are Recognized by GXMR-CAR. Microorganisms 2021, 9, 1886. [Google Scholar] [CrossRef]
- Dos Santos, M.H.; Machado, M.P.; Kumaresan, P.R.; da Silva, T.A. Modification of Hinge/Transmembrane and Signal Transduction Domains Improves the Expression and Signaling Threshold of GXMR-CAR Specific to Cryptococcus spp. Cells 2022, 11, 3386. [Google Scholar] [CrossRef]
- Machado, M.P.; Dos Santos, M.H.; Guimaraes, J.G.; de Campos, G.Y.; Oliveira Brito, P.K.M.; Ferreira, C.M.G.; Rezende, C.P.; Frota, N.F.; Soares, S.G.; Kumaresan, P.R.; et al. GXMR-CAR containing distinct GXM-specific single-chain variable fragment (scFv) mediated the cell activation against Cryptococcus spp. And had difference in the strength of tonic signaling. Bioengineered 2023, 14, 2281059. [Google Scholar] [CrossRef]
- Schett, G.; Mackensen, A.; Mougiakakos, D. CAR T-cell therapy in autoimmune diseases. Lancet 2023, 402, 2034–2044. [Google Scholar] [CrossRef]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.C. CAR T-Cell-Based gene therapy for cancers: New perspectives, challenges, and clinical developments. Front. Immunol. 2022, 13, 925985. [Google Scholar] [CrossRef]
- Pazina, T.; Shemesh, A.; Brusilovsky, M.; Porgador, A.; Campbell, K.S. Regulation of the Functions of Natural Cytotoxicity Receptors by Interactions with Diverse Ligands and Alterations in Splice Variant Expression. Front. Immunol. 2017, 8, 369. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okafor, E.C.; Nielsen, K. State of the Field: Cytotoxic Immune Cell Responses in C. neoformans and C. deneoformans Infection. J. Fungi 2024, 10, 712. https://doi.org/10.3390/jof10100712
Okafor EC, Nielsen K. State of the Field: Cytotoxic Immune Cell Responses in C. neoformans and C. deneoformans Infection. Journal of Fungi. 2024; 10(10):712. https://doi.org/10.3390/jof10100712
Chicago/Turabian StyleOkafor, Elizabeth C., and Kirsten Nielsen. 2024. "State of the Field: Cytotoxic Immune Cell Responses in C. neoformans and C. deneoformans Infection" Journal of Fungi 10, no. 10: 712. https://doi.org/10.3390/jof10100712
APA StyleOkafor, E. C., & Nielsen, K. (2024). State of the Field: Cytotoxic Immune Cell Responses in C. neoformans and C. deneoformans Infection. Journal of Fungi, 10(10), 712. https://doi.org/10.3390/jof10100712





