Infective Endocarditis: Predictive Factors for Diagnosis and Mortality in Surgically Treated Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
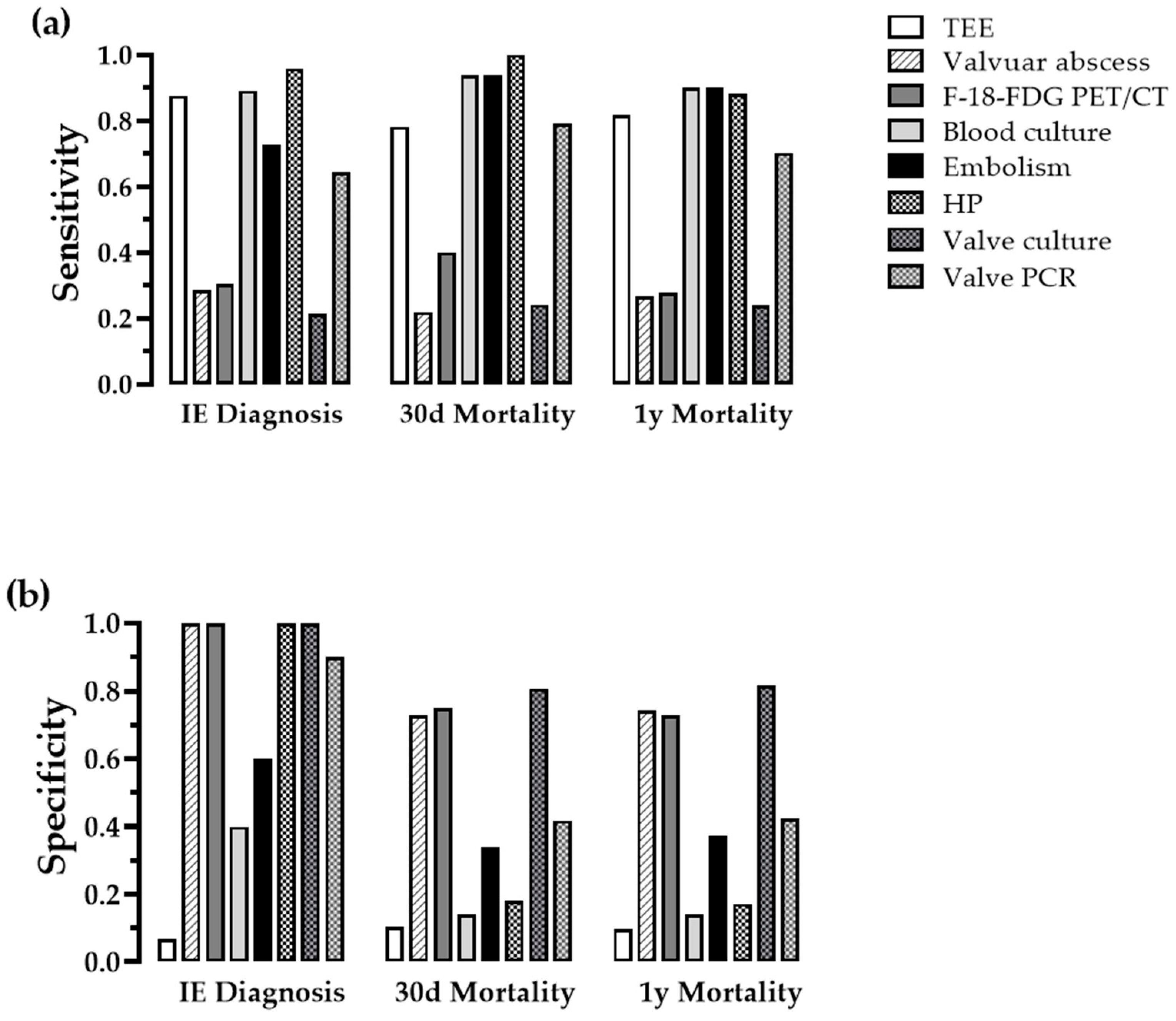
3.2. Examination of Qualitative Parameters
3.3. Prediction of IE by Quantitative Parameters
3.4. Prediction of Mortality
4. Discussion
4.1. Microbiological Testing Is a Mainstay in Diagnosing IE
4.2. TEE and Establishment of a Cut-off Value for Vegetation Size
4.3. Patient Population and Affected Valves
4.4. Nt-Probnp Had Predictive Power for Mortality, Presence of Systemic Embolism Was Associated with Mortality
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anantha Narayanan, M.; Mahfood Haddad, T.; Kalil, A.C.; Kanmanthareddy, A.; Suri, R.M.; Mansour, G.; Destache, C.J.; Baskaran, J.; Mooss, A.N.; Wichman, T.; et al. Early versus late surgical intervention or medical management for infective endocarditis: A systematic review and meta-analysis. Heart 2016, 102, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, Y.J.; Kim, S.H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Sonneville, R.; Hajage, D.; Novy, E.; Tubach, F.; Vignon, P.; Perez, P.; Lavoué, S.; Kouatchet, A.; Pajot, O.; et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur. Heart J. 2014, 35, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Hasbun, R.; Vikram, H.R.; Barakat, L.A.; Buenconsejo, J.; Quagliarello, V.J. Complicated left-sided native valve endocarditis in adults: Risk classification for mortality. JAMA 2003, 289, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Alla, F.; Selton-Suty, C.; Beguinot, I.; Bouvet, A.; Briancon, S.; Casalta, J.P.; Danchin, N.; Delahaye, F.; Etienne, J.; et al. Changing profile of infective endocarditis: Results of a 1-year survey in France. JAMA 2002, 288, 75–81. [Google Scholar] [CrossRef]
- Stavi, V.; Brandstaetter, E.; Sagy, I.; Sapunar, S.; Nevzorov, R.; Bartal, C.; Barski, L. Comparison of Clinical Characteristics and Prognosis in Patients with Right- and Left-sided Infective Endocarditis. Rambam Maimonides Med. J. 2019, 10, e0003. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Fosbol, E.L.; Park, L.P.; Chu, V.H.; Athan, E.; Delahaye, F.; Freiberger, T.; Lamas, C.; Miro, J.M.; Strahilevitz, J.; Tribouilloy, C.; et al. The association between vegetation size and surgical treatment on 6-month mortality in left-sided infective endocarditis. Eur. Heart J. 2019, 40, 2243–2251. [Google Scholar] [CrossRef]
- Hill, E.E.; Herijgers, P.; Claus, P.; Vanderschueren, S.; Peetermans, W.E.; Herregods, M.C. Abscess in infective endocarditis: The value of transesophageal echocardiography and outcome: A 5-year study. Am. Heart J. 2007, 154, 923–928. [Google Scholar] [CrossRef]
- Vieira, M.L.; Grinberg, M.; Pomerantzeff, P.M.; Andrade, J.L.; Mansur, A.J. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Heart 2004, 90, 1020–1024. [Google Scholar] [CrossRef]
- Sag, S.J.M.; Menhart, K.; Grosse, J.; Hitzenbichler, F.; Hanses, F.; Mohr, A.; Salzberger, B.; Zerdzitzki, M.; Hilker, M.; Rupprecht, L.; et al. Diagnostic value of FDG PET/CT imaging in patients with surgically managed infective endocarditis: Results of a retrospective analysis at a tertiary center. J. Nucl. Cardiol. 2020, 29, 1191–1204. [Google Scholar] [CrossRef]
- Durack, D.T.; Lukes, A.S.; Bright, D.K. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Duke Endocarditis Service. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cabrera, E.; Fernandez-Hidalgo, N.; Almirante, B.; Ivanova-Georgieva, R.; Noureddine, M.; Plata, A.; Lomas, J.M.; Galvez-Acebal, J.; Hidalgo-Tenorio, C.; Ruiz-Morales, J.; et al. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation 2013, 127, 2272–2284. [Google Scholar] [CrossRef] [PubMed]
- Schou, M.; Gustafsson, F.; Kjaer, A.; Hildebrandt, P.R. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur. Heart J. 2007, 28, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Soon, E.; Doughty, N.J.; Treacy, C.M.; Ross, R.M.; Toshner, M.; Upton, P.D.; Sheares, K.; Morrell, N.W.; Pepke-Zaba, J. Log-transformation improves the prognostic value of serial NT-proBNP levels in apparently stable pulmonary arterial hypertension. Pulm. Circ. 2011, 1, 244–249. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Tattevin, P.; Watt, G.; Revest, M.; Arvieux, C.; Fournier, P.E. Update on blood culture-negative endocarditis. Med. Mal. Infect. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Lamas, C.C.; Fournier, P.E.; Zappa, M.; Brandao, T.J.; Januario-da-Silva, C.A.; Correia, M.G.; Barbosa, G.I.; Golebiovski, W.F.; Weksler, C.; Lepidi, H.; et al. Diagnosis of blood culture-negative endocarditis and clinical comparison between blood culture-negative and blood culture-positive cases. Infection 2016, 44, 459–466. [Google Scholar] [CrossRef]
- Lepidi, H.; Durack, D.T.; Raoult, D. Diagnostic methods current best practices and guidelines for histologic evaluation in infective endocarditis. Infect. Dis. Clin. N. Am. 2002, 16, 339–361. [Google Scholar] [CrossRef]
- Chu, V.H.; Park, L.P.; Athan, E.; Delahaye, F.; Freiberger, T.; Lamas, C.; Miro, J.M.; Mudrick, D.W.; Strahilevitz, J.; Tribouilloy, C.; et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: A prospective study from the International Collaboration on Endocarditis. Circulation 2015, 131, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Bouza, E.; Marin, M.; Alcala, L.; Rodriguez Creixems, M.; Valerio, M.; Pinto, A.; Group for the Management of Infective Endocarditis of the Gregorio Maranon, H. Heart valves should not be routinely cultured. J. Clin. Microbiol. 2008, 46, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Lepidi, H.; Rovery, C.; Casalta, J.P.; Habib, G.; Collard, F.; Fournier, P.E.; Raoult, D. Diagnosis of infectious endocarditis in patients undergoing valve surgery. Am. J. Med. 2005, 118, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.J.; Drinkovic, D.; Pottumarthy, S.; MacCulloch, D.; Kerr, A.R.; West, T. Bacteriological outcome after valve surgery for active infective endocarditis: Implications for duration of treatment after surgery. Clin. Infect. Dis. 2005, 41, 187–194. [Google Scholar] [CrossRef]
- Geissdorfer, W.; Moos, V.; Moter, A.; Loddenkemper, C.; Jansen, A.; Tandler, R.; Morguet, A.J.; Fenollar, F.; Raoult, D.; Bogdan, C.; et al. High frequency of Tropheryma whipplei in culture-negative endocarditis. J. Clin. Microbiol. 2012, 50, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mohananey, D.; Mohadjer, A.; Pettersson, G.; Navia, J.; Gordon, S.; Shrestha, N.; Grimm, R.A.; Rodriguez, L.L.; Griffin, B.P.; Desai, M.Y. Association of Vegetation Size With Embolic Risk in Patients With Infective Endocarditis: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2018, 178, 502–510. [Google Scholar] [CrossRef]
- Slipczuk, L.; Codolosa, J.N.; Davila, C.D.; Romero-Corral, A.; Yun, J.; Pressman, G.S.; Figueredo, V.M. Infective endocarditis epidemiology over five decades: A systematic review. PLoS ONE 2013, 8, e82665. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Mahmoud, I.; Dykun, I.; Totzeck, M.; Rath, P.M.; Ruhparwar, A.; Buer, J.; Rassaf, T. Diagnostic value of the modified Duke criteria in suspected infective endocarditis -The PRO-ENDOCARDITIS study. Int. J. Infect. Dis. 2021, 104, 556–561. [Google Scholar] [CrossRef]
- Bertolino, L.; Ursi, M.P.; Iossa, D.; Karruli, A.; D’Amico, F.; Zampino, R.; Dialetto, G.; De Feo, M.; Durante-Mangoni, E.; Monaldi Hospital Cardiovascular Infection Study Group. Dissecting the correlates of N-terminal prohormone brain natriuretic peptide in acute infective endocarditis. Infection 2022, 50, 1465–1474. [Google Scholar] [CrossRef]
- Jordal, S.; Kittang, B.R.; Salminen, P.R.; Eide, G.E.; Kommedal, O.; Wendelbo, O.; Haaverstad, R.; Sjursen, H. Infective endocarditis in Western Norway: A 20-year retrospective survey. Infect. Dis. 2018, 50, 757–763. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, D.H.; Lee, M.Z.; Yun, S.C.; Kim, Y.J.; Song, J.M.; Song, J.K.; Lee, J.W.; Sohn, D.W. Impact of early surgery on embolic events in patients with infective endocarditis. Circulation 2010, 122, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- El-Dalati, S.; Murthy, V.L.; Owczarczyk, A.B.; Fagan, C.; Riddell, J.t.; Cinti, S.; Weinberg, R.L. Correlating cardiac F-18 FDG PET/CT results with intra-operative findings in infectious endocarditis. J. Nucl. Cardiol. 2019, 28, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.P.; Gouriet, F.; Riberi, A.; Avierinos, J.F.; et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: Increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J. Am. Coll. Cardiol. 2013, 61, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Schirone, L.; Iaccarino, A.; Saade, W.; D’Abramo, M.; De Bellis, A.; Frati, G.; Sciarretta, S.; Mestres, C.A.; Greco, E. Cerebrovascular Complications and Infective Endocarditis: Impact of Available Evidence on Clinical Outcome. BioMed Res. Int. 2018, 2018, 4109358. [Google Scholar] [CrossRef] [PubMed]
- Brandao, T.J.; Januario-da-Silva, C.A.; Correia, M.G.; Zappa, M.; Abrantes, J.A.; Dantas, A.M.; Golebiovski, W.; Barbosa, G.I.; Weksler, C.; Lamas, C.C. Histopathology of valves in infective endocarditis, diagnostic criteria and treatment considerations. Infection 2017, 45, 199–207. [Google Scholar] [CrossRef]



| Parameter | n = 215 |
|---|---|
| Age (years) | 64.2 ± 13.3 |
| Male | 160 (74.4) |
| BMI | 28.2 ± 11.9 |
| Logistic EuroSCORE II | 15.6 ± 10.9 |
| Coronary artery disease | 50 (23.2) |
| Peripheral occlusive disease | 28 (13.0) |
| Diabetes mellitus | 41 (19.1) |
| Malignancies | 31 (14.4) |
| Serum kreatinin (mg/dL) | 1.6 ± 2.2 |
| Chronic hemodialysis | 18 (8.4) |
| Immunosuppression | 15 (7.0) |
| i.v. drug abuse | 9 (4.2) |
| NYHA functional class III/IV | 104 (48.4) |
| Native valves | 160 (74.4) |
| IE affected valves | |
| Aortic valve only | 114 (52.3) |
| Mitral valve only | 80 (37.2) |
| Combined aortic and mitral | 21 (9.8) |
| Regurgitation degree II/III | |
| Aortic valve | 78 (57.7) |
| Mitral valve | 68 (67.3) |
| LVEF > 50% | 142 (66.0) |
| LVEF < 30% | 19 (8.8) |
| Embolism | 151 (70.2) |
| Cerebral | 77 (35.8) |
| Renal | 38 (17.7) |
| Spleen | 21 (9.8) |
| Eyes | 13 (6.0) |
| Other | 10 (4.6) |
| Fever | 185 (86.0) |
| WBC (1/nL) | 11.4 ± 5.5 |
| CRP (mg/L) | 90.1 ± 77.9 |
| PCT (ng/mL) | 4.3 ± 13.3 |
| Vegetation size (mm) | 14.8 ± 5.8 |
| NT-proBNP (pg/mL) | 4398 (1310, 11,830) |
| Log(NT-proBNP) | 3.62 ± 0.70 |
| IE Diagnosis | Mortality 30-Days | Mortality 1-Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Pos. | Neg. | p | Pos. | Neg. | p | Pos. | Neg. | p |
| TEE | >0.999 | 0.079 | 0.102 | ||||||
| pos. | 175 | 14 | 25 | 164 | 49 | 140 | |||
| neg. | 25 | 1 | 7 | 19 | 11 | 15 | |||
| Abscess | 0.013 | 0.665 | >0.999 | ||||||
| pos. | 57 | 0 | 7 | 50 | 16 | 40 | |||
| neg. | 143 | 15 | 25 | 133 | 44 | 115 | |||
| PET/CT | 0.176 | 0.440 | >0.999 | ||||||
| pos. | 17 | 0 | 4 | 13 | 5 | 12 | |||
| neg. | 39 | 6 | 6 | 39 | 13 | 32 | |||
| Blood culture | 0.006 | 0.269 | 0.503 | ||||||
| pos. | 178 | 9 | 30 | 157 | 54 | 133 | |||
| neg. | 22 | 6 | 2 | 26 | 6 | 22 | |||
| Embolism | 0.016 | 0.001 | <0.001 | ||||||
| pos. | 145 | 6 | 30 | 121 | 54 | 97 | |||
| neg. | 55 | 9 | 2 | 62 | 6 | 58 | |||
| Histopathology | <0.001 | 0.121 | 0.759 | ||||||
| pos. | 94 | 0 | 14 | 81 | 22 | 72 | |||
| neg. | 4 | 14 | 0 | 18 | 3 | 15 | |||
| Valve culture | 0.129 | 0.613 | 0.420 | ||||||
| pos. | 37 | 0 | 7 | 30 | 13 | 24 | |||
| neg. | 137 | 11 | 22 | 126 | 41 | 107 | |||
| Valve PCR | 0.001 | 0.070 | 0.161 | ||||||
| pos. | 103 | 1 | 19 | 85 | 33 | 71 | |||
| neg. | 57 | 9 | 5 | 61 | 10 | 14 | 52 | ||
| Simple logistic regression analysis on outcome IE. | |||||||
| Parameter | Cut-off | Sens. | Spec. | 80% Sens. | 80% Spec. | AUC | pValue |
| WBC (1/nL) | 8.75 | 0.67 | 0.73 | 7.34 | 11.55 | 0.680 | 0.024 |
| CRP (mg/L) | 66.10 | 0.57 | 0.73 | 28.72 | 93.67 | 0.589 | 0.272 |
| PCT (ng/mL) | 0.50 | 0.60 | 0.64 | 0.41 | 1.22 | 0.681 | 0.057 |
| Vegetation size (mm) | 11.50 | 0.69 | 0.86 | 9.49 | 11.50 | 0.800 | <0.001 |
| log(NT-proBNP) | 3.37 | 0.68 | 0.83 | 3.06 | 3.37 | 0.691 | 0.114 |
| Multivariate logistic regression analysis on outcome IE. | |||||||
| Parameter | OR (95%-CI) | pValue | C Index | ||||
| WBC (1/nL) | 1.37 (0.98, 2.16) | 0.110 | |||||
| CRP (mg/L) | 1.00 (0.98, 1.03) | 0.707 | 0.909 | ||||
| Vegetation size (mm) | 1.58 (1.12, 2.64) | 0.037 | |||||
| log(NT-proBNP) | 1.91 (0.41–12.83) | 0.449 | |||||
| Simple logistic regression analysis on outcome 30-day mortality. | |||||||
| Parameter | Cut-off | Sens. | Spec. | 80% Sens. | 80% Spec. | AUC | pValue |
| WBC (1/nL) | 11.27 | 0.50 | 0.62 | 7.71 | 14.85 | 0.543 | 0.430 |
| CRP (mg/L) | 76.60 | 0.85 | 0.58 | 77.66 | 138.69 | 0.719 | <0.001 |
| PCT (ng/mL) | 1.63 | 0.54 | 0.74 | 0.21 | 2.43 | 0.624 | 0.046 |
| Vegetation size (mm) | 10.53 | 0.83 | 0.28 | 10.53 | 19.50 | 0.553 | 0.413 |
| log(NT-proBNP) | 3.61 | 0.88 | 0.52 | 3.61 | 4.08 | 0.750 | <0.001 |
| Simple logistic regression analysis on outcome 1-year mortality. | |||||||
| Parameter | Cut-off | Sen.s | Spec. | 80% Sens. | 80% Spec. | AUC | pValue |
| WBC (1/nL) | 12.51 | 0.42 | 0.72 | 7.75 | 14.89 | 0.547 | 0.278 |
| CRP (mg/L) | 59.78 | 0.77 | 0.55 | 49.76 | 142.91 | 0.620 | 0.006 |
| PCT (ng/mL) | 1.21 | 0.72 | 0.49 | 0.81 | 2.03 | 0.627 | 0.012 |
| Vegetation size (mm) | 16.50 | 0.44 | 0.67 | 8.51 | 19.50 | 0.552 | 0.296 |
| log(NT-proBNP) | 3.37 | 0.92 | 0.45 | 3.60 | 4.04 | 0.745 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ruegamer, T.; Brochhausen, C.; Menhart, K.; Hiergeist, A.; Kraemer, L.; Hellwig, D.; Maier, L.S.; Schmid, C.; Jantsch, J.; et al. Infective Endocarditis: Predictive Factors for Diagnosis and Mortality in Surgically Treated Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 467. https://doi.org/10.3390/jcdd9120467
Li J, Ruegamer T, Brochhausen C, Menhart K, Hiergeist A, Kraemer L, Hellwig D, Maier LS, Schmid C, Jantsch J, et al. Infective Endocarditis: Predictive Factors for Diagnosis and Mortality in Surgically Treated Patients. Journal of Cardiovascular Development and Disease. 2022; 9(12):467. https://doi.org/10.3390/jcdd9120467
Chicago/Turabian StyleLi, Jing, Tamara Ruegamer, Christoph Brochhausen, Karin Menhart, Andreas Hiergeist, Lukas Kraemer, Dirk Hellwig, Lars S. Maier, Christof Schmid, Jonathan Jantsch, and et al. 2022. "Infective Endocarditis: Predictive Factors for Diagnosis and Mortality in Surgically Treated Patients" Journal of Cardiovascular Development and Disease 9, no. 12: 467. https://doi.org/10.3390/jcdd9120467
APA StyleLi, J., Ruegamer, T., Brochhausen, C., Menhart, K., Hiergeist, A., Kraemer, L., Hellwig, D., Maier, L. S., Schmid, C., Jantsch, J., & Schach, C. (2022). Infective Endocarditis: Predictive Factors for Diagnosis and Mortality in Surgically Treated Patients. Journal of Cardiovascular Development and Disease, 9(12), 467. https://doi.org/10.3390/jcdd9120467







