Phylogenetic and Morphological Perspectives on Crepidotus subg. Dochmiopus: Exploratively Unveiling Hidden Diversity in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Morphological Examination
2.2. DNA Extraction, PCR Amplification and DNA Sequencing
2.3. Sequence Alignment and Molecular Phylogeny
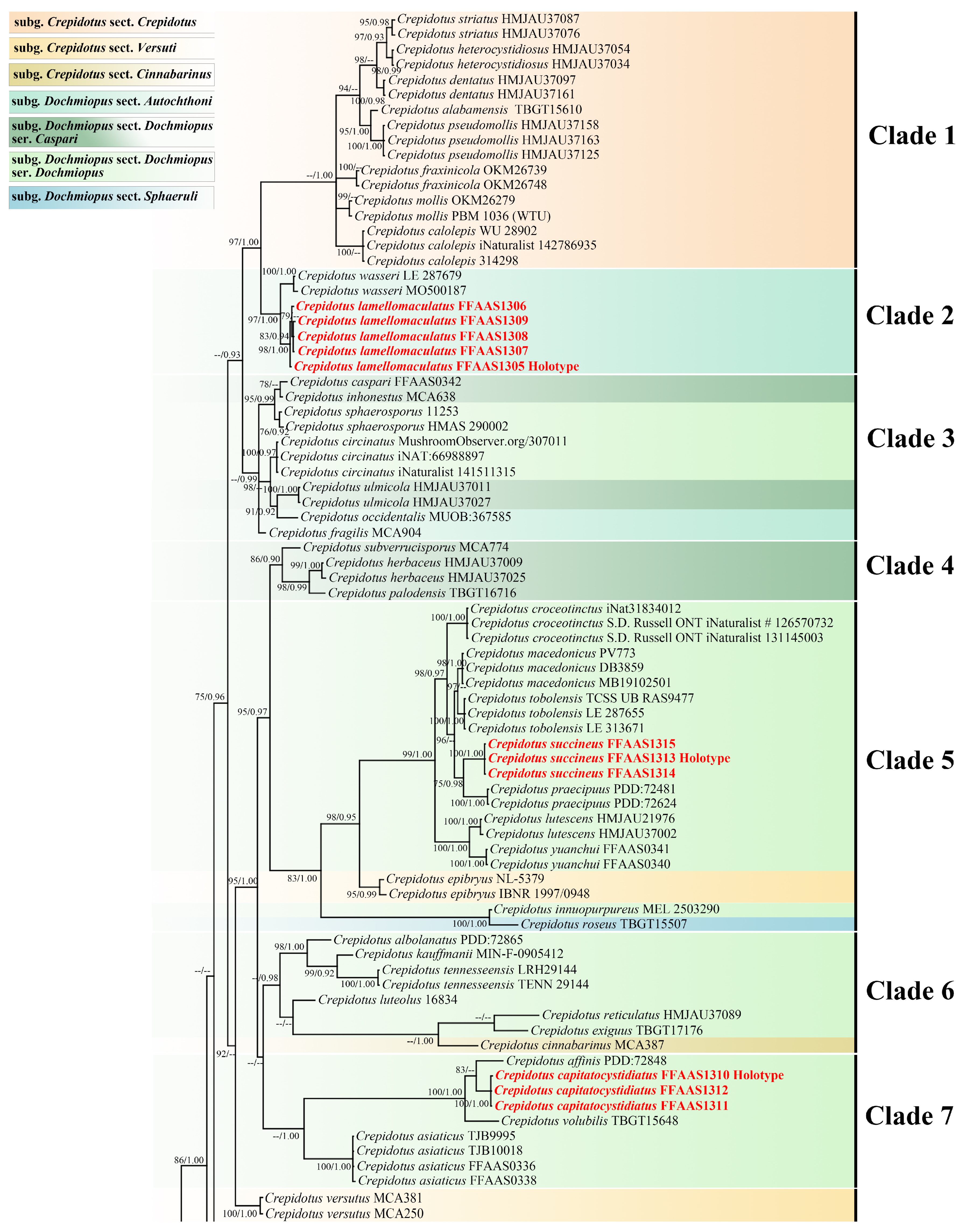
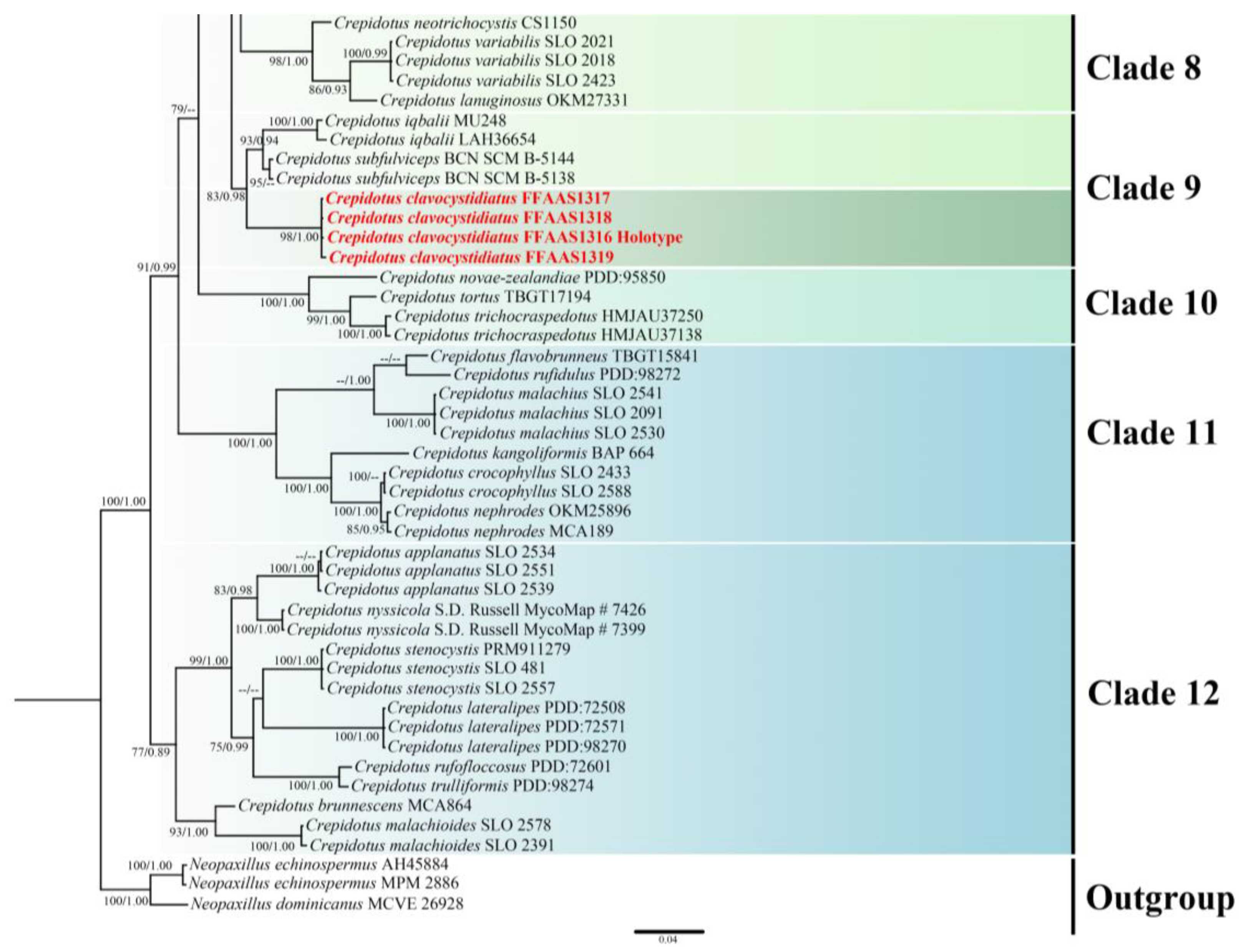
3. Results
3.1. Phylogenetic Analysis
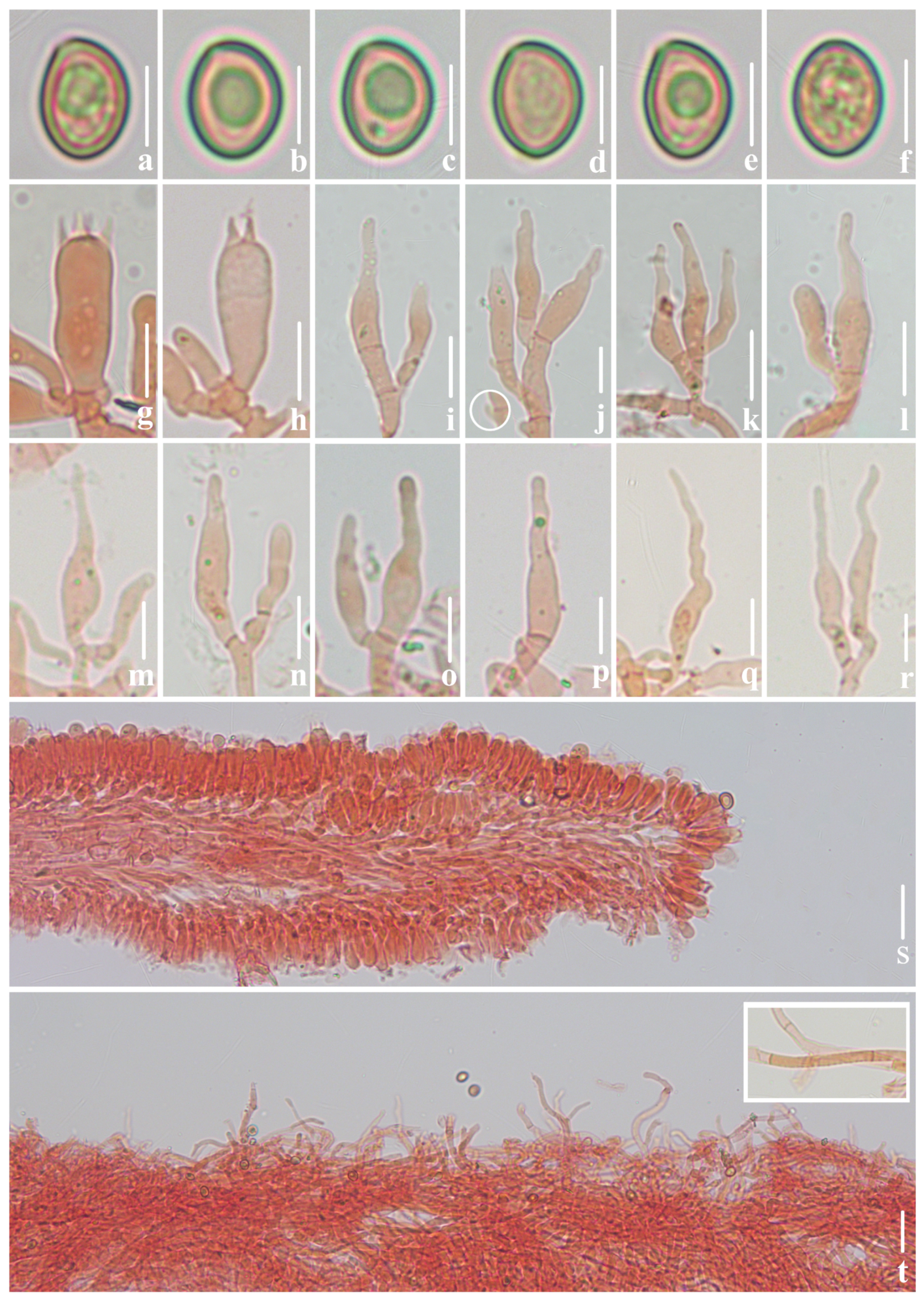
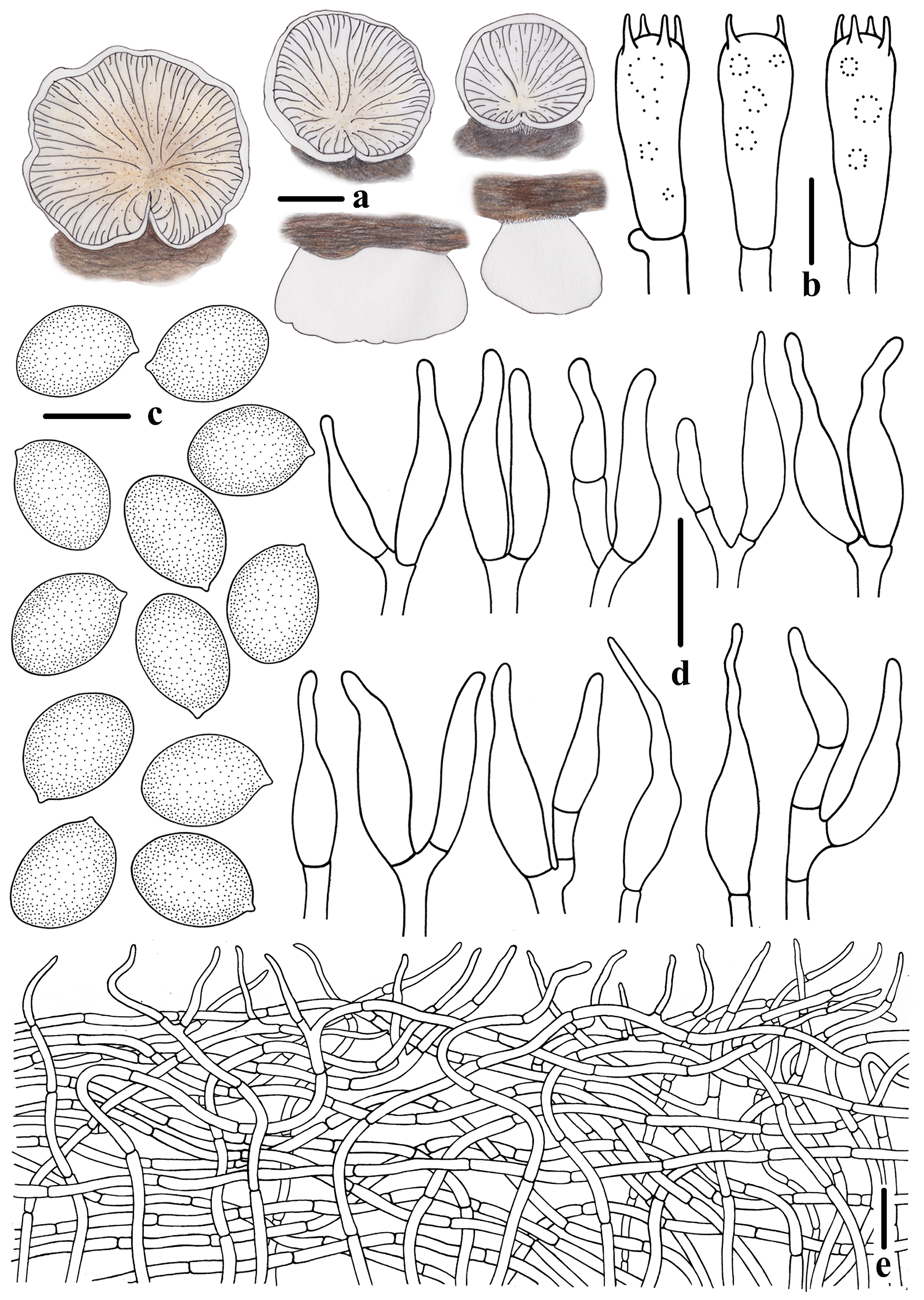
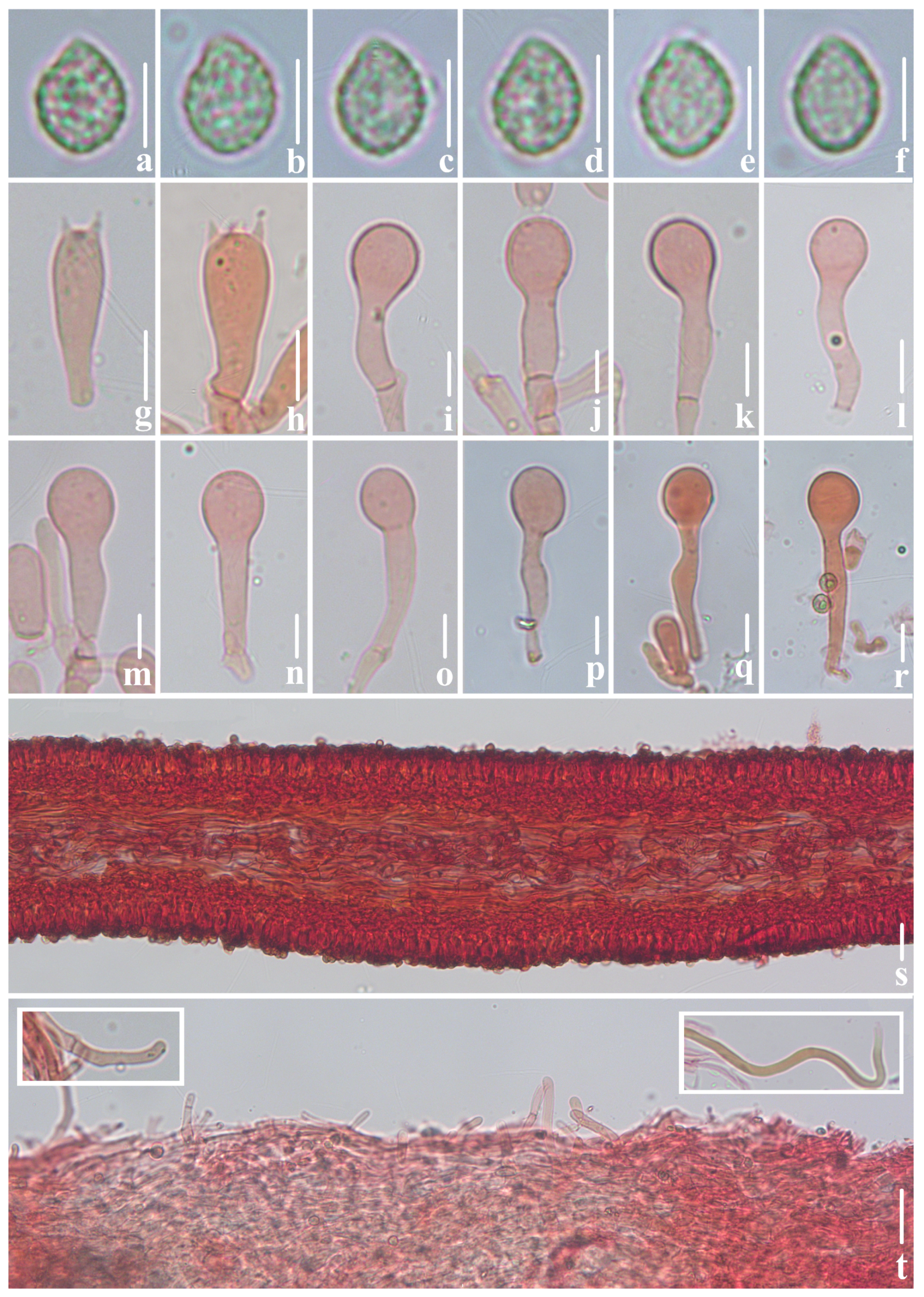
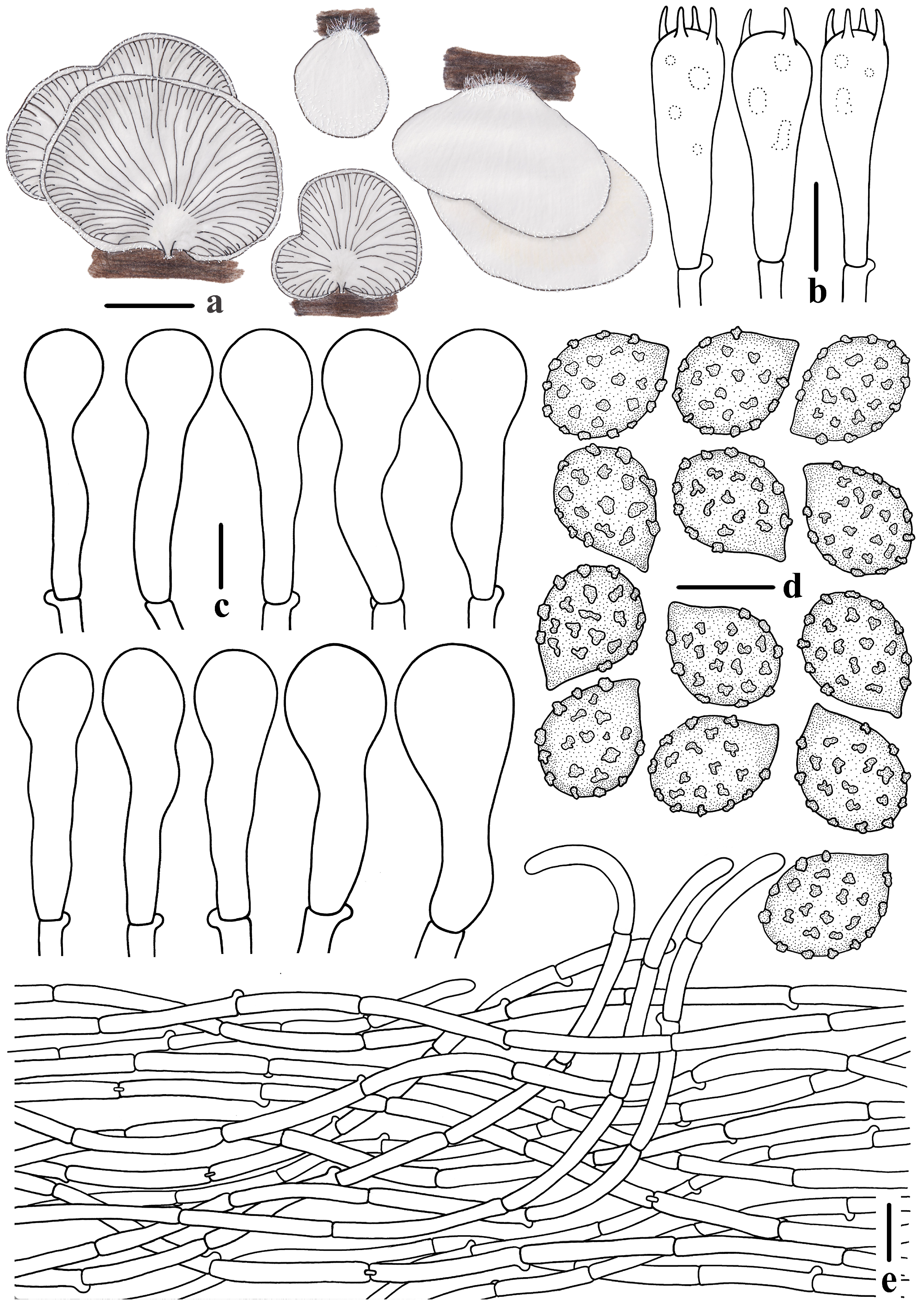
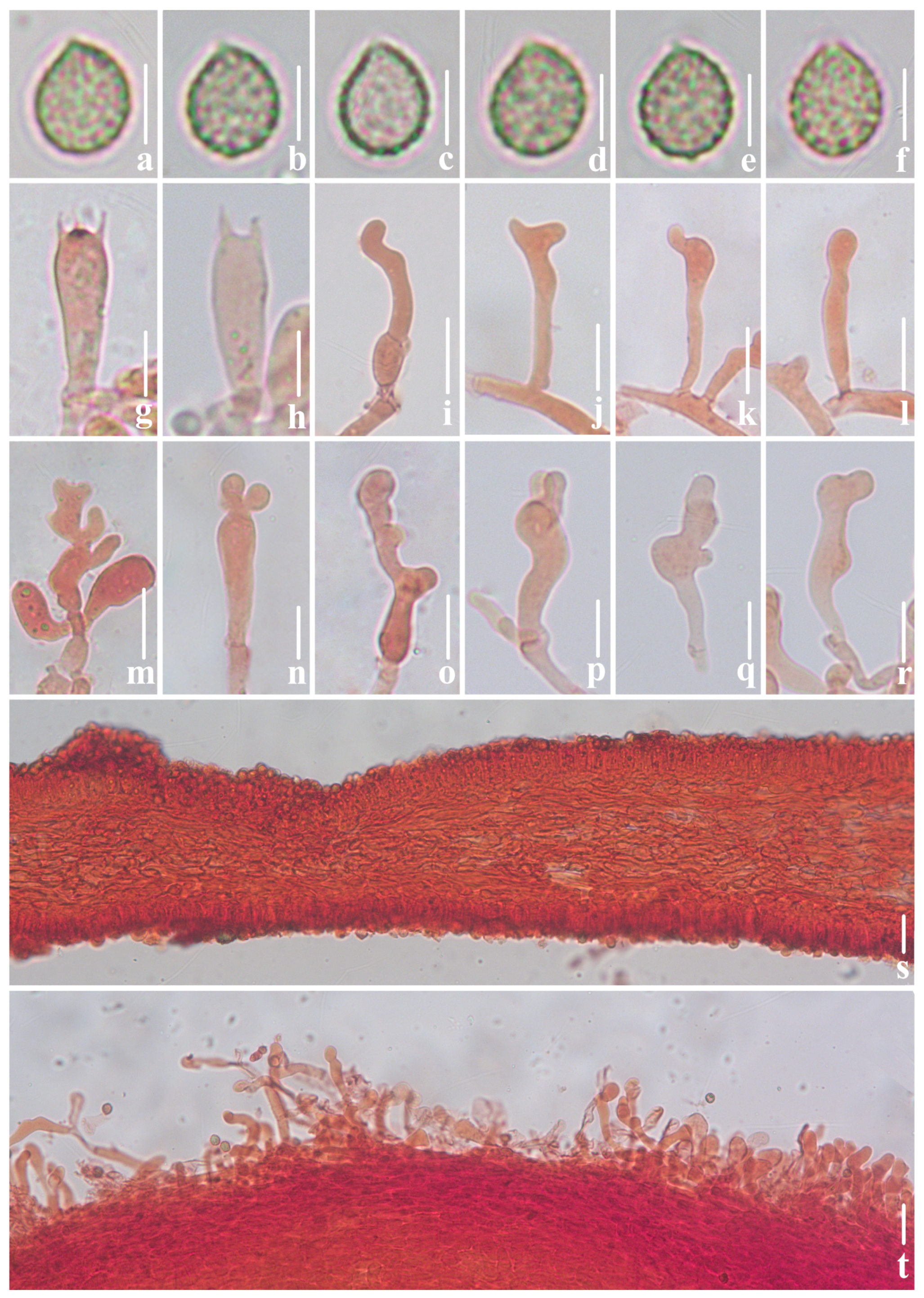
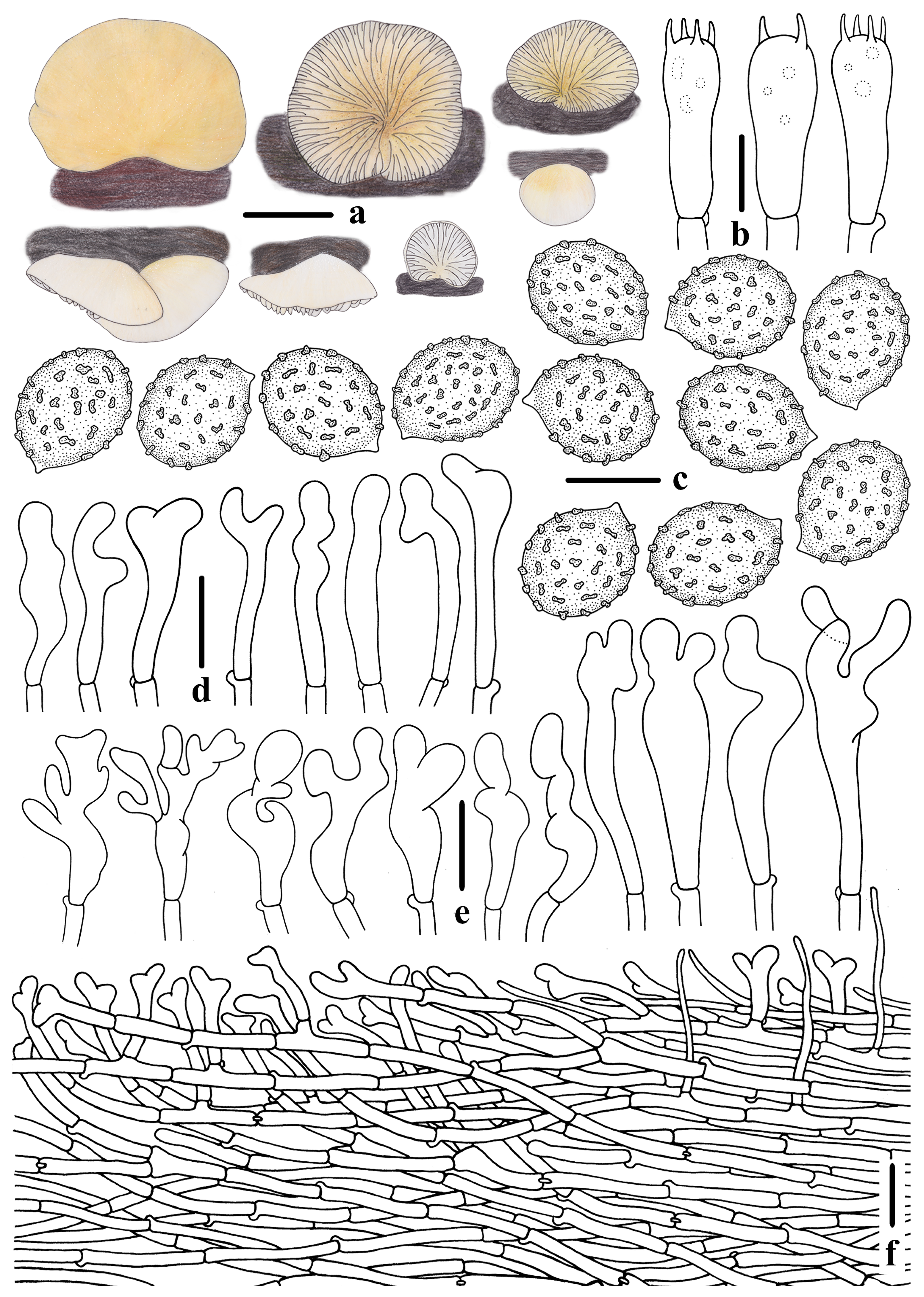
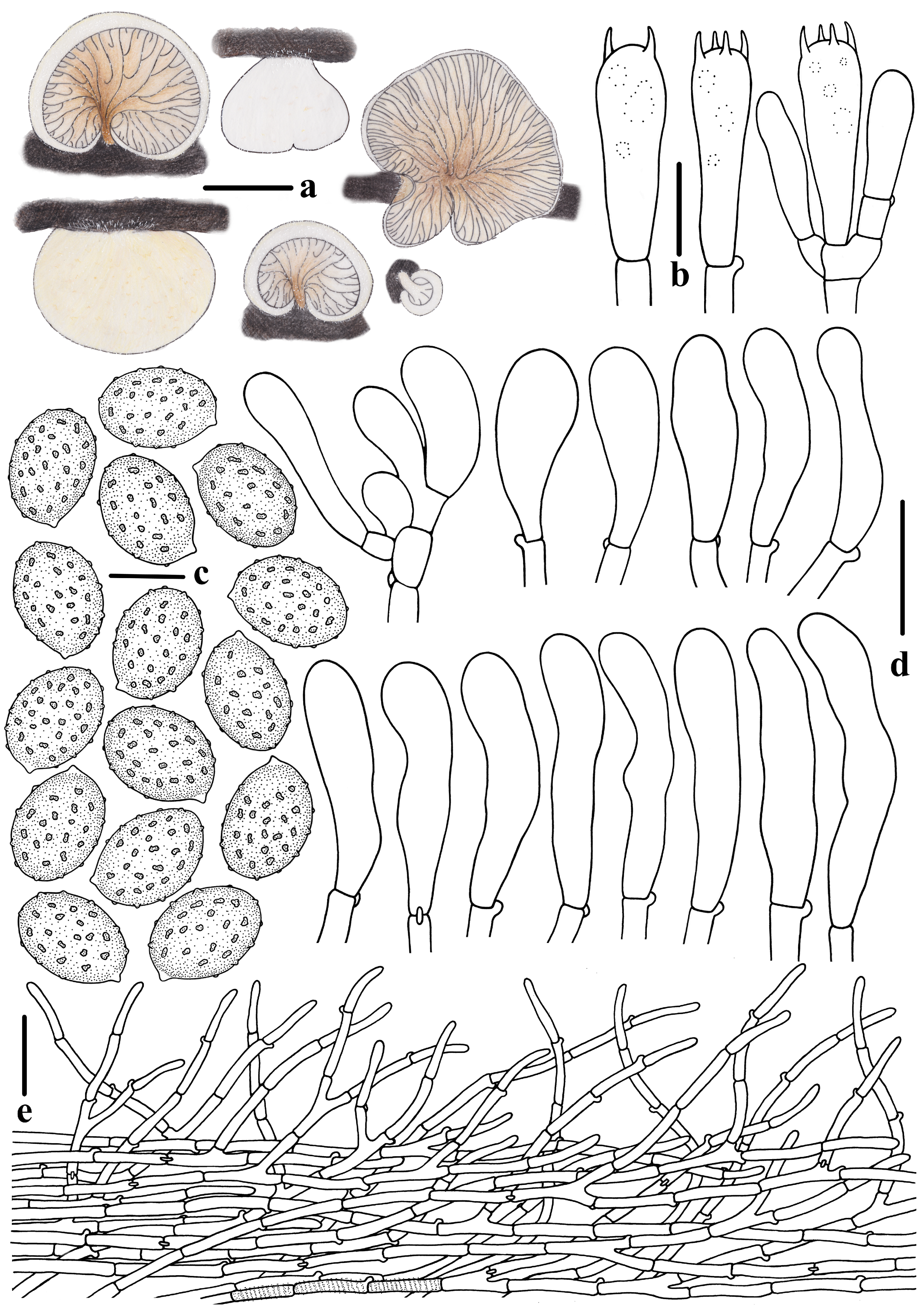
3.2. Taxonomy
4. Discussion
| Key to the elongated-spored species of C. subg. Dochmiopus known in China | |
| 1 Basidiospores smooth to nearly smooth | 2 |
| 1 Basidiospores ornamented | 10 |
| 2 Pileocystidia present | C. autochthonus |
| 2 Pileocystidia absent | 3 |
| 3 Pileipellis gelatinous | C. betulae |
| 3 Pileipellis non-gelatinous | 4 |
| 4 Cheilocystidia vine-shaped | C. trichocraspedotus |
| 4 Cheilocystidia not vine-shaped | 5 |
| 5 Pileipellis hyphae clampless | C. albidus |
| 5 Pileipellis hyphae clamped | 6 |
| 6 Lamellae edge not fimbriate | 7 |
| 6 Lamellae edge fimbriate | 8 |
| 7 Pileipellis a cutis | C. caspari |
| 7 Pileipellis a tomentose | C. lamellomaculatus |
| 8 Basidiospores width < 4.5 μm | C. albissimus |
| 8 Basidiospores width ≥ 4.5 μm | 9 |
| 9 Pileipellis a cutis, pileus greyish white to silver-grey | C. occidentalis |
| 9 Pileipellis a trichoderm, pileus white to light orange-yellow | C. ulmicola |
| 10 Inhabit on living plants | C. herbaceus |
| 10 Inhabit on rotten branches and woods | 11 |
| 11 Pileus reddish | C. reticulatus |
| 11 Pileus not reddish | 12 |
| 12 Pleurocystidia present | C. luteicolor |
| 12 Pleurocystidia absent | 13 |
| 13 Pileocystidia present | 14 |
| 13 Pileocystidia absent | 15 |
| 14 Pileus buff yellow to apricot yellow | C. succineus |
| 14 Pileus white | C. vulgaris |
| 15 Basidiospores subglobose to ellipsoid, Qm ≤ 1.60 | 16 |
| 15 Basidiospores amygdaliform, oblong to cylindrical, Qm > 1.60 | 26 |
| 16 Pileus brown, at point of attachment smooth | C. payettensis |
| 16 Pileus not brown, at point of attachment villous, fibrillose or tomentose | 17 |
| 17 Pileus surface smooth | 18 |
| 17 Pileus surface not smooth | 20 |
| 18 Pileus translucent striate | C. capitatocystidiatus |
| 18 Pileus not translucent striate | 19 |
| 19 Lamellae edge not fimbriate, cheilocystidia apex bifurcate | C. furcaticystidiosus |
| 19 Lamellae edge fimbriate, cheilocystidia apex branched or stag antlers | C. macedonicus |
| 20 Pileus hygrophanous | 21 |
| 20 Pileus non-hygrophanous | 22 |
| 21 Cheilocystidia lageniform | C. lutescens |
| 21 Cheilocystidia narrowly clavate to narrowly utriform | C. croceotinctus |
| 22 Pileipellis with incrusted hyphae | 23 |
| 22 Pileipellis without incrusted hyphae | 24 |
| 23 Cheilocystidia clavate or ventricose, sessile | C. kauffmanii |
| 23 Cheilocystidia clavate to narrowly clavate, pruinose stipe | C. clavocystidiatus |
| 24 Pileus orange-yellow to brownish-yellow | C. yuanchui |
| 24 Pileus white to cream | 25 |
| 25 Basidiospores subglobose to broadly ellipsoid, Q ≤ 1.30 | C. cesatii |
| 25 Basidiospores ellipsoid to ovoid, Q > 1.30 | C. subverrucisporus |
| 26 Pileus light smoky or rusty brown | C. herbarum |
| 26 Pileus white to light yellow or orange | 27 |
| 27 Basidiospores length ≥ 8.0 μm | C. luteolus |
| 27 Basidiospores length < 8.0 μm | 28 |
| 28 Cheilocystidia with a long cylindrical protuberance at apex | C. tomentellus |
| 28 Cheilocystidia without a long cylindrical protuberance at apex | 29 |
| 29 Cheilocystidia filiform, narrowly lageniform, at times diverticular or knobby in the upper portion | C. neotrichocystis |
| 29 Cheilocystidia clavate, utriform, strangulated, sometimes stag antlers | C. variabilis |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senn-Irlet, B. The Genus Crepidotus (Fr.) Staude in Europe. Persoonia 1995, 16, 1–80. [Google Scholar]
- Bandala, V.; Montoya, L. A taxonomic revision of some American Crepidotus. Mycologia 2000, 92, 341–353. [Google Scholar] [CrossRef]
- Aime, M.C.; Miller, O.K., Jr. Delayed germination of basidiospores in temperate species of Crepidotus (Fr.) Staude. Can. J. Bot. 2002, 80, 280–287. [Google Scholar] [CrossRef]
- Na, Q.; Liu, Z.; Zeng, H.; Cheng, X.; Ge, Y. Crepidotus yuanchui sp. nov. and C. caspari found in subalpine areas of China. Mycoscience 2022, 63, 1–11. [Google Scholar] [CrossRef]
- Hesler, L.R.; Smith, A.H. North American Species of Crepidotus; Hafner Publishing Company: New York, NY, USA, 1965. [Google Scholar]
- Consiglio, G.; Setti, L. Il Genere Crepidotus in Europa; A. M. B. Fondazione Centro Studi Micologici: Vicenza, Italy, 2008. [Google Scholar]
- Ge, Y.; Yang, S.; Bau, T. Crepidotus lutescens sp. nov. (Inocybaceae, Agaricales), an ochraceous salmon colored species from northeast of China. Phytotaxa 2017, 297, 189. [Google Scholar] [CrossRef]
- Guzmán-Dávalos, L.; Pradeep, C.K.; Vrinda, K.B.; Manoj Kumar, A.; Ramírez-Cruz, V.; Herrera, M.; Villalobos-Arámbula, A.R.; Soytong, K.; Baroni, T.J.; Aime, M.C. A new stipitate species of Crepidotus from India and Thailand, with notes on other tropical species. Mycologia 2017, 109, 804–814. [Google Scholar] [CrossRef]
- Manoj Kumar, A.; Vrinda, K.B.; Pradeep, C.K. New and Noteworthy Crepidotoid Agarics from India. Cryptogam. Mycol. 2018, 39, 287–298. [Google Scholar] [CrossRef]
- Manoj Kumar, A.; Vrinda, K.B.; Pradeep, C.K. Two new species of Crepidotus (Basidiomycota, Agaricales) from peninsular India. Phytotaxa 2018, 372, 67. [Google Scholar] [CrossRef]
- Manoj Kumar, A.; Pradeep, C.K.; Aime, M.C. New species and new records of Crepidotus (Crepidotaceae) from India. Mycol. Prog. 2022, 21, 311–326. [Google Scholar] [CrossRef]
- Ge, Y.; Bau, T. Descriptions of six new species of Crepidotus from China. Mycosystema 2020, 39, 238–255. [Google Scholar] [CrossRef]
- Izhar, A.; Usman, M.; Khalid, A.N. Crepidotus iqbalii (Crepidotaceae, Agaricales) a new stipitate species, from Pakistan. Phytotaxa 2021, 500, 95–107. [Google Scholar] [CrossRef]
- Takahashi, H. New species of Clitocybe and Crepidotus (Agaricales) from eastern Honshu, Japan. Mycoscience 2003, 44, 103–107. [Google Scholar] [CrossRef]
- Liu, S.-L.; Zhao, P.; Cai, L.; Shen, S.; Wei, H.-W.; Na, Q.; Han, M.; Wei, R.; Ge, Y.; Ma, H.; et al. Catalogue of fungi in China 1. New Taxa of Plant-Inhabiting Fungi. Mycology 2024, 3, 1–58. [Google Scholar] [CrossRef]
- Pilát, A. Revision of the types of some extra-European species of the genus Crepidotus Fr. Trans. Br. Mycol. Soc. 1950, 33, 215–249. [Google Scholar] [CrossRef]
- Montoya, L.; Mata, M. New species and records of Crepidotus from Costa Rica and Mexico. Fungal Divers. 2008, 32, 9–29. [Google Scholar]
- Jančovičová, S.; Adamčíková, K.; Caboň, M.; Adamčík, S. How variable is Crepidotus variabilis? Phytotaxa 2020, 449, 3. [Google Scholar] [CrossRef]
- Aime, M.C. Biosystematic studies in Crepidotus and the Crepidotaceae (Basidiomycetes, Agaricales). Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2001. [Google Scholar]
- Zhang, P.; Ge, Y.; Bau, T. Two new species of Crepidotus (Crepidotaceae) from China. Phytotaxa 2022, 552, 22–34. [Google Scholar] [CrossRef]
- Ridgway, R. Color Standards and Color Nomenclature; August Hoen Company: Washington, DC, USA, 1912; pp. 1–172. [Google Scholar]
- Na, Q.; Hu, Y.; Zeng, H.; Song, Z.; Ding, H.; Cheng, X.; Ge, Y. Updated taxonomy on Gerronema (Porotheleaceae, Agaricales) with three new taxa and one new record from China. MycoKeys 2022, 89, 87–120. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, Z.; Zeng, H.; Cheng, X.; Na, Q. Updated description of Atheniella (Mycenaceae, Agaricales), including three new species with brightly coloured pilei from Yunnan Province, southwest China. MycoKeys 2021, 81, 139–164. [Google Scholar] [CrossRef]
- Na, Q.; Hu, Y.; Liu, Z.; Zeng, H.; Qi, L.; Ding, H.; Cheng, X.; Ge, Y. The first reported occurrence of Leucoinocybe (Porotheleaceae, Agaricales) in China: Leucoinocybe lishuiensis sp. nov. from Zhejiang Province. Nova Hedwig. 2021, 113, 453–469. [Google Scholar] [CrossRef]
- Carl Zeiss Microscopy GmbH. ZEN 2.3 (Blue Edition); Carl Zeiss Microscopy GmbH: Jena, Germany, 2016. [Google Scholar]
- Liu, Z.; Na, Q.; Cheng, X.; Wu, X.; Ge, Y. Mycena yuezhuoi sp. nov. (Mycenaceae, Agaricales), a purple species from the peninsula areas of China. Phytotaxa 2021, 511, 2. [Google Scholar] [CrossRef]
- Bandala, V.M.; Montoya, L. A revision of some Crepidotus species related to Mexican Taxa. Mycol. Res. 2000, 104, 495–506. [Google Scholar] [CrossRef]
- Dramani, R.; Hegbe, A.D.M.T.; Tabe, A.; Badou, A.S.; Furneaux, B.; Ryberg, M.; Yorou, N.S. How are basidiospore size measurements affected by drying? Curr. Res. Environ. Appl. Mycol. 2020, 10, 63–70. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D.; Sninsky, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; Volume 31, pp. 315–322. [Google Scholar] [CrossRef]
- Hopple, J.S., Jr.; Vilgalys, R. Phylogenetic Relationships in the Mushroom Genus Coprinus and Dark-Spored Allies Based on Sequence Data from the Nuclear Gene Coding for the Large Ribosomal Subunit RNA: Divergent Domains, Outgroups, and Monophyly. Mol. Phylogenetics Evol. 1999, 13, 1–19. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, Y.; Zeng, H.; Cheng, X.; Na, Q. Four new species of Mycena sect. Calodontes (Agaricales, Mycenaceae) from northeast China. MycoKeys 2022, 93, 23–56. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Alzohairy, A.M. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Horak, E. Fungi of New Zealand Volume 6 Agaricales (Basidiomycota) of New Zealand 2 Brown Spored Genera; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2018; ISBN 978-94-91751-13-4. [Google Scholar]
- Jančovičová, S.; Adamčíková, K.; Caboň, M.; Adamčík, S. Phylogeny of Crepidotus applanatus Look-Alikes Reveals a Convergent Morphology Evolution and a New Species C. pini. J. Fungi 2022, 8, 489. [Google Scholar] [CrossRef]
- Jančovičová, S. New records and epitypification of Crepidotus malachioides (Crepidotaceae, Agaricales). Sydowia Int. J. Mycol. 2014, 66, 79–97. [Google Scholar] [CrossRef]
- Aime, M.C. Generic Concepts in the Crepidotaceae as Inferred from Nuclear Large Subunit Ribosomal DNA Sequences, Morphology, and Basidiospore Dormancy Patterns. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1998. [Google Scholar]
- Aime, M.; Vilgals, R.; Miller, O. The Crepidotaceae (Basidiomycota, Agaricales): Phylogeny and taxonomy of the genera and revision of the family based on molecular evidence. Am. J. Bot. 2005, 92, 74–82. [Google Scholar] [CrossRef]
- Varga, T.; Krizsán, K.; Földi, C.; Dima, B.; Sánchez-García, M.; Sánchez-Ramírez, S.; Szöllősi, G.J.; Szarkándi, J.G.; Papp, V.; Albert, L.; et al. Megaphylogeny resolves global patterns of mushroom evolution. Nat. Ecol. Evol. 2019, 3, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Moncalvo, J.-M.; Vilgalys, R.; Redhead, S.A.; Johnson, J.E.; James, T.Y.; Catherine Aime, M.; Hofstetter, V.; Verduin, S.J.W.; Larsson, E.; Baroni, T.J.; et al. One hundred and seventeen clades of euagarics. Mol. Phylogenetics Evol. 2002, 23, 357–400. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Osieck, E.R.; Jurjevi, Ž.; Boers, J.; Van Iperen, A.L.; Starink-Willemse, M.; Dima, B.; Balashov, S.; Bulgakov, T.S.; Johnston, P.R.; et al. Fungal Planet Description Sheets: 1284–1382. Persoonia 2021, 47, 178–374. [Google Scholar] [CrossRef]
- Desjardin, D. Dark-spored species of Agaricineae from Republic of São Tomé and Príncipe, West Africa. Mycosphere 2016, 7, 359–391. [Google Scholar] [CrossRef]
- Osmundson, T.W.; Robert, V.A.; Schoch, C.L.; Baker, L.J.; Smith, A.; Robich, G.; Mizzan, L.; Garbelotto, M.M. Filling Gaps in Biodiversity Knowledge for Macrofungi: Contributions and Assessment of an Herbarium Collection DNA Barcode Sequencing Project. PLoS ONE 2013, 8, e62419. [Google Scholar] [CrossRef]
- Liu, L.-N.; Razaq, A.; Atri, N.S.; Bau, T.; Belbahri, L.; Bouket, A.C.; Chen, L.-P.; Deng, C.; Ilyas, S.; Khalid, A.N.; et al. Fungal Systematics and Evolution: FUSE 4. Sydowia 2018, 70, 286. [Google Scholar] [CrossRef]
- Matheny, P.B.; Vellinga, E.C.; Bougher, N.L.; Ceska, O.; Moreau, P.-A.; Neves, M.A.; Ammirati, J.F. Taxonomy of displaced species of Tubaria. Mycologia 2007, 99, 569–585. [Google Scholar] [CrossRef]
- Sammut, C. Further additions to the Mycobiota of Malta. Ecol. Mediterr. 2021, 47, 85–135. [Google Scholar] [CrossRef]
- Jančovičová, S.; Adamčík, S.; Looney, B.P.; Caboň, M.; Čaplovičová, M.; Kopáni, M.; Pennycook, S.R.; Adamčíková, K. Delimitation of European Crepidotus stenocystis as different from the North American species C. brunnescens (Inocybaceae, Agaricales). Phytotaxa 2017, 328, 17. [Google Scholar] [CrossRef]
- Aime, M.C.; Vila, J.; Moreau, P.-A. Crepidotus subfulviceps comb. nov., a stipitate Crepidotus from temperate North America and Europe. Mycotaxon 2009, 110, 283–287. [Google Scholar] [CrossRef]
- Horak, E.; Matheny, P.B.; Desjardin, D.E.; Soytong, K. The genus Inocybe (Inocybaceae, Agaricales, Basidiomycota) in Thailand and Malaysia. Phytotaxa 2015, 230, 201–238. [Google Scholar] [CrossRef]
- Kapitonov, V.I. Crepidotus tobolensis Kapitonov, Biketova & Zmitr., sp. nov./Fungal Planet 918. Persoonia Mol. Phylogeny Evol. Fungi 2019, 2, 404–405. [Google Scholar]
- Malysheva, E.F.; Kiyashko, A.A.; Malysheva, V.F.; Shikalova, E.A. A survey of rare species of agaricoid fungi (Basidiomycota) from South Siberia, Russia. Turczaninowia 2022, 25, 52–72. [Google Scholar] [CrossRef]
- Kapitonov, V.I. Crepidotus wasseri Kapitonov, Biketova, Zmitrovich & Á. Kovács, sp. nov./Fungal Planet 1236. Persoonia Mol. Phylogeny Evol. Fungi 2021, 46, 434–435. [Google Scholar]
- Vizzini, A.; Angelini, C.; Ercole, E. A new Neopaxillus species (Agaricomycetes) from the Dominican Republic and the status of Neopaxillus within the Agaricales. Mycologia 2012, 104, 138–147. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 2016, 32, 1933–1942. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Nylander, J.A.A.; Wilgenbusch, J.C.; Warren, D.L.; Swofford, D.L. AWTY (are we there yet?): A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 2008, 24, 581–583. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Jančovičová, S.; Adamčíková, K.; Caboň, M.; Graddy, M.G.; Matheny, P.B.; Noffsinger, C.R.; Wheeler, T.B.; Adamčík, S. Taxonomic reintroduction of the holarctic saprotrophic fungus Crepidotus cinnamomeus. Mycol Prog. 2024, 23, 49. [Google Scholar] [CrossRef]
- Ge, Y. Taxonomy and Molecular Phylogeny of Crepidotaceae in China. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2017. [Google Scholar]
- Gonou-Zagou, Z.; Delivorias, P. Studies on Basidiomycetes in Greece 1: The Genus Crepidotus. Mycotaxon 2005, 94, 15–42. [Google Scholar]
- Krisai-Greilhuber, I.; Senn-Irlet, B.; Voglmayr, H. Notes on Crepidotus from Mexico and the South-eastern USA. Persoonia Mol. Phylogeny Evol. Fungi 2002, 17, 515–539. [Google Scholar]
- Fan, Y.G. Studies on Conservation Biology of Endangered Macrofungi in Changbai Mountain Nature Reserve. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2007. [Google Scholar]
- Wang, W. Biodiversity of Macrofungi in Changbai Mountain Region. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2014. [Google Scholar]
- Zhang, P. Diversity of Macrofungi in the Greater and Lesser Khinggan Moutains. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2019. [Google Scholar]
- Wang, F.J. Diversity of Macrofungi in Western Hubei. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2019. [Google Scholar]
- Wang, J.R. Biodiversity of Macrofungi in Shandong Province. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2013. [Google Scholar]
- Clémençon, H. Cytology and Plectology of the Hymenomycetes, 2nd ed.; Gebr. Borntraeger Verlagsbuchhandlung: Stuttgart, Germany, 2012. [Google Scholar]





| Taxa | Voucher/Strain No. | Location | GenBank Sequence ID | Reference | |
|---|---|---|---|---|---|
| ITS | nLSU | ||||
| C. affinis | PDD:72848 | New Zealand | KY827291 | – | [34] |
| C. alabamensis | TBGT15610 | India | MK459545 | MK459543 | [34] |
| C. albolanatus | PDD:72865 | New Zealand | KY827292 | – | [34] |
| C. applanatus | SLO 2534 | Slovakia | OM832521 | OM832556 | [35] |
| C. applanatus | SLO 2539 | Slovakia | OM832523 | OM832558 | [35] |
| C. applanatus | SLO 2551 | Slovakia | OM832526 | OM832560 | [35] |
| C. asiaticus | TJB9995 | Thailand | MF077337 | MF077336 | [8] |
| C. asiaticus | TJB10018 | Thailand | MF077339 | MF077338 | [8] |
| C. asiaticus | FFAAS0336 | China | MW580871 | – | Direct Sub. |
| C. asiaticus | FFAAS0338 | China | MW580872 | – | Direct Sub. |
| C. brunnescens | MCA864 | Not indicated | – | AF367936 | Unpublished |
| C. calolepis | WU 28902 | Hungary | KF879617 | – | [36] |
| C. calolepis | iNaturalist 142786935 | USA | OR824560 | – | Unpublished |
| C. calolepis | 314298 | USA | MH188448 | – | Direct Sub. |
| C. capitatocystidiatus | FFAAS1310 Holotype | China | PQ061270 | PQ061255 | This study |
| C. capitatocystidiatus | FFAAS1311 | China | PQ061271 | PQ061256 | This study |
| C. capitatocystidiatus | FFAAS1312 | China | PQ061272 | PQ061257 | This study |
| C. caspari | FFAAS0342 | China | MZ401361 | MW581521 | [4] |
| C. cinnabarinus | MCA387 | USA | – | AF205686 | [37] |
| C. circinatus | MushroomObserver. org/307011 | USA | MH087459 | – | Direct Sub. |
| C. circinatus | iNAT:66988897 | USA | OQ147188 | – | Direct Sub. |
| C. circinatus | iNaturalist 141511315 | USA | OR824682 | – | Direct Sub. |
| C. clavocystidiatus | FFAAS1316 Holotype | China | PQ061276 | PQ061261 | This study |
| C. clavocystidiatus | FFAAS1317 | China | PQ061277 | PQ061262 | This study |
| C. clavocystidiatus | FFAAS1318 | China | PQ061278 | PQ061263 | This study |
| C. clavocystidiatus | FFAAS1319 | China | PQ061279 | PQ061264 | This study |
| C. croceotinctus | iNat31834012 | USA | MN498116 | – | Direct Sub. |
| C. croceotinctus | S.D. Russell ONT iNaturalist # 126570732 | USA | OP470431 | – | Direct Sub. |
| C. croceotinctus | S.D. Russell ONT iNaturalist 131145003 | USA | OP549098 | – | Direct Sub. |
| C. crocophyllus | SLO 2433 | Slovakia | OM832529 | OM832562 | [35] |
| C. crocophyllus | SLO 2588 | Slovakia | OM832530 | OM832563 | [35] |
| C. dentatus | HMJAU37097 | China | MH320736 | – | [12] |
| C. dentatus | HMJAU37161 | China | MH320737 | – | [12] |
| C. epibryus | IBNR 1997/0948 | Russia | – | AF367934 | [38] |
| C. epibryus | NL-5379 | Hungary | – | MK277884 | [39] |
| C. exiguus | TBGT17176 | India | – | MK567974 | [11] |
| C. flavobrunneus | TBGT15841 | India | – | MK567981 | [11] |
| C. fragilis | MCA 904 | USA | – | AF367931 | [38] |
| C. fraxinicola | OKM26739 | USA | – | AF205699 | [37] |
| C. fraxinicola | OKM26748 | USA | – | AF205697 | [37] |
| C. herbaceus | HMJAU37009 | China | MW080327 | – | [20] |
| C. herbaceus | HMJAU37025 | China | MW080326 | – | [20] |
| C. heterocystidiosus | HMJAU37054 | China | MF461342 | – | [12] |
| C. heterocystidiosus | HMJAU37034 | China | MF461344 | – | [12] |
| C. inhonestus | MCA638 | Not indicated | – | AF205704 | [40] |
| C. innuopurpureus | MEL 2503290 | Australia | NR_182391 | MZ870346 | [41] |
| C. iqbalii | MU248 | Pakistan | OQ672617 | – | Direct Sub. |
| C. iqbalii | LAH36654 | Pakistan | MT973498 | MW888515 | [13] |
| C. kangoliformis | BAP 664 | São Tomé and Príncipe | KX017199 | – | [42] |
| C. kauffmanii | MIN-F-0905412 | USA | – | MK277887 | [39] |
| C. lamellomaculatus | FFAAS1306 | China | PQ061266 | PQ061251 | This study |
| C. lamellomaculatus | FFAAS1309 | China | PQ061269 | PQ061254 | This study |
| C. lamellomaculatus | FFAAS1308 | China | PQ061268 | PQ061253 | This study |
| C. lamellomaculatus | FFAAS1307 | China | PQ061267 | PQ061252 | This study |
| C. lamellomaculatus | FFAAS1305 Holotype | China | PQ061265 | PQ061250 | This study |
| C. lanuginosus | OKM27331 | USA | – | AF367940 | Unpublished |
| C. lateralipes | PDD:72508 | New Zealand | KY827293 | – | [34] |
| C. lateralipes | PDD:72571 | New Zealand | KY827294 | – | [34] |
| C. lateralipes | PDD:98270 | New Zealand | KY827295 | – | [34] |
| C. luteolus | 16834 | Italy | JF907963 | – | [43] |
| C. lutescens | HMJAU 21976 | China | KU762016 | – | [7] |
| C. lutescens | HMJAU 37002 | China | KU762017 | – | [7] |
| C. macedonicus | PV773 | Hungary | MH780921 | – | [44] |
| C. macedonicus | DB3859 | Hungary | MH780922 | – | [44] |
| C. macedonicus | MB19102501 | Italy | PP131267 | PP125747 | Direct Sub. |
| C. malachioides | SLO 2578 | Slovakia | OM832538 | OM832568 | [35] |
| C. malachioides | SLO 2391 | Slovakia | OM832536 | OM832567 | [35] |
| C. malachius | SLO 2541 | Slovakia | OM832546 | OM832575 | [35] |
| C. malachius | SLO 2091 | Slovakia | OM832541 | OM832571 | [35] |
| C. malachius | SLO 2530 | Slovakia | OM832543 | OM832573 | [35] |
| C. mollis | OKM26279 | USA | – | AF205677 | [37] |
| C. mollis | PBM 1036 (WTU) | USA | – | DQ986293 | [45] |
| C. neotrichocystis | CS1150 | Malta | OL672745 | OL672702 | [46] |
| C. nephrodes | OKM25896 | Not indicated | – | AF205693 | [37] |
| C. nephrodes | MCA189 | Not indicated | – | AF205670 | [37] |
| C. novae-zealandiae | PDD:95850 | New Zealand | HQ533046 | – | Direct Sub. |
| C. nyssicola | S.D. Russell MycoMap # 7426 | USA | MN906237 | – | Direct Sub. |
| C. nyssicola | S.D. Russell MycoMap # 7399 | USA | MN906236 | – | Direct Sub. |
| C. occidentalis | MUOB:367585 | USA | OK376745 | – | Direct Sub. |
| C. palodensis | TBGT16716 | India | MH844890 | MH310743 | [10] |
| C. praecipuus | PDD:72624 | New Zealand | KY827312 | – | [34] |
| C. praecipuus | PDD:72481 | New Zealand | KY827311 | – | [34] |
| C. pseudomollis | HMJAU37158 | China | MH320739 | – | [12] |
| C. pseudomollis | HMJAU37163 | China | MH320740 | – | [12] |
| C. pseudomollis | HMJAU37125 | China | MH320738 | – | [12] |
| C. reticulatus | HMJAU37089 | China | MF461346 | – | Direct Sub. |
| C. roseus | TBGT15507 | India | MK567976 | MK567977 | [11] |
| C. rufidulus | PDD 98272 | New Zealand | NR_159823 | – | [34] |
| C. rufofloccosus | PDD 72601 | New Zealand | NR_159822 | – | [34] |
| C. sphaerosporus | 11253 | Italy | JF907960 | – | Direct Sub. |
| C. sphaerosporus | HMAS 290002 | China | MK966514 | – | Direct Sub. |
| C. stenocystis | PRM911279 | Czech Republic | MF621030 | MF621024 | [47] |
| C. stenocystis | SLO 481 | Slovakia | OM832552 | – | [35] |
| C. stenocystis | SLO 2557 | Slovakia | OM832553 | OM832581 | [35] |
| C. striatus | HMJAU37087 | China | MH320742 | – | [12] |
| C. striatus | HMJAU37076 | China | MH320741 | – | [12] |
| C. subfulviceps | BCN SCM B-5144 | Spain | – | FJ947116 | [48] |
| C. subfulviceps | BCN SCM B-5138 | Spain | – | FJ947117 | [48] |
| C. subverrucisporus | MCA774 | USA | – | AF367948 | [19] |
| C. succineus | FFAAS1313 Holotype | China | PQ061273 | PQ061258 | This study |
| C. succineus | FFAAS1314 | China | PQ061274 | PQ061259 | This study |
| C. succineus | FFAAS1315 | China | PQ061275 | PQ061260 | This study |
| C. tennesseensis | TENN 29144 | USA | FJ601806 | – | Unpublished |
| C. tennesseensis | LRH29144 | USA | NR_119720 | GQ892981 | [49] |
| C. tobolensis | TCSS UB RAS9477 | Russia | MK522392 | – | [50] |
| C. tobolensis | LE 287655 | Russia | MK522393 | MK560762 | [50] |
| C. tobolensis | LE313671 | Russia | OL739885 | – | [51] |
| C. tortus | TBGT17194 | India | MK462161 | MK462162 | [11] |
| C. trichocraspedotus | HMJAU37250 | China | MH320744 | – | [12] |
| C. trichocraspedotus | HMJAU37138 | China | MH320743 | – | [12] |
| C. trulliformis | PDD:98274 | New Zealand | KY827298 | – | [34] |
| C. ulmicola | HMJAU37011 | China | KX456184 | – | [20] |
| C. ulmicola | HMJAU37027 | China | MW080328 | – | [20] |
| C. variabilis | SLO 2021 | Slovakia | MT055889 | OM832585 | [18] |
| C. variabilis | SLO 2018 | Slovakia | MT055890 | OM832583 | [18] |
| C. variabilis | SLO 2423 | Slovakia | MT055887 | OM832584 | [18] |
| C. versutus | MCA381 | USA | – | AF205683 | [37] |
| C. versutus | MCA250 | USA | – | AF205695 | [37] |
| C. volubilis | TBGT15648 | India | MH845231 | MH310742 | [11] |
| C. wasseri | LE 287679 | Russia | MW722981 | MW723022 | [52] |
| C. wasseri | MO500187 | USA | OR203555 | – | Direct Sub. |
| C. yuanchui | FFAAS0340 | China | MZ401362 | – | [4] |
| C. yuanchui | FFAAS0341 | China | MZ401363 | MW581519 | [4] |
| N. dominicanus | MCVE 26928 | Dominican Republic | JN033216 | JN033217 | [53] |
| N. echinospermus | AH45884 | Brazil | KY468512 | KY468511 | Direct Sub. |
| N. echinospermus | MPM 2886 | Brazil | – | JN033222 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Na, Q.; Wei, R.; Zeng, H.; Hu, Y.; Zhang, L.; Du, J.; Zou, L.; Tang, W.; Cheng, X.; et al. Phylogenetic and Morphological Perspectives on Crepidotus subg. Dochmiopus: Exploratively Unveiling Hidden Diversity in China. J. Fungi 2024, 10, 710. https://doi.org/10.3390/jof10100710
Han M, Na Q, Wei R, Zeng H, Hu Y, Zhang L, Du J, Zou L, Tang W, Cheng X, et al. Phylogenetic and Morphological Perspectives on Crepidotus subg. Dochmiopus: Exploratively Unveiling Hidden Diversity in China. Journal of Fungi. 2024; 10(10):710. https://doi.org/10.3390/jof10100710
Chicago/Turabian StyleHan, Menghui, Qin Na, Renxiu Wei, Hui Zeng, Yaping Hu, Libo Zhang, Jinhong Du, Li Zou, Weimin Tang, Xianhao Cheng, and et al. 2024. "Phylogenetic and Morphological Perspectives on Crepidotus subg. Dochmiopus: Exploratively Unveiling Hidden Diversity in China" Journal of Fungi 10, no. 10: 710. https://doi.org/10.3390/jof10100710
APA StyleHan, M., Na, Q., Wei, R., Zeng, H., Hu, Y., Zhang, L., Du, J., Zou, L., Tang, W., Cheng, X., & Ge, Y. (2024). Phylogenetic and Morphological Perspectives on Crepidotus subg. Dochmiopus: Exploratively Unveiling Hidden Diversity in China. Journal of Fungi, 10(10), 710. https://doi.org/10.3390/jof10100710






