Prognostic Value of Combined Radiomic Features from Follow-Up DWI and T2-FLAIR in Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
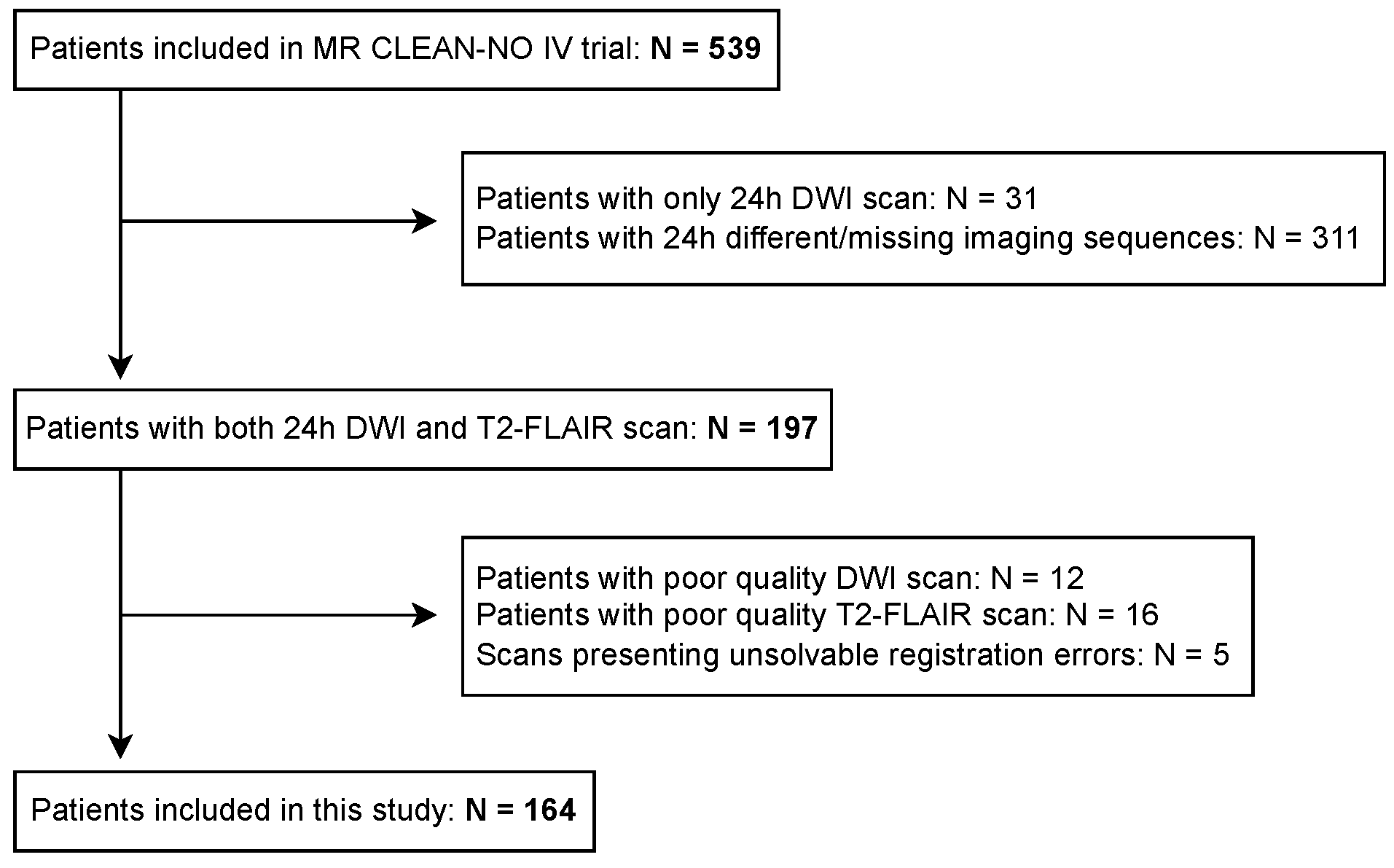
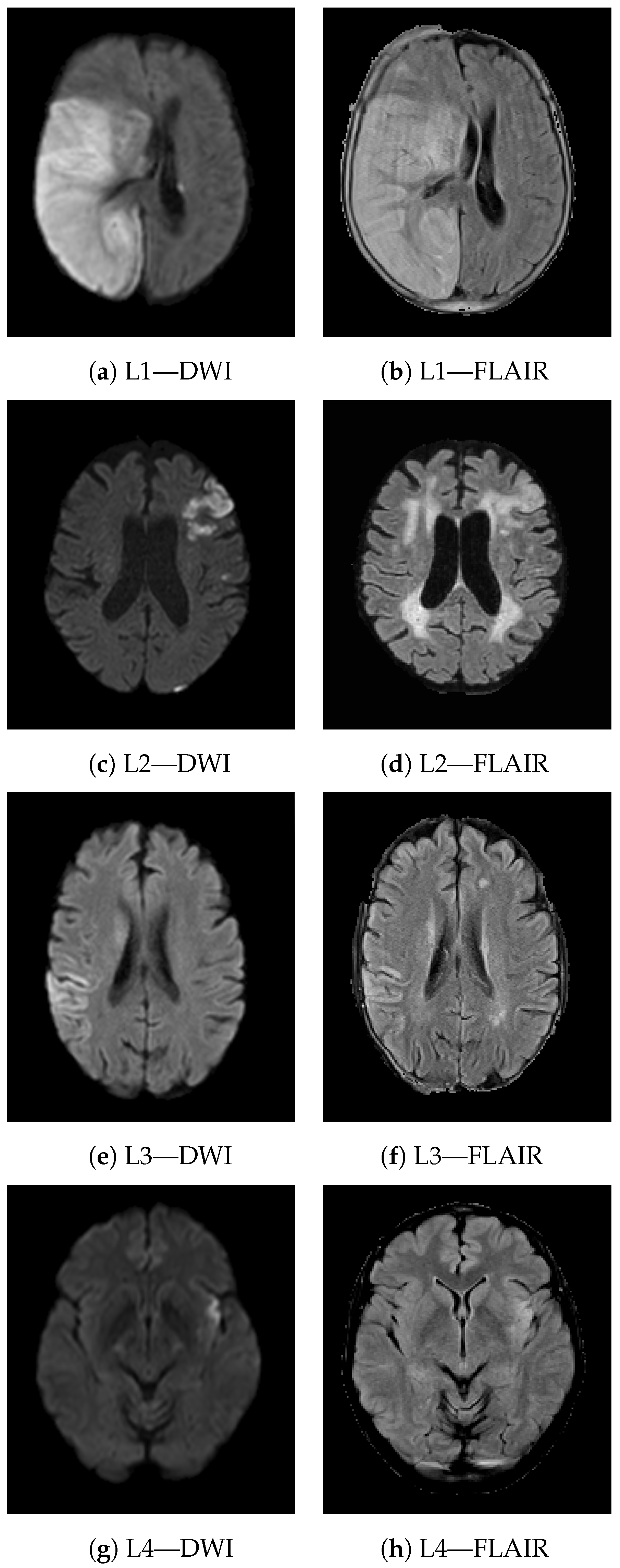
2.1. Data Collection
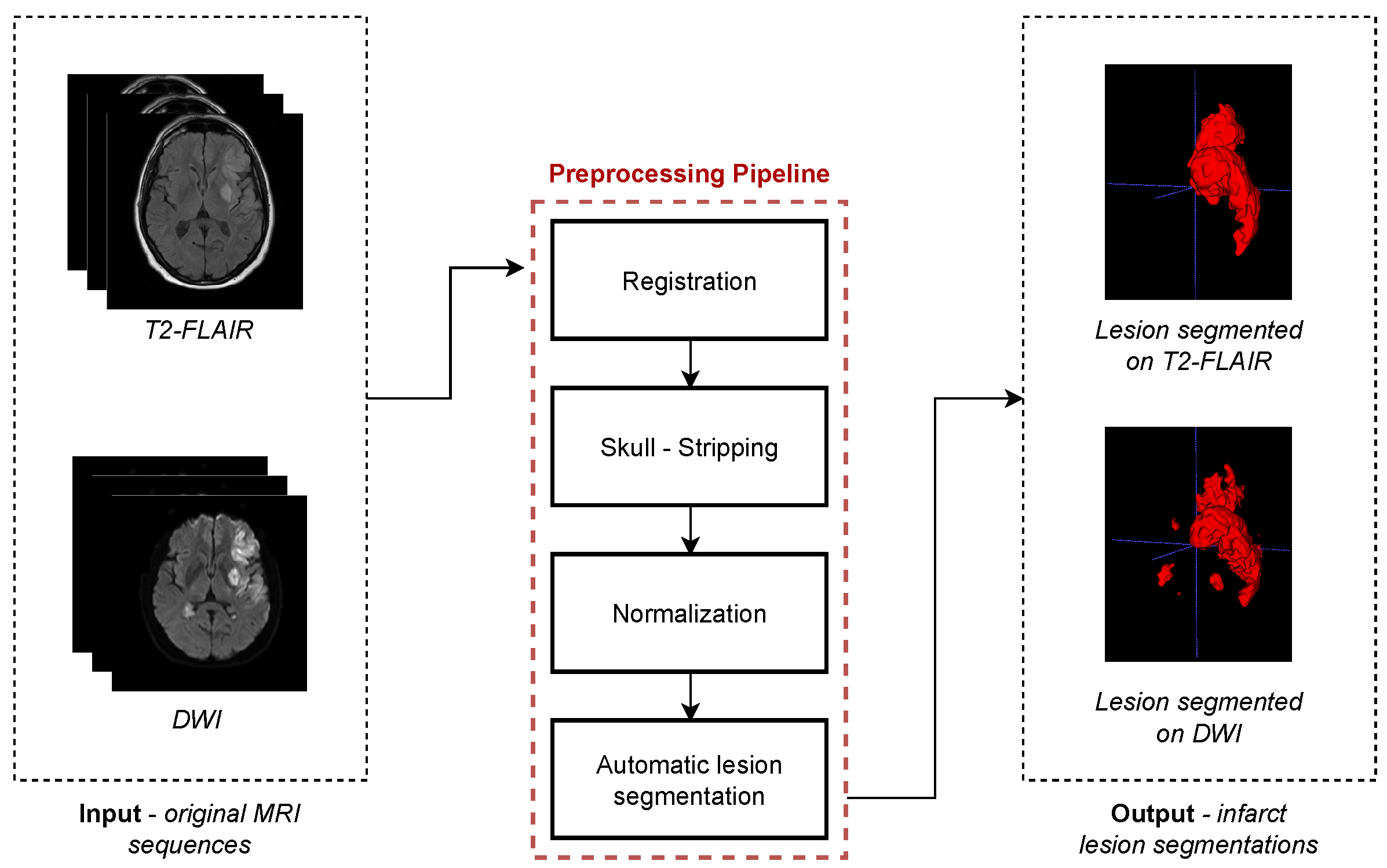
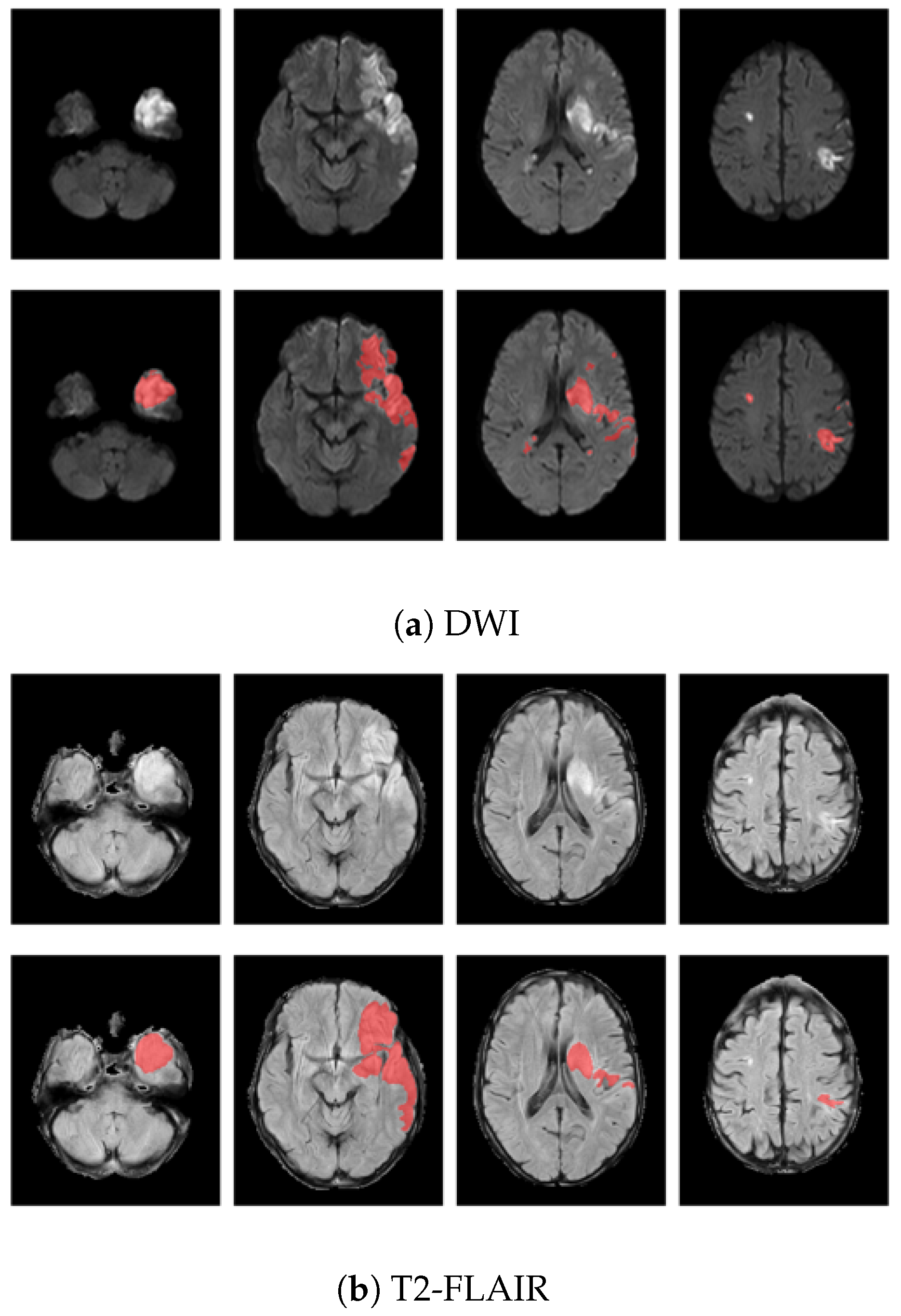
2.2. Image Preprocessing
2.3. Features
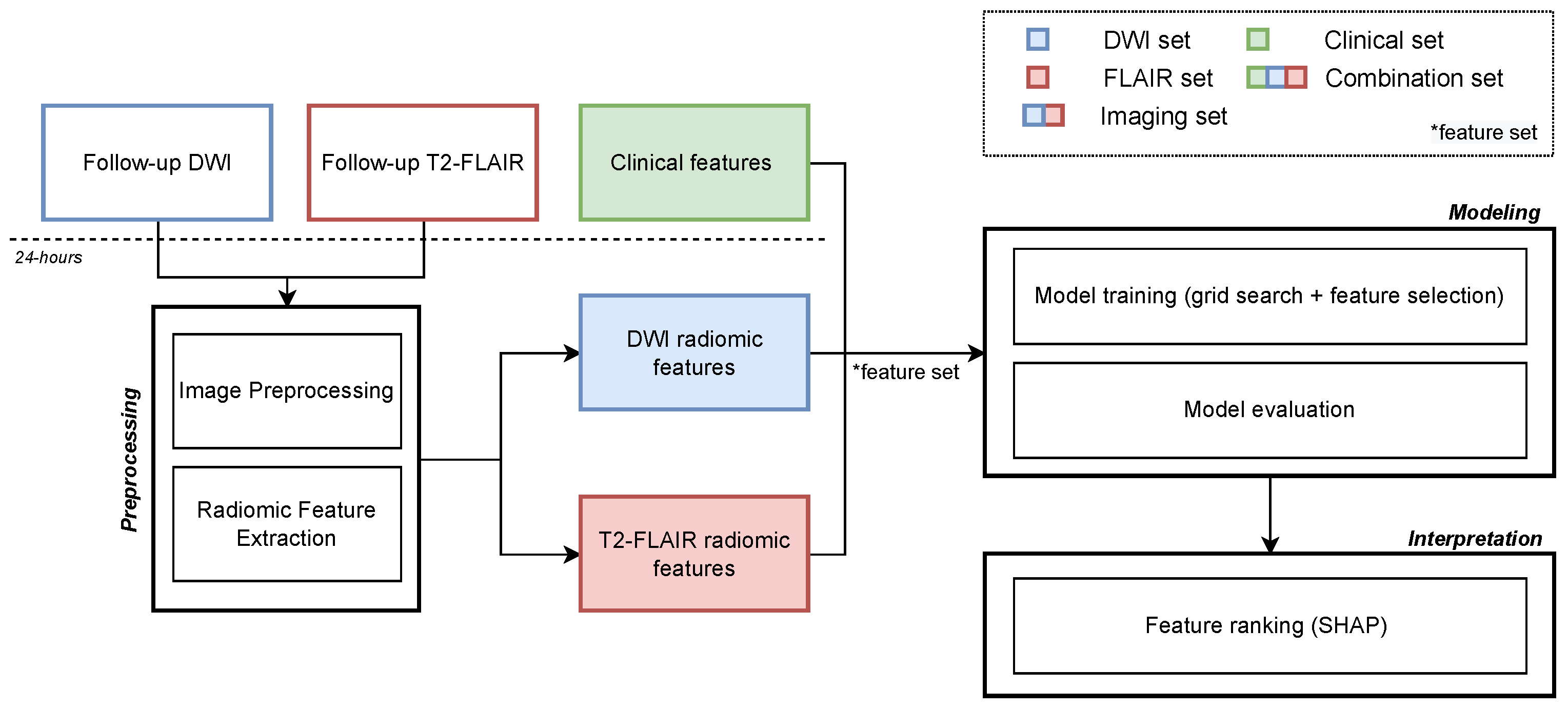
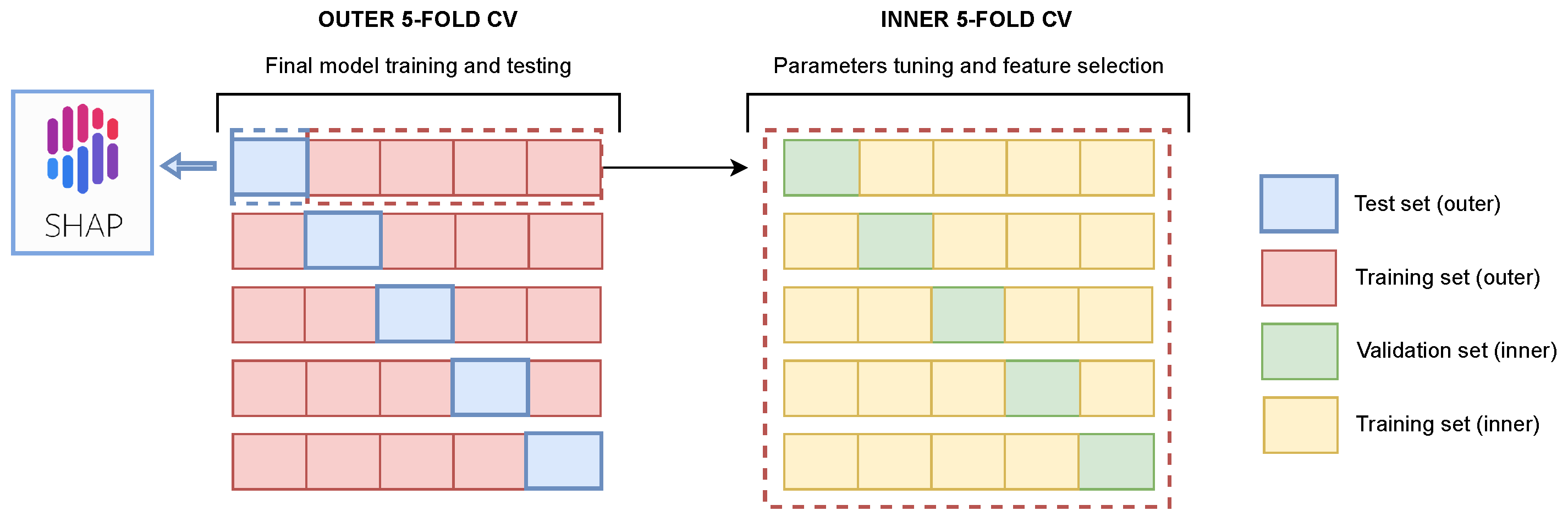
2.4. Image Analysis Pipeline and Experimental Setup
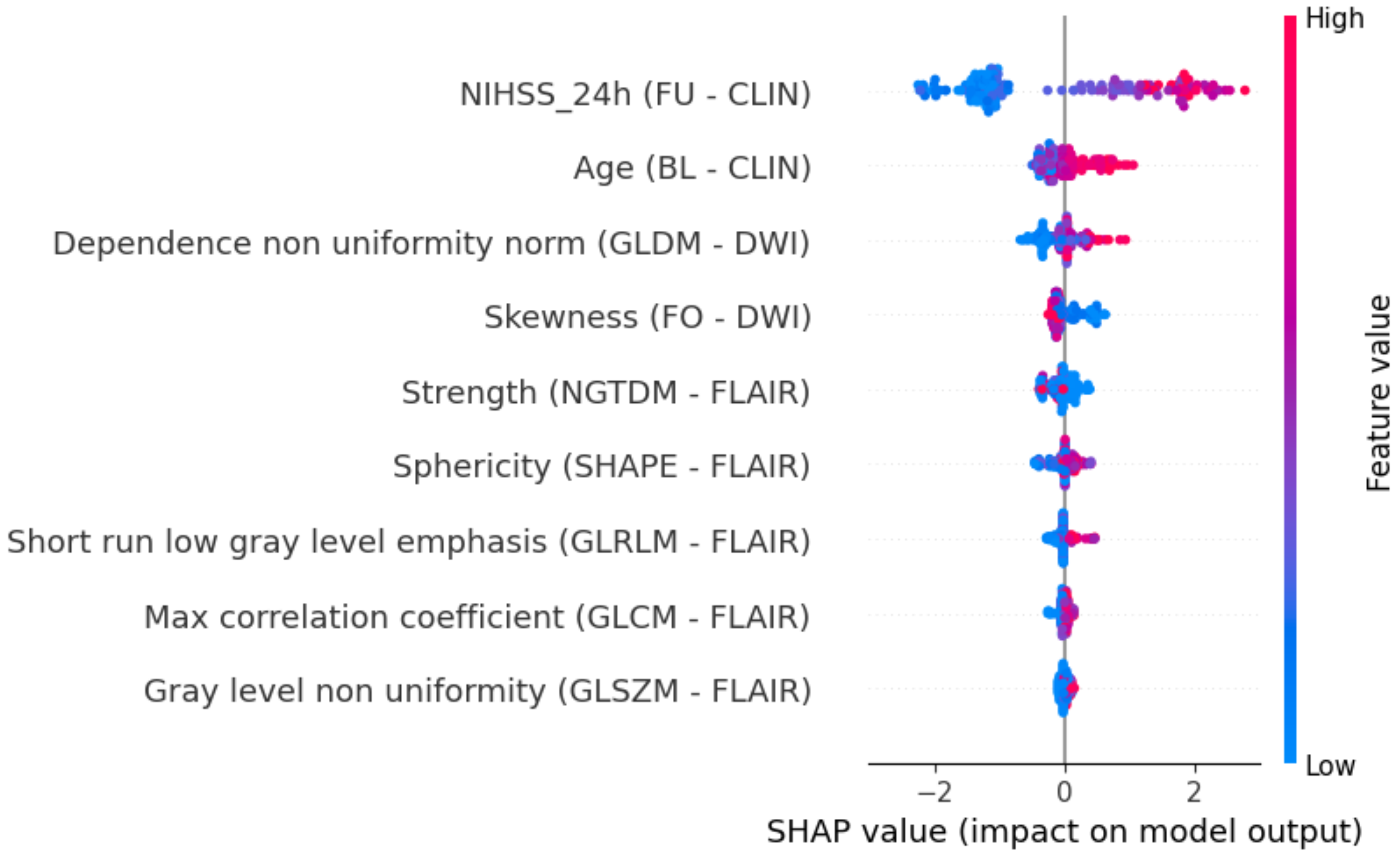
2.5. Explainability
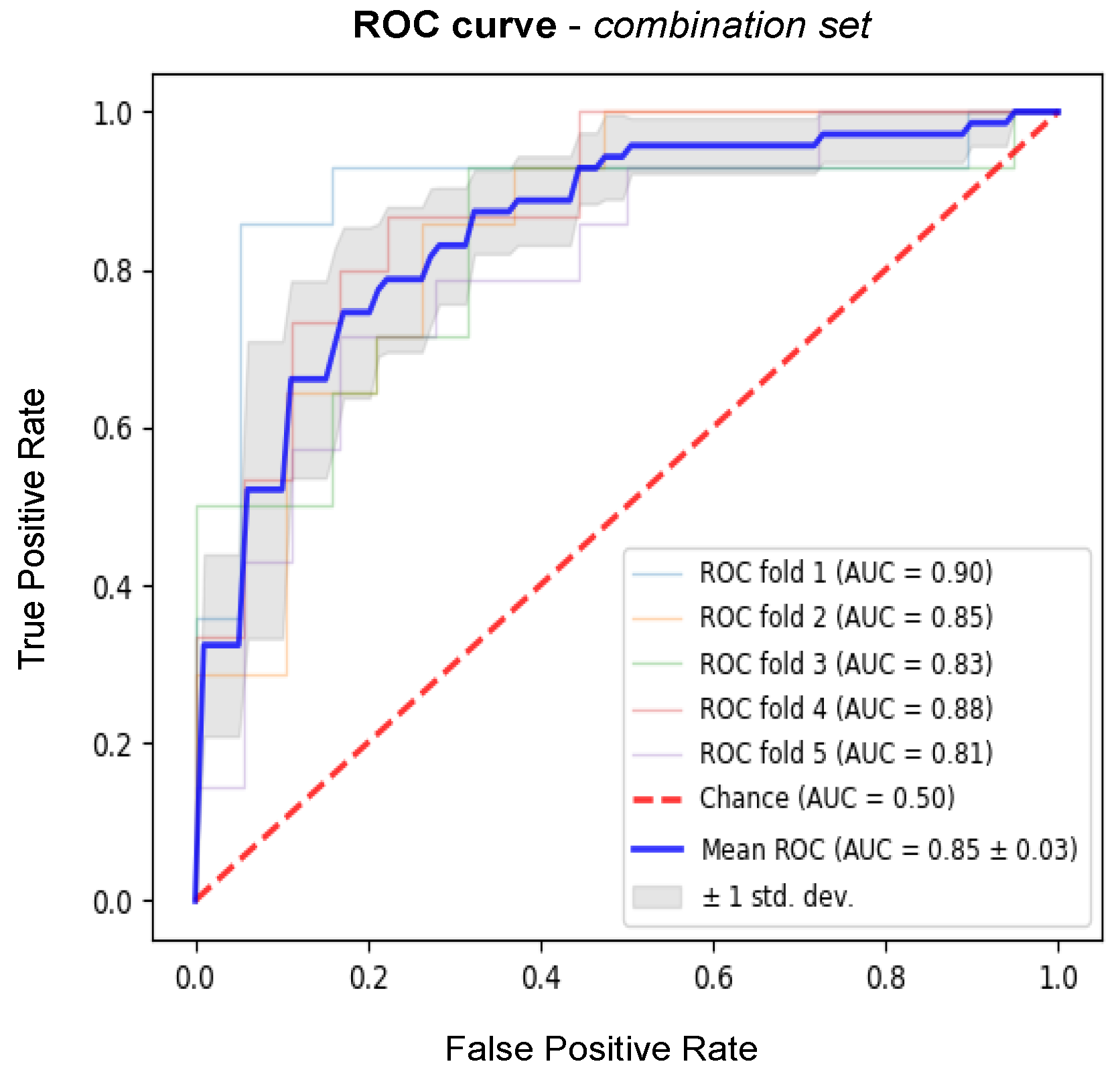
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wittenauer, R.; Smith, L. Background paper 6.6 ischaemic and haemorrhagic stroke. In Priority Medicines for Europe and the World. A Public Health Approach to Innovation; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Rabinstein, A.A.; Albers, G.W.; Brinjikji, W.; Koch, S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int. J. Stroke 2019, 14, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yang, Y.; Liu, B.; Castro, S.A.; Shi, T. Patients With Acute Ischemic Stroke Who Receive Brain Magnetic Resonance Imaging Demonstrate Favorable In-Hospital Outcomes. J. Am. Heart Assoc. 2020, 9, e016987. [Google Scholar] [CrossRef] [PubMed]
- DiBella, E.; Sharma, A.; Richards, L.; Prabhakaran, V.; Majersik, J.; Hashemizadeh Kolowri, S. Beyond diffusion tensor MRI methods for improved characterization of the brain after ischemic stroke: A Review. Am. J. Neuroradiol. 2022, 43, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kwah, L.K.; Herbert, R.D. Prediction of walking and arm recovery after stroke: A critical review. Brain Sci. 2016, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- MEDICA, E.M. Factors enhancing Activities of Daily Living after stroke in specialized rehabilitation. An observational multicenter study within the Sunnaas International Network. Eur. J. Phys. Rehabil. Med. 2017, 53, 725–734. [Google Scholar]
- Thijs, V.N.; Lansberg, M.G.; Beaulieu, C.; Marks, M.P.; Moseley, M.E.; Albers, G.W. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke 2000, 31, 2597–2602. [Google Scholar] [CrossRef]
- Zaidi, S.F.; Aghaebrahim, A.; Urra, X.; Jumaa, M.A.; Jankowitz, B.; Hammer, M.; Nogueira, R.; Horowitz, M.; Reddy, V.; Jovin, T.G. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke 2012, 43, 3238–3244. [Google Scholar] [CrossRef]
- Yoo, A.J.; Chaudhry, Z.A.; Nogueira, R.G.; Lev, M.H.; Schaefer, P.W.; Schwamm, L.H.; Hirsch, J.A.; González, R.G. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 2012, 43, 1323–1330. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Zheng, L.; Zhao, J.; Song, B.; Dai, Y. Texture analysis based on ADC maps and T2-FLAIR images for the assessment of the severity and prognosis of ischaemic stroke. Clin. Imaging 2020, 67, 152–159. [Google Scholar] [CrossRef]
- Hilbert, A.; Ramos, L.A.; van Os, H.J.; Olabarriaga, S.D.; Tolhuisen, M.L.; Wermer, M.J.; Barros, R.S.; van der Schaaf, I.; Dippel, D.; Roos, Y.; et al. Data-efficient deep learning of radiological image data for outcome prediction after endovascular treatment of patients with acute ischemic stroke. Comput. Biol. Med. 2019, 115, 103516. [Google Scholar] [CrossRef]
- Nishi, H.; Oishi, N.; Ishii, A.; Ono, I.; Ogura, T.; Sunohara, T.; Chihara, H.; Fukumitsu, R.; Okawa, M.; Yamana, N.; et al. Deep Learning–Derived High-Level Neuroimaging Features Predict Clinical Outcomes for Large Vessel Occlusion. Stroke 2020, 51, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Birenbaum, D.; Bancroft, L.W.; Felsberg, G.J. Imaging in acute stroke. West. J. Emerg. Med. 2011, 12, 67. [Google Scholar]
- Ayata, C.; Ropper, A.H. Ischaemic brain oedema. J. Clin. Neurosci. 2002, 9, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Shlobin, N.A.; Baig, A.A.; Waqas, M.; Patel, T.R.; Dossani, R.H.; Wilson, M.; Cappuzzo, J.M.; Siddiqui, A.H.; Tutino, V.M.; Levy, E.I. Artificial Intelligence for Large Vessel Occlusion Stroke: A Systematic Review. World Neurosurg. 2021, 159, 207–220.e1. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, T.; Zhang, M.; Xia, N.; Liu, J.; Yang, Y. Radiomics in stroke neuroimaging: Techniques, applications, and challenges. Aging Dis. 2021, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4768–4777. [Google Scholar]
- LeCouffe, N.E.; Kappelhof, M.; Treurniet, K.M.; Rinkel, L.A.; Bruggeman, A.E.; Berkhemer, O.A.; Wolff, L.; van Voorst, H.; Tolhuisen, M.L.; Dippel, D.W.; et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N. Engl. J. Med. 2021, 385, 1833–1844. [Google Scholar] [CrossRef]
- Treurniet, K.M.; LeCouffe, N.E.; Kappelhof, M.; Emmer, B.J.; van Es, A.C.; Boiten, J.; Lycklama, G.J.; Keizer, K.; Lonneke, S.; Lingsma, H.F.; et al. MR CLEAN-NO IV: Intravenous treatment followed by endovascular treatment versus direct endovascular treatment for acute ischemic stroke caused by a proximal intracranial occlusion—Study protocol for a randomized clinical trial. Trials 2021, 22, 141. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Shinohara, R.T.; Sweeney, E.M.; Goldsmith, J.; Shiee, N.; Mateen, F.J.; Calabresi, P.A.; Jarso, S.; Pham, D.L.; Reich, D.S.; Crainiceanu, C.M.; et al. Statistical normalization techniques for magnetic resonance imaging. Neuroimage Clin. 2014, 6, 9–19. [Google Scholar] [CrossRef]
- Hazlett, Y.P.P.J.C. H Gimpel Smith R Ho S Gee JC Gerig G User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116. [Google Scholar]
- Li, H.; Jiang, G.; Zhang, J.; Wang, R.; Wang, Z.; Zheng, W.S.; Menze, B. Fully convolutional network ensembles for white matter hyperintensities segmentation in MR images. NeuroImage 2018, 183, 650–665. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.G.; Lee, S.I. Consistent Individualized Feature Attribution for Tree Ensembles. arXiv 2018, arXiv:1802.03888. [Google Scholar]
- XGBoost Documentation. Available online: https://xgboost.readthedocs.io/en/stable/parameter.html (accessed on 12 July 2022).
- Pyradiomics Documentation. Available online: https://pyradiomics.readthedocs.io/en/latest/features.html (accessed on 12 July 2022).
- Hendrix, P.; Melamed, I.; Collins, M.; Lieberman, N.; Sharma, V.; Goren, O.; Zand, R.; Schirmer, C.M.; Griessenauer, C.J. NIHSS 24 h after mechanical thrombectomy predicts 90-day functional outcome. Clin. Neuroradiol. 2022, 32, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Mlynash, M.; Sainani, K.L.; Albers, G.W.; Lansberg, M.G. Ordinal Prediction Model of 90-Day Modified Rankin Scale in Ischemic Stroke. Front. Neurol. 2021, 12, 727171. [Google Scholar] [CrossRef]
- Stokum, J.A.; Gerzanich, V.; Simard, J.M. Molecular pathophysiology of cerebral edema. J. Cereb. Blood Flow Metab. 2016, 36, 513–538. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, W.N.; Slot, K.B.; Fernandes, P.; Sandercock, P.; Wardlaw, J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: A systematic review and meta-analysis of 55 studies. Stroke 2012, 43, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, C.; Wang, S.; Ai, Z.; Shen, T.; Zhang, H.; Duan, S.; Yin, X.; Chen, Y.C. MRI Radiomics Features From Infarction and Cerebrospinal Fluid for Prediction of Cerebral Edema after Acute Ischemic Stroke. Front. Aging Neurosci. 2022, 14, 782036. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, Z.; Wu, S.; Cao, Z.; Xu, Y.; Lv, Y. Predictive value of magnetic resonance imaging-based texture analysis for hemorrhage transformation in large cerebral infarction. Front. Neurosci. 2022, 16, 923708. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Hong, D.; Chen, F.; Ji, X.; Cao, G. White matter injury in ischemic stroke. Prog. Neurobiol. 2016, 141, 45–60. [Google Scholar] [CrossRef]
- Hong, S.; Giese, A.K.; Schirmer, M.D.; Bonkhoff, A.K.; Bretzner, M.; Rist, P.; Dalca, A.V.; Regenhardt, R.W.; Etherton, M.R.; Donahue, K.L.; et al. Excessive White Matter Hyperintensity Increases Susceptibility to Poor Functional Outcomes After Acute Ischemic Stroke. Front. Neurol. 2021, 12, 700616. [Google Scholar] [CrossRef] [PubMed]
- Boers, A.M.; Jansen, I.G.; Beenen, L.F.; Devlin, T.G.; San Roman, L.; Heo, J.H.; Ribó, M.; Brown, S.; Almekhlafi, M.A.; Liebeskind, D.S.; et al. Association of follow-up infarct volume with functional outcome in acute ischemic stroke: A pooled analysis of seven randomized trials. J. Neurointerventional Surg. 2018, 10, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Almenkerk, S.v.; Smalbrugge, M.; Depla, M.F.; Eefsting, J.A.; Hertogh, C.M. What predicts a poor outcome in older stroke survivors? A systematic review of the literature. Disabil. Rehabil. 2013, 35, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
| Feature Set | Acc | Sens | Spec | Prec | AUC |
|---|---|---|---|---|---|
| DWI | |||||
| FLAIR | |||||
| Imaging | |||||
| Clinical | |||||
| Combination |
| Feature Set | AUC | p-Value |
|---|---|---|
| Combination—DWI | – | <0.001 |
| Combination—FLAIR | – | <0.001 |
| Combination—Imaging | – | <0.001 |
| Combination—Clinical | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerbasi, A.; Konduri, P.; Tolhuisen, M.; Cavalcante, F.; Rinkel, L.; Kappelhof, M.; Wolff, L.; Coutinho, J.M.; Emmer, B.J.; Costalat, V.; et al. Prognostic Value of Combined Radiomic Features from Follow-Up DWI and T2-FLAIR in Acute Ischemic Stroke. J. Cardiovasc. Dev. Dis. 2022, 9, 468. https://doi.org/10.3390/jcdd9120468
Gerbasi A, Konduri P, Tolhuisen M, Cavalcante F, Rinkel L, Kappelhof M, Wolff L, Coutinho JM, Emmer BJ, Costalat V, et al. Prognostic Value of Combined Radiomic Features from Follow-Up DWI and T2-FLAIR in Acute Ischemic Stroke. Journal of Cardiovascular Development and Disease. 2022; 9(12):468. https://doi.org/10.3390/jcdd9120468
Chicago/Turabian StyleGerbasi, Alessia, Praneeta Konduri, Manon Tolhuisen, Fabiano Cavalcante, Leon Rinkel, Manon Kappelhof, Lennard Wolff, Jonathan M. Coutinho, Bart J. Emmer, Vincent Costalat, and et al. 2022. "Prognostic Value of Combined Radiomic Features from Follow-Up DWI and T2-FLAIR in Acute Ischemic Stroke" Journal of Cardiovascular Development and Disease 9, no. 12: 468. https://doi.org/10.3390/jcdd9120468
APA StyleGerbasi, A., Konduri, P., Tolhuisen, M., Cavalcante, F., Rinkel, L., Kappelhof, M., Wolff, L., Coutinho, J. M., Emmer, B. J., Costalat, V., Arquizan, C., Hofmeijer, J., Uyttenboogaart, M., van Zwam, W., Roos, Y., Quaglini, S., Bellazzi, R., Majoie, C., & Marquering, H., on behalf of the MR CLEAN-NO IV Trial Investigators (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in The Netherlands). (2022). Prognostic Value of Combined Radiomic Features from Follow-Up DWI and T2-FLAIR in Acute Ischemic Stroke. Journal of Cardiovascular Development and Disease, 9(12), 468. https://doi.org/10.3390/jcdd9120468






_Uyttenboogaart.png)


