Predicting Fungemia in the ICU: Unveiling the Value of Weekly Fungal Surveillance and Yeast Colonisation Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection Criteria
2.2. Sample Collection
2.3. Fungal Surveillance Cultures
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Colonisation Patterns of Yeast Species
3.3. Fungemia Predictive Values for Colonisation by Each Yeast Species
3.4. Anatomical Site Preferences of Yeast Species
3.5. Yeast Colonisation Risk
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vazquez, J. Invasive Fungal Infections in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2010, 31, 079–086. [Google Scholar] [CrossRef] [PubMed]
- Leroy, O.; Gangneux, J.-P.; Montravers, P.; Mira, J.-P.; Gouin, F.; Sollet, J.-P.; Carlet, J.; Reynes, J.; Rosenheim, M.; Regnier, B.; et al. Epidemiology, Management, and Risk Factors for Death of Invasive Candida Infections in Critical Care: A Multicenter, Prospective, Observational Study in France (2005–2006). Crit. Care Med. 2009, 37, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, M.; Sayın-Kutlu, S.; Alp-Çavuş, S.; Öztürk, Ş.B.; Taşbakan, M.; Özhak, B.; Kaya, O.; Kutsoylu, O.E.; Şenol-Akar, Ş.; Turhan, Ö.; et al. Mortality-Associated Factors of Candidemia: A Multi-Center Prospective Cohort in Turkey. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Paiva, J.-A.; Pereira, J.M.; Tabah, A.; Mikstacki, A.; de Carvalho, F.B.; Koulenti, D.; Ruckly, S.; Çakar, N.; Misset, B.; Dimopoulos, G.; et al. Characteristics and Risk Factors for 28-Day Mortality of Hospital Acquired Fungemias in ICUs: Data from the EUROBACT Study. Crit. Care 2016, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Blot, S.I.; Vandewoude, K.H.; Hoste, E.A.; Colardyn, F.A. Effects of Nosocomial Candidemia on Outcomes of Critically Ill Patients. Am. J. Med. 2002, 113, 480–485. [Google Scholar] [CrossRef]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to Initiation of Fluconazole Therapy Impacts Mortality in Patients with Candidemia: A Multi-Institutional Study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef]
- Morrell, M.; Fraser, V.J.; Kollef, M.H. Delaying the Empiric Treatment of Candida Bloodstream Infection until Positive Blood Culture Results Are Obtained: A Potential Risk Factor for Hospital Mortality. Antimicrob. Agents Chemother. 2005, 49, 3640–3645. [Google Scholar] [CrossRef]
- Playford, E.G.; Webster, A.C.; Sorrell, T.C.; Craig, J.C. Antifungal Agents for Preventing Fungal Infections in Non-Neutropenic Critically Ill and Surgical Patients: Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Antimicrob. Chemother. 2006, 57, 628–638. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Graybill, J.R.; Playford, E.G.; Watcharananan, S.P.; Oh, M.D.; Ja’alam, K.; Huang, S.; Nangia, V.; Kurup, A.; Padiglione, A.A. Consensus Statement on the Management of Invasive Candidiasis in Intensive Care Units in the Asia-Pacific Region. Int. J. Antimicrob. Agents 2009, 34, 205–209. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An International Call-to-Arms. Clin. Infect. Dis. 2017, 64, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.E.; Abdolrasouli, A.; Hagen, F. Fungal Nomenclature: Managing Change Is the Name of the Game. Open Forum Infect. Dis. 2023, 10, ofac559. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, Z.-D.; Zhao, X.-M.; Zhao, W.-N.; Feng, Z.-X.; Yurkov, A.; Alwasel, S.; Boekhout, T.; Bensch, K.; Hui, F.-L.; et al. Phylogenomic Analysis of the Candida auris-Candida haemuli Clade and Related Taxa in the Metschnikowiaceae, and Proposal of Thirteen New Genera, Fifty-Five New Combinations and Nine New Species. Persoonia-Mol. Phylogeny Evol. Fungi 2024, 52, 22–43. [Google Scholar] [CrossRef]
- Pappas, P.G.; Rex, J.H.; Lee, J.; Hamill, R.J.; Larsen, R.A.; Powderly, W.; Kauffman, C.A.; Hyslop, N.; Mangino, J.E.; Chapman, S.; et al. A Prospective Observational Study of Candidemia: Epidemiology, Therapy, and Influences on Mortality in Hospitalized Adult and Pediatric Patients. Clin. Infect. Dis. 2003, 37, 634–643. [Google Scholar] [CrossRef]
- Quindós, G.; Marcos-Arias, C.; San-Millán, R.; Mateo, E.; Eraso, E. The Continuous Changes in the Aetiology and Epidemiology of Invasive Candidiasis: From Familiar Candida albicans to Multiresistant Candida auris. Int. Microbiol. 2018, 21, 107–119. [Google Scholar] [CrossRef]
- Pfaller, M.A. Nosocomial Candidiasis: Emerging Species, Reservoirs, and Modes of Transmission. Clin. Infect. Dis. 1996, 22 (Suppl. 2), S89–S94. [Google Scholar] [CrossRef]
- Lau, A.F.; Kabir, M.; Chen, S.C.A.; Playford, E.G.; Marriott, D.J.; Jones, M.; Lipman, J.; McBryde, E.; Gottlieb, T.; Cheung, W.; et al. Candida Colonization as a Risk Marker for Invasive Candidiasis in Mixed Medical-Surgical Intensive Care Units: Development and Evaluation of a Simple, Standard Protocol. J. Clin. Microbiol. 2015, 53, 1324. [Google Scholar] [CrossRef]
- Charles, P.E.; Dalle, F.; Aube, H.; Doise, J.M.; Quenot, J.P.; Aho, L.S.; Chavanet, P.; Blettery, B. Candida spp. Colonization Significance in Critically Ill Medical Patients: A Prospective Study. Intensive Care Med. 2005, 31, 393–400. [Google Scholar] [CrossRef]
- Alenazy, H.; Alghamdi, A.; Pinto, R.; Daneman, N. Candida Colonization as a Predictor of Invasive Candidiasis in Non-Neutropenic ICU Patients with Sepsis: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 102, 357–362. [Google Scholar] [CrossRef]
- Playford, E.G.; Lipman, J.; Kabir, M.; McBryde, E.S.; Nimmo, G.R.; Lau, A.; Sorrell, T.C. Assessment of Clinical Risk Predictive Rules for Invasive Candidiasis in a Prospective Multicentre Cohort of ICU Patients. Intensive Care Med. 2009, 35, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D.; Monod, M.; Suter, P.M.; Frenk, E.; Auckenthaler, R. Candida Colonization and Subsequent Infections in Critically III Surgical Patients. Ann. Surg. 1994, 220, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pelz, R.K.; Lipsett, P.A.; Swoboda, S.M.; Diener-West, M.; Hammond, J.M.; Hendrix, C.W. The Diagnostic Value of Fungal Surveillance Cultures in Critically Ill Patients. Surg. Infect. 2000, 1, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Swoboda, S.M.; Johnson, E.A.; Merz, W.G.; Pelz, R.K.; Lipsett, P.A.; Hendrix, C.W. The Association between Anatomic Site of Candida Colonization, Invasive Candidiasis, and Mortality in Critically Ill Surgical Patients. Diagn. Microbiol. Infect. Dis. 2006, 55, 293–301. [Google Scholar] [CrossRef]
- Posteraro, B.; De Pascale, G.; Tumbarello, M.; Torelli, R.; Pennisi, M.; Bello, G.; Maviglia, R.; Fadda, G.; Sanguinetti, M.; Antonelli, M. Early Diagnosis of Candidemia in Intensive Care Unit Patients with Sepsis: A Prospective Comparison of (1→3)-β-D-Glucan Assay, Candida Score, and Colonization Index. Crit. Care 2011, 15, R249. [Google Scholar] [CrossRef]
- Ahmed, A.; Baronia, A.; Azim, A.; Sharma, P.; Yadav, R.; Marak, R.S.K.; Singh, R. External Validation of Risk Prediction Scores for Invasive Candidiasis in a Medical/Surgical Intensive Care Unit: An Observational Study. Indian J. Crit. Care Med. 2017, 21, 514–520. [Google Scholar] [CrossRef]
- Jordà-Marcos, R.; Álvarez-Lerma, F.; Jurado, M.; Palomar, M.; Nolla-Salas, J.; León, M.A.; León, C. Risk Factors for Candidaemia in Critically Ill Patients: A Prospective Surveillance Study. Mycoses 2007, 50, 302–310. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Cornely, O.A.; DonneNy, J.P.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID Guideline for the Diagnosis and Management of Candida Diseases 2012: Developing European Guidelines in Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2012, 18, 1–8. [Google Scholar] [CrossRef]
- Patolia, S.; Kennedy, E.; Zahir, M.; Patolia, S.; Gulati, N.; Narendra, D.; Vadde, R.; Pokharel, S.; Schmidt, F.M.; Enriquez, D.; et al. Risk Factors for Candida Blood Stream Infection in Medical ICU and Role of Colonization-A Retrospective Study Introduction. Br. J. Med. Pract. 2013, 6, a618. [Google Scholar]
- León, C.; Álvarez-Lerma, F.; Ruiz-Santana, S.; León, M.Á.; Nolla, J.; Jordá, R.; Saavedra, P.; Palomar, M. Fungal Colonization and/or Infection in Non-Neutropenic Critically Ill Patients: Results of the EPCAN Observational Study. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First Hospital Outbreak of the Globally Emerging Candida auris in a European Hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
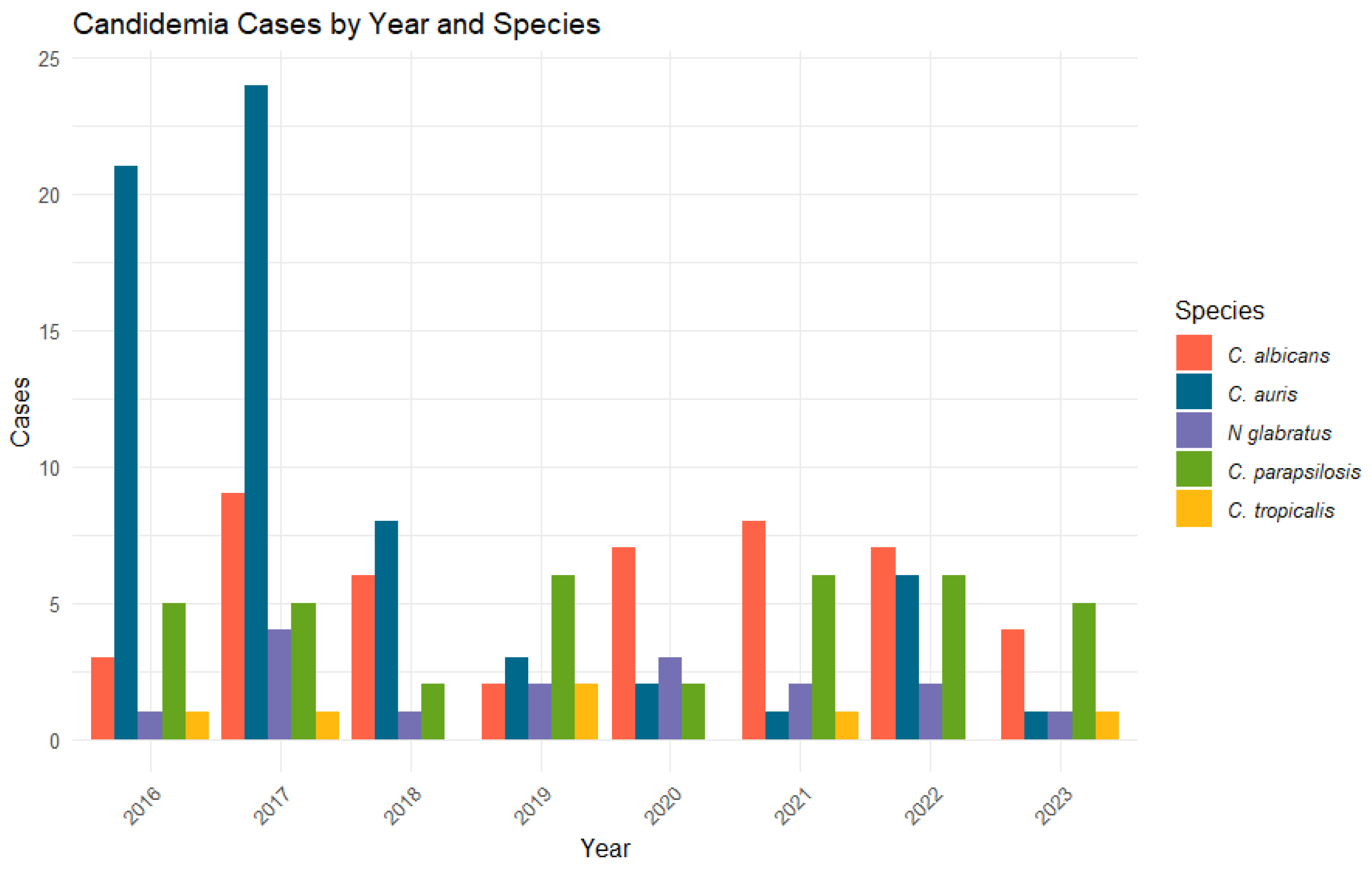




| Variable n (%) | Fungemia (n = 171) | No Fungemia (n = 3035) | OR | p-Value |
|---|---|---|---|---|
| Male sex | 122 (71.34%) | 1950 (64.25%) | 1.38 [0.99–1.95] | 0.059 |
| Age ≥ 65 | 57 (33.33%) | 1124 (37.03%) | 0.85 [0.61–1.17] | 0.32 |
| Surgical ICU admission | 111 (64.9%) | 1275 (42.0%) | 2.54 [1.85–3.53] | <0.001 |
| No positive previous colonisation cultures | 8 (4.68%) | 1297 (42.73%) | 0.06 [0.02–0.11] | <0.001 |
| 1–2 previous colonisation cultures | 96 (56.14%) | 1394 (45.93%) | 1.51 [1.11–2.06] | 0.004 |
| >2 previous colonisation cultures | 68 (39.76%) | 344 (11.33%) | 5.16 [3.71–7.14] | <0.001 |
| Mortality | 80 (48.48%) | 904 (29.72%) | 2.22 [1.62–3.05] | <0.001 |
| Days of admission (mean ± SD) | 25.42 ± 18.58 | 22.54 ± 13.34 | - | 0.047 |
| Reason for admission | ||||
| Heart surgery | 35 (20.47%) | 253 (8.34%) | 2.88 [1.88–4.16] | <0.001 |
| Polytrauma | 26 (15.2%) | 193 (6.36%) | 2.65 [1.66–4.06] | <0.001 |
| Respiratory infection | 20 (11.7%) | 620 (20.43%) | 0.51 [0.31–0.81] | 0.005 |
| Abdominal surgery | 15 (8.77%) | 126 (4.15%) | 2.23 [1.22–3.80] | 0.004 |
| Cardiovascular disease | 15 (8.77%) | 562 (18.52%) | 0.42 [0.23–0.70] | 0.001 |
| Neurosurgery | 14 (8.19%) | 78 (2.57%) | 3.41 [1.80–5.98] | <0.001 |
| Abdominal infection | 10 (5.85%) | 120 (3.95%) | 1.52 [0.73–2.83] | 0.22 |
| Neoplasia | 10 (5.85%) | 110 (3.62%) | 1.67 [0.80–3.11] | 0.136 |
| Neurologic disease | 8 (4.68%) | 503 (16.57%) | 0.25 [0.11–0.48] | <0.001 |
| Septic shock | 5 (2.92%) | 102 (3.36%) | 0.89 [0.30–2.01] | 0.756 |
| Solid organ transplantation | 4 (2.34%) | 90 (2.97%) | 0.81 [0.24–1.97] | 0.81 |
| Haematologic disease | 2 (1.17%) | 90 (2.97%) | 0.41 [0.06–1.32] | 0.236 |
| Renal disease | 0 (0%) | 40 (1.32%) | 0 [0.00–1.71] | 0.272 |
| Other | 7 (4.09%) | 148 (4.88%) | 0.85 [0.35–1.71] | 0.64 |
| p | OR | S | E | PPV | NPV | |
|---|---|---|---|---|---|---|
| Colonisation | ||||||
| C. auris | <0.001 | 29.63 [40.57–21.64] | 80.30% | 87.91% | 12.33% | 99.53% |
| C. albicans | <0.001 | 13.13 [23.89–7.22] | 93.48% | 47.82% | 2.56% | 99.80% |
| C. parapsilosis | <0.001 | 14.31 [20.58–9.95] | 70.27% | 85.82% | 5.51% | 99.59% |
| N. glabratus | <0.001 | - | 100.00% | 63.25% | 1.36% | 100.00% |
| C. tropicalis | <0.001 | 83.74 [251.11–27.93] | 83.33% | 94.37% | 2.72% | 99.97% |
| Any yeast species | <0.001 | 6.4 [9.22–4.44] | 95.32% | 23.90% | 6.64% | 98.90% |
| IC ≥ 0.5 | ||||||
| C. auris | <0.001 | 24.86 [32.49–19.02] | 65.15% | 93.01% | 16.48% | 99.21% |
| C. albicans | <0.001 | 5.54 [7.76–3.95] | 73.91% | 66.15% | 3.10% | 99.43% |
| C. parapsilosis | <0.001 | 12.72 [17.96–9.02] | 40.54% | 94.91% | 8.57% | 99.27% |
| N. glabratus | <0.001 | 11.73 [20.93–6.57] | 75.00% | 79.63% | 1.83% | 99.84% |
| C. tropicalis | <0.001 | 18.87 [45.19–7.88] | 33.33% | 97.42% | 2.38% | 99.87% |
| Any yeast species | <0.001 | 3.94 [4.88–3.19] | 84.21% | 42.50% | 7.68% | 97.93% |
| ICC ≥ 0.4 | ||||||
| C. auris | <0.001 | 13.39 [17.84–10.05] | 30.30% | 96.86% | 16.95% | 98.50% |
| C. albicans | <0.001 | 3.42 [4.65–2.52] | 39.13% | 84.19% | 3.50% | 98.95% |
| C. parapsilosis | <0.001 | 18.76 [28.19–12.48] | 24.32% | 98.32% | 14.52% | 99.10% |
| N. glabratus | <0.001 | 21.42 [36.86–12.45] | 68.75% | 90.69% | 3.59% | 99.83% |
| C. tropicalis | <0.001 | 58.33 [141.67–24.02] | 33.33% | 99.15% | 6.90% | 99.87% |
| Any yeast species | <0.001 | 2.81 [3.29–2.39] | 54.97% | 69.69% | 9.33% | 96.46% |
| Yeast Species | Sample | p | OR | E | S | NPV | VPP |
|---|---|---|---|---|---|---|---|
| Total | |||||||
| Urine | <0.001 | 3.3 [3.91–2.79] | 76.04% | 50.98% | 95.70% | 12.91% | |
| Tracheal/Bronchial | <0.001 | 2.46 [2.04–2.96] | 58.01% | 64.03% | 92.99% | 15.64% | |
| Rectal | <0.001 | 2.68 [2.17–3.32] | 36.07% | 82.61% | 96.41% | 9.07% | |
| Oropharyngeal | <0.001 | 2.43 [1.87–3.15] | 28.99% | 85.60% | 95.81% | 9.59% | |
| Axillary | <0.001 | 2.85 [2.17–3.72] | 75.30% | 48.28% | 97.53% | 6.71% | |
| Inguinal | <0.001 | 4.04 [2.88–5.68] | 49.71% | 80.36% | 98.60% | 5.43% | |
| C. auris | |||||||
| Urine | <0.001 | 21.88 [16.6–28.83] | 96.25% | 46.03% | 98.46% | 25.44% | |
| Tracheal/Bronchial | <0.001 | 15.2 [11.15–20.7] | 96.23% | 37.29% | 96.88% | 32.84% | |
| Rectal | <0.001 | 18.91 [14.27–25.04] | 88.65% | 70.77% | 99.02% | 15.75% | |
| Oropharyngeal | <0.001 | 9.42 [7.01–12.66] | 94.32% | 36.21% | 97.41% | 20.00% | |
| Axillary | <0.001 | - | 94.55% | 100.00% | 100.00% | 11.11% | |
| Inguinal | <0.001 | - | 94.55% | 100.00% | 100.00% | 10.31% | |
| C. albicans | |||||||
| Urine | 0.0143 | 2.41 [1.67–3.5] | 70.70% | 50.00% | 98.28% | 4.04% | |
| Tracheal/Bronchial | <0.001 | 7.49 [5.42–10.35] | 89.22% | 47.50% | 98.98% | 7.14% | |
| Rectal | 0.001 | 2.84 [2.04–3.97] | 60.49% | 65.00% | 98.95% | 2.92% | |
| Oropharyngeal | <0.001 | 12.6 [6.03–26.32] | 50.20% | 92.59% | 99.74% | 3.23% | |
| Axillary | 0.066 | 2.72 [1.54–4.79] | 91.58% | 20.00% | 98.92% | 2.88% | |
| Inguinal | <0.001 | 5.19 [3.16–8.52] | 70.54% | 68.42% | 99.47% | 2.71% | |
| C. parapsilosis | |||||||
| Urine | <0.001 | 14.14 [9.62–20.78] | 95.38% | 40.63% | 98.38% | 18.84% | |
| Tracheal/Bronchial | <0.001 | 14.27 [9.42–21.63] | 97.21% | 29.03% | 99.02% | 12.33% | |
| Rectal | <0.001 | 10.66 [7.42–15.32] | 94.75% | 37.14% | 98.95% | 10.16% | |
| Oropharyngeal | <0.001 | 13.06 [8.71–19.57] | 92.89% | 50.00% | 99.08% | 10.83% | |
| Axillary | <0.001 | 5.7 [3.3–9.86] | 92.61% | 31.25% | 99.27% | 4.03% | |
| Inguinal | <0.001 | 10.54 [6.34–17.51] | 91.33% | 50.00% | 99.45% | 5.48% | |
| N. glabratus | |||||||
| Urine | 0.048 | 3.08 [1.7–5.61] | 88.52% | 28.57% | 99.09% | 2.76% | |
| Tracheal/Bronchial | 0.150 | 2.49 [1.29–4.79] | 90.12% | 21.43% | 99.47% | 1.30% | |
| Rectal | <0.001 | 16.94 [7.94–36.13] | 70.76% | 87.50% | 99.87% | 2.12% | |
| Oropharyngeal | <0.001 | 38.86 [13.65–110.65] | 77.94% | 91.67% | 99.92% | 3.19% | |
| Axillary | 0.006 | 5.91 [2.83–12.33] | 90.79% | 37.50% | 99.66% | 1.97% | |
| Inguinal | <0.001 | 21.68 [7.43–63.25] | 75.59% | 87.50% | 99.92% | 1.76% | |
| C. tropicalis | |||||||
| Urine | <0.001 | 43.49 [17.18–110.06] | 98.49% | 40.00% | 99.87% | 5.41% | |
| Tracheal/Bronchial | <0.001 | 91.77 [28.59–294.53] | 96.83% | 75.00% | 99.92% | 7.14% | |
| Rectal | 0.691 | - | 96.93% | 0.00% | 99.77% | 0.00% | |
| Oropharyngeal | <0.001 | - | 95.27% | 100.00% | 100.00% | 2.70% | |
| Axillary | 0.916 | - | 99.63% | 0.00% | 99.81% | 0.00% | |
| Inguinal | 0.814 | - | 98.19% | 0.00% | 99.81% | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Urquiza, P.; Pemán, J.; Gordon, M.; Favier, P.; Muñoz-Brell, P.; López-Hontangas, J.L.; Ruiz-Gaitán, A. Predicting Fungemia in the ICU: Unveiling the Value of Weekly Fungal Surveillance and Yeast Colonisation Monitoring. J. Fungi 2024, 10, 674. https://doi.org/10.3390/jof10100674
Suárez-Urquiza P, Pemán J, Gordon M, Favier P, Muñoz-Brell P, López-Hontangas JL, Ruiz-Gaitán A. Predicting Fungemia in the ICU: Unveiling the Value of Weekly Fungal Surveillance and Yeast Colonisation Monitoring. Journal of Fungi. 2024; 10(10):674. https://doi.org/10.3390/jof10100674
Chicago/Turabian StyleSuárez-Urquiza, Pedro, Javier Pemán, Monica Gordon, Patricio Favier, Paula Muñoz-Brell, Jose Luis López-Hontangas, and Alba Ruiz-Gaitán. 2024. "Predicting Fungemia in the ICU: Unveiling the Value of Weekly Fungal Surveillance and Yeast Colonisation Monitoring" Journal of Fungi 10, no. 10: 674. https://doi.org/10.3390/jof10100674
APA StyleSuárez-Urquiza, P., Pemán, J., Gordon, M., Favier, P., Muñoz-Brell, P., López-Hontangas, J. L., & Ruiz-Gaitán, A. (2024). Predicting Fungemia in the ICU: Unveiling the Value of Weekly Fungal Surveillance and Yeast Colonisation Monitoring. Journal of Fungi, 10(10), 674. https://doi.org/10.3390/jof10100674







