From Mice to Mainframes: Experimental Models for Investigation of the Intracardiac Nervous System
Abstract
1. Introduction
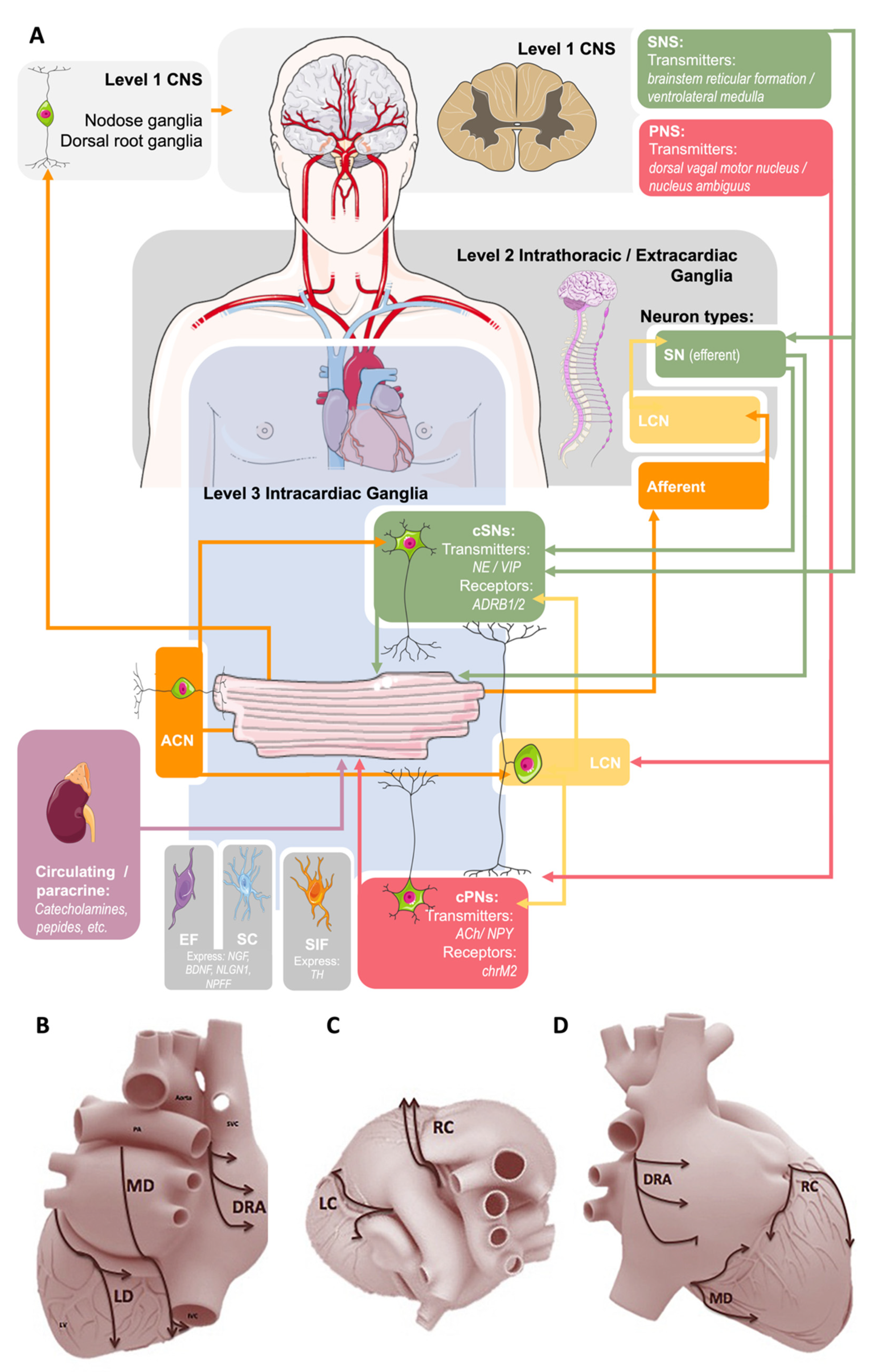
1.1. Extrinsic Cardiac Regulation—The CNS and Intrathoracic Nervous System
1.2. Intrinsic Cardiac Regulation—The IcNS
1.2.1. Intracardiac Neurons
1.2.2. Intracardiac Schwann Cells (SC)
1.2.3. Small Intensely Fluorescent (SIF) Cells
1.2.4. Intracardiac Endoneurial Fibroblasts (EF)
1.3. Therapeutic Potential of IcNS Targets
2. Mammalian Models of the IcNS
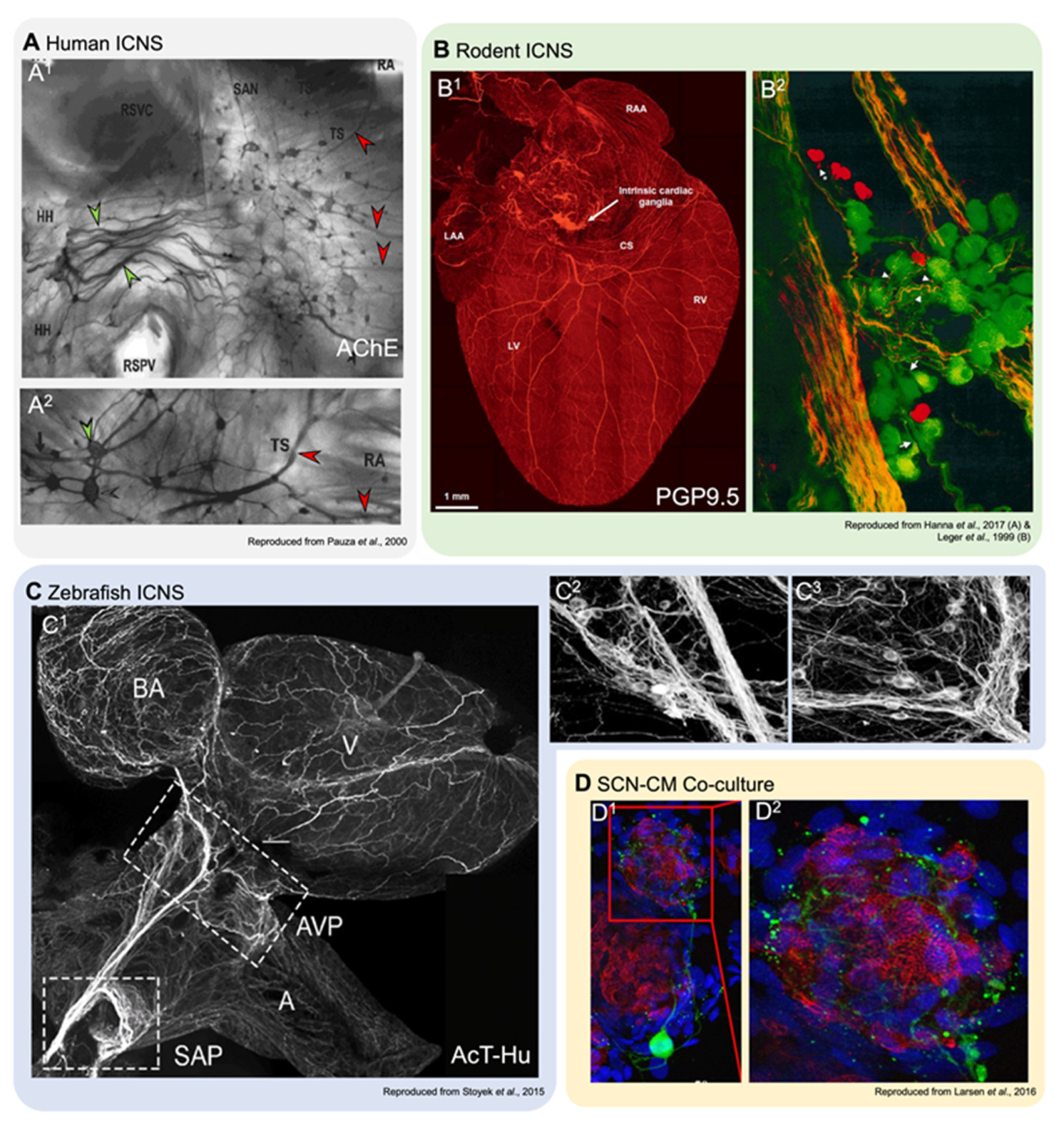
2.1. Anatomy of the IcNS
2.2. Function of the IcNS
2.3. Considerations for Investigations Using Mammalian Models
3. Zebrafish for Investigations of the IcNS
3.1. Zebrafish IcNS Anatomy
3.2. Zebrafish IcNS Function
3.3. Considerations for IcNS Investigations in Zebrafish
4. Cell Culture and Computational Modelling Studies
4.1. Neuron-CM Co-Cultures to Study the IcNS
4.2. In Silico Investigations of IcNS Function
4.3. Considerations for Co-Culture and Computational IcNS Models
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scarpa, A. Tabulae neurologicae: Ad illustrandam historiam anatomicam: Cardiacorum nervorum, noni nervorum cerebri, glossopharyngaei, et pharyngaei ex octavo cerebri. Apud Balthassarem Comini. 1794. [Google Scholar] [CrossRef]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement; D. Appleton & Company: New York, NY, USA, 1915; p. 92. [Google Scholar] [CrossRef]
- Langley, J.N. The Autonomic Nervous System Part I; W Heffer and Sons Ltd.: Cambridge, UK, 1921; p. 80. [Google Scholar]
- Nilsson, S. Autonomic Nerve Function in the Vertebrates; Springer: Berlin/Heidelberg, Germany, 1983; Volume 13, p. 253. [Google Scholar]
- Nilsson, S. Comparative anatomy of the autonomic nervous system. Auton. Neurosci. Basic Clin. 2011, 165, 3–9. [Google Scholar] [CrossRef]
- Donald, J. Autonomic nervous system. In The Physiology of Fishes, 3rd ed; Evans, D.H., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 407–439. [Google Scholar]
- Jänig, W. Integrative Action of the Autonomic Nervous System; Cambridge University Press: Cambridge, UK, 2006; p. 610. [Google Scholar]
- Armour, J.A. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp. Physiol. 2008, 93, 165–176. [Google Scholar] [CrossRef]
- Lakkireddy, D. Cardiac neuroanatomy for the cardiac electrophysiologist. J. Atr. Fibrillation 2020, 13, 29–34. [Google Scholar] [CrossRef]
- Fedele, L.; Brand, T. The intrinsic cardiac nervous system and its role in cardiac pacemaking and conduction. J. Cardiovasc. Dev. Dis. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Kember, G.; Armour, J.; Zamir, M. Neural control of heart rate: The role of neuronal networking. J. Theor. Biol. 2011, 277, 41–47. [Google Scholar] [CrossRef]
- Leger, J.; Croll, R.P.; Smith, F.M. Regional distribution and extrinsic innervation of intrinsic cardiac neurons in the guinea pig. J. Comp. Neurol. 1999, 407, 303–317. [Google Scholar] [CrossRef]
- Steele, P.A.; Choate, J.K. Innervation of the pacemaker in guinea-pig sinoatrial node. J. Auton. Nerv. Syst. 1994, 47, 177–187. [Google Scholar] [CrossRef]
- Yuan, B.-X.; Ardell, J.L.; Hopkins, D.A.; Losier, A.M.; Armour, J.A. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1994, 239, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Pauza, D.H.; Skripka, V.; Pauziene, N.; Stropus, R. Morphology, distribution, and variability of the epicardiac neural gangli-onated subplexuses in the human heart. Anat. Rec. 2000, 259, 353–382. [Google Scholar] [CrossRef]
- Rajendran, P.S.; Nakamura, K.; Ajijola, O.; Vaseghi, M.; Armour, J.A.; Ardell, J.L.; Shivkumar, K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J. Physiol. 2015, 594, 321–341. [Google Scholar] [CrossRef]
- Pauziene, N.; Rysevaite-Kyguoliene, K.; Alaburda, P.; Pauza, A.G.; Skukauskaite, M.; Masaityte, A.; Laucaityte, G.; Saburkina, I.; Inokaitis, H.; Plisiene, J.; et al. Neuroanatomy of the pig cardiac ventricles. a stereomicroscopic, confocal and electron microscope study. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2017, 300, 1756–1780. [Google Scholar] [CrossRef]
- Pauziene, N.; Pauza, D.H.; Stropus, R. Morphology of human intracardiac nerves: An electron microscope study. J. Anat. 2000, 197, 437–459. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Stoyek, M.R.; Rose, R.; Quinn, T.A. Intrinsic regulation of sinoatrial node function and the zebrafish as a model of stretch effects on pacemaking. Prog. Biophys. Mol. Biol. 2017, 130, 198–211. [Google Scholar] [CrossRef]
- Macdonald, E.A.; Rose, R.A.; Quinn, T.A. Neurohumoral control of sinoatrial node activity and heart rate: Insight from experimental models and findings from humans. Front. Physiol. 2020, 11, 170. [Google Scholar] [CrossRef]
- Campos, I.D.; Pinto, V.; Sousa, N.; Pereira, V.H. A brain within the heart: A review on the intracardiac nervous system. J. Mol. Cell. Cariol. 2018, 119, 1–9. [Google Scholar] [CrossRef]
- Hopkins, D.A.; Armour, J.A. Localization of sympathetic postganglionic and parasympathetic preganglionic neurons which innervate different regions of the dog heart. J. Comp. Neurol. 1984, 229, 186–198. [Google Scholar] [CrossRef]
- Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Brain Struct. Funct. 2005, 209, 425–438. [Google Scholar] [CrossRef]
- Smith, F.M.; McGuirt, A.S.; Leger, J.; Armour, J.A.; Ardell, J.L. Effects of chronic cardiac decentralization on functional properties of canine intracardiac neurons in vitro. Am. J. Physiol. Integr. Comp. Physiol. 2001, 281, R1474–R1482. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Quinn, T.A.; Croll, R.P.; Smith, F.M. Zebrafish heart as a model to study the integrative autonomic control of pacemaker function. Am. J. Physiol. Hearth Circ. Physiol. 2016, 311, H676–H688. [Google Scholar] [CrossRef]
- Ardell, J.L.; Butler, C.K.; Smith, F.M.; Hopkins, D.A.; Armour, J.A. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am. J. Physiol. Circ. Physiol. 1991, 260, H713–H721. [Google Scholar] [CrossRef]
- Hildreth, V.; Webb, S.; Bradshaw, L.; Brown, N.A.; Anderson, R.H.; Henderson, D.J. Cells migrating from the neural crest contribute to the innervation of the venous pole of the heart. J. Anat. 2007, 212, 1–11. [Google Scholar] [CrossRef]
- Végh, A.M.D.; Duim, S.N.; Smits, A.M.; Poelmann, R.E.; Harkel, A.D.J.T.; DeRuiter, M.C.; Goumans, M.J.; Jongbloed, M.R.M. Part and parcel of the cardiac autonomic nerve system: Unravelling its cellular building blocks during development. J. Cardiovasc. Dev. Dis. 2016, 3, 28. [Google Scholar] [CrossRef]
- Nam, J.; Onitsuka, I.; Hatch, J.; Uchida, Y.; Ray, S.; Huang, S.; Li, W.; Zang, H.; Ruiz-Lozano, P.; Mukouyama, Y.-S. Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development 2013, 140, 1475–1485. [Google Scholar] [CrossRef]
- Hortells, L.; Meyer, E.C.; Thomas, Z.M.; Yutzey, K.E. Periostin-expressing Schwann cells and endoneurial cardiac fibroblasts contribute to sympathetic nerve fasciculation after birth. J. Mol. Cell. Cardiol. 2021, 154, 124–136. [Google Scholar] [CrossRef]
- Fregoso, S.; Hoover, D. Development of cardiac parasympathetic neurons, glial cells, and regional cholinergic innervation of the mouse heart. Neuroscience 2012, 221, 28–36. [Google Scholar] [CrossRef]
- Habecker, B.A.; Anderson, M.E.; Birren, S.J.; Fukuda, K.; Herring, N.; Hoover, D.B.; Kanazawa, H.; Paterson, D.J.; Ripplinger, C. Molecular and cellular neurocardiology: Development, and cellular and molecular adaptations to heart disease. J. Physiol. 2016, 594, 3853–3875. [Google Scholar] [CrossRef]
- Armour, J.A.; Murphy, D.A.; Yuan, B.-X.; MacDonald, S.; Hopkins, D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1997, 247, 289–298. [Google Scholar] [CrossRef]
- Wake, E.; Brack, K. Characterization of the intrinsic cardiac nervous system. Auton. Neurosci. 2016, 199, 3–16. [Google Scholar] [CrossRef]
- Ardell, J.L.; Armour, J.A. Neurocardiology: Structure-based function. Compr. Physiol. 2016, 6, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.M. Extrinsic inputs to intrinsic neurons in the porcine heart in vitro. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R455–R467. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Randall, W.C.; Bieger, D.; Wurster, R.D.; Hopkins, D.A.; Armour, J.A. Activity of in vivo canine cardiac plexus neurons. Am. J. Physiol. Circ. Physiol. 1988, 255, H789–H800. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Terui, N.; Kollai, M. Neural control of the heart: Significance of double innervation re-examined. J. Auton. Nerv. Syst. 1983, 7, 279–294. [Google Scholar] [CrossRef]
- Koizumi, K.; Terui, N.; Kollai, M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. J. Auton. Nerv. Syst. 1985, 12, 251–259. [Google Scholar] [CrossRef]
- Kollai, M.; Koizumi, K. Reciprocal and non-reciprocal action of the vagal and sympathetic nerves innervating the heart. J. Auton. Nerv. Syst. 1979, 1, 33–52. [Google Scholar] [CrossRef]
- Salzer, J.L. Schwann Cell Myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef]
- Harty, B.L.; Monk, K.R. Unwrapping the unappreciated: Recent progress in Remak Schwann cell biology. Curr. Opin. Neurobiol. 2017, 47, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.A.; Squiers, G.T.; McLellan, M.A.; Bolisetty, M.T.; Robson, P.; Rosenthal, N.A.; Pinto, A.R. Single-Cell Transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep. 2018, 22, 600–610. [Google Scholar] [CrossRef]
- Slavíková, J.; Kuncová, J.; Reischig, J.; Dvoráková, M. Catecholaminergic neurons in the rat intrinsic cardiac nervous system. Neurochem. Res. 2003, 28, 593–598. [Google Scholar] [CrossRef]
- White, S.K.; Frohlich, G.M.; Sado, D.M.; Maestrini, V.; Fontana, M.; Treibel, T.; Tehrani, S.; Flett, A.S.; Meier, P.; Ariti, C.; et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2015, 8, 178–188. [Google Scholar] [CrossRef]
- Zhu, C.; Hanna, P.; Rajendran, P.S.; Shivkumar, K. Neuromodulation for ventricular tachycardia and atrial fibrillation. JACC Clin. Electrophysiol. 2019, 5, 881–896. [Google Scholar] [CrossRef]
- Bardsley, E.N.; Paterson, D.J. Neurocardiac regulation: From cardiac mechanisms to novel therapeutic approaches. J. Physiol. 2020, 598, 2957–2976. [Google Scholar] [CrossRef]
- Shivkumar, K.; Ajijola, O.; Anand, I.; Armour, J.A.; Chen, P.-S.; Esler, M.; De Ferrari, G.M.; Fishbein, M.C.; Goldberger, J.J.; Harper, R.M.; et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J. Physiol. 2016, 594, 3911–3954. [Google Scholar] [CrossRef]
- Hanna, P.; Rajendran, P.S.; Ajijola, O.; Vaseghi, M.; Armour, J.A.; Ardell, J.L.; Shivkumar, K. Cardiac neuroanatomy—imaging nerves to define functional control. Auton. Neurosci. 2017, 207, 48–58. [Google Scholar] [CrossRef]
- Larsen, H.E.; Lefkimmiatis, K.; Paterson, D.J. Sympathetic neurons are a powerful driver of myocyte function in cardiovascular disease. Sci. Rep. 2016, 6, 38898. [Google Scholar] [CrossRef]
- Taggart, P.; Critchley, H.; van Duijvendoden, S.; Lambiase, P.D. Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Auton. Neurosci. 2016, 199, 54–65. [Google Scholar] [CrossRef]
- Ajijola, O.A.; Wisco, J.J.; Lambert, H.W.; Mahajan, A.; Stark, E.; Fishbein, M.C.; Shivkumar, K. Extracardiac neural remodeling in humans with cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2012, 5, 1010–1116. [Google Scholar] [CrossRef]
- Hanna, P.; Shivkumar, K.; Ardell, J.L. Calming the nervous heart: Autonomic therapies in heart failure. Card. Fail. Rev. 2018, 4, 92–98. [Google Scholar] [CrossRef]
- Das, S.; Gordián-Vélez, W.J.; Ledebur, H.C.; Mourkioti, F.; Rompolas, P.; Chen, H.I.; Serruya, M.D.; Cullen, D.K. Innervation: The missing link for biofabricated tissues and organs. NPJ Regen. Med. 2020, 5, 1–19. [Google Scholar] [CrossRef]
- Awad, M.; Czer, L.S.C.; Hou, M.; Golshani, S.S.; Goltche, M.; De Robertis, M.; Kittleson, M.; Patel, J.; Azarbal, B.; Kransdorf, E.; et al. Early denervation and later reinnervation of the heart following cardiac transplantation: A review. J. Am. Heart Assoc. 2016, 5, e004070. [Google Scholar] [CrossRef]
- Stavrakis, S.; Kulkarni, K.; Singh, J.P.; Katritsis, D.G.; Armoundas, A.A. Autonomic modulation of cardiac arrhythmias. JACC Clin. Electrophysiol. 2020, 6, 467–483. [Google Scholar] [CrossRef]
- Saburkina, I.; Gukauskiene, L.; Rysevaite, K.; Brack, K.E.; Pauza, A.G.; Pauziene, N.; Pauza, D.H. Morphological pattern of intrinsic nerve plexus distributed on the rabbit heart and interatrial septum. J. Anat. 2014, 224, 583–593. [Google Scholar] [CrossRef]
- Saburkina, I.; Rysevaite, K.; Pauziene, N.; Mischke, K.; Schauerte, P.; Jalife, J.; Pauza, D.H. Epicardial neural ganglionated plexus of ovine heart: Anatomic basis for experimental cardiac electrophysiology and nerve protective cardiac surgery. Heart Rhythm. 2010, 7, 942–950. [Google Scholar] [CrossRef]
- Pauziene, N.; Alaburda, P.; Rysevaite-Kyguoliene, K.; Pauza, A.G.; Inokaitis, H.; Masaityte, A.; Rudokaite, G.; Saburkina, I.; Plisiene, J.; Pauza, D.H. Innervation of the rabbit cardiac ventricles. J. Anat. 2015, 228, 26–46. [Google Scholar] [CrossRef]
- Furnival, C.M.; Linden, R.J.; Snow, H.M. The inotropic effect on the heart of stimulating the vagus in the dog, duck and toad. J. Physiol. 1973, 230, 155–170. [Google Scholar] [CrossRef]
- Pauza, D.H.; Saburkina, I.; Rysevaite, K.; Inokaitis, H.; Jokubauskas, M.; Jalife, J.; Pauziene, N. Neuroanatomy of the murine cardiac conduction system: A combined stereomicroscopic and fluorescence immunohistochemical study. Auton. Neurosci. 2013, 176, 32–47. [Google Scholar] [CrossRef]
- Rysevaite, K.; Saburkina, I.; Pauziene, N.; Vaitkevicius, R.; Noujaim, S.F.; Jalife, J.; Pauza, D.H. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Hearth Rhythm. 2011, 8, 731–738. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Croll, R.P.; Smith, F.M. Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J. Comp. Neurol. 2015, 523, 1683–1700. [Google Scholar] [CrossRef]
- Lazzara, R.; Scherlag, B.J.; Robinson, M.J.; Samet, P. Selective in situ parasympathetic control of the canine sinoatrial and atrioventricular nodes. Circ. Res. 1973, 32, 393–401. [Google Scholar] [CrossRef]
- Ardell, J.L.; Randall, W.C. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am. J. Physiol. Circ. Physiol. 1986, 251, H764–H773. [Google Scholar] [CrossRef]
- Hou, Y.; Scherlag, B.J.; Lin, J.; Zhang, Y.; Lu, Z.; Truong, K.; Patterson, E.; Lazzara, R.; Jackman, W.M.; Po, S.S. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: Effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J. Am. Coll. Cardiol. 2007, 50, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.C.; Brown, D.R.; Li, S.-G.; Olmstead, M.E.; Kilgore, J.M.; Sprinkle, A.G.; Randall, W.C.; Ardell, J.L. Ablation of posterior atrial ganglionated plexus potentiates sympathetic tachycardia to behavioral stress. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R779–R787. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Randall, W.C.; Cannon, W.J.; Schmacht, D.C.; Tasdemiroglu, E.; Taşdemiroǧlu, E. Differential sympathetic regulation of automatic, conductile, and contractile tissue in dog heart. Am. J. Physiol. Circ. Physiol. 1988, 255, H1050–H1059. [Google Scholar] [CrossRef]
- Dickerson, L.W.; Rodak, D.J.; Fleming, T.J.; Gatti, P.J.; Massari, V.; McKenzie, J.C.; Gillis, A.R. Parasympathetic neurons in the cranial medial ventricular fat pad on the dog heart selectively decrease ventricular contractility. J. Auton. Nerv. Syst. 1998, 70, 129–141. [Google Scholar] [CrossRef]
- Armour, J.A.; Collier, K.; Kember, G.; Ardell, J.L. Differential selectivity of cardiac neurons in separate intrathoracic autonomic ganglia. Am. J. Physiol. Integr. Comp. Physiol. 1998, 274, R939–R949. [Google Scholar] [CrossRef]
- Armour, J.A.; Hopkins, D.A. Activity of canine in situ left atrial ganglion neurons. Am. J. Physiol. Circ. Physiol. 1990, 259, H1207–H1215. [Google Scholar] [CrossRef]
- Armour, J.A.; Hopkins, D.A. Activity of in vivo canine ventricular neurons. Am. J. Physiol. Circ. Physiol. 1990, 258, H326–H336. [Google Scholar] [CrossRef]
- Beaumont, E.; Salavatian, S.; Southerland, E.M.; Vinet, A.; Jacquemet, V.; Armour, J.A.; Ardell, J.L. Network interactions within the canine intrinsic cardiac nervous system: Implications for reflex control of regional cardiac function. J. Physiol. 2013, 591, 4515–4533. [Google Scholar] [CrossRef]
- McAllen, R.M.; Salo, L.M.; Paton, J.F.R.; Pickering, A.E. Processing of central and reflex vagal drives by rat cardiac ganglion neurones: An intracellular analysis. J. Physiol. 2011, 589, 5801–5818. [Google Scholar] [CrossRef] [PubMed]
- Edwards, F.R.; Hirst, G.D.; Klemm, M.F.; Steele, A.P. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J. Physiol. 1995, 486, 453–471. [Google Scholar] [CrossRef]
- Davis, H.; Herring, N.; Paterson, D.J. Downregulation of M current is coupled to membrane excitability in sympathetic neurons before the onset of hypertension. Hypertension 2020, 76, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Patel, K.; Schmid, P.; Lund, D. Location, distribution and projections of intracardiac ganglion cells in the rat. J. Auton. Nerv. Syst. 1987, 20, 91–101. [Google Scholar] [CrossRef]
- Achanta, S.; Gorky, J.; Leung, C.; Moss, A.; Robbins, S.; Eisenman, L.; Chen, J.; Tappan, S.; Heal, M.; Farahani, N.; et al. A comprehensive integrated anatomical and molecular atlas of rat intrinsic cardiac nervous system. iScience 2020, 23, 101140. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Kowalik, G.; Mendelowitz, D.; Kay, M.W. Optogenetic control of cardiac autonomic neurons in transgenic mice. In Methods in Molecular Biology; Springer: New York, NY, USA, 2020; pp. 309–321. [Google Scholar]
- Moreno, A.; Endicott, K.; Skancke, M.; Dwyer, M.K.; Brennan, J.; Efimov, I.R.; Trachiotis, G.; Mendelowitz, D.; Kay, M.W. Sudden heart rate reduction upon optogenetic release of acetylcholine from cardiac parasympathetic neurons in perfused hearts. Front. Physiol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Batulevicius, D.; Skripkiene, G.; Batulevičienė, V.; Skripka, V.; Dabuzinskiene, A.; Pauza, D.H. Distribution, structure and projections of the frog intracardiac neurons. Auton. Neurosci. 2012, 168, 14–24. [Google Scholar] [CrossRef]
- Gibbins, I.L.; Morris, J.L. Structure of peripheral synapses: Autonomic ganglia. Cell Tissue Res. 2006, 326, 205–220. [Google Scholar] [CrossRef]
- Koster, R.; Sassen, W.A. A molecular toolbox for genetic manipulation of zebrafish. Adv. Genom. Genet. 2015, 5, 151–163. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nat. Cell Biol. 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Nawrocki, L.; Bremiller, R.; Streisinger, G.; Kaplan, M. Larval and adult visual pigments of the zebrafish, Brachydanio rerio. Vis. Res. 1985, 25, 1569–1576. [Google Scholar] [CrossRef]
- Dooley, K. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nat. Cell Biol. 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Brette, F.; Luxan, G.; Cros, C.; Dixey, H.; Wilson, C.; Shiels, H.A. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2008, 374, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Genge, C.E.; Lin, E.; Lee, L.; Sheng, X.; Rayani, K.; Gunawan, M.; Stevens, C.M.; Li, A.Y.; Talab, S.S.; Claydon, T.W.; et al. The zebrafish heart as a model of mammalian cardiac function. Rev. Physiol. Biochem. Pharmacol. 2016, 171, 99–136. [Google Scholar] [CrossRef]
- Liu, J.; Stainier, D.Y.R. Zebrafish in the study of early cardiac development. Circ. Res. 2012, 110, 870–874. [Google Scholar] [CrossRef]
- Nemtsas, P.; Wettwer, E.; Christ, T.; Weidinger, G.; Ravens, U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J. Mol. Cell. Cardiol. 2010, 48, 161–171. [Google Scholar] [CrossRef]
- Vornanen, M.; Hassinen, M. Zebrafish heart as a model for human cardiac electrophysiology. Channels 2016, 10, 101–110. [Google Scholar] [CrossRef]
- Brown, D.; Samsa, L.; Ito, C.; Ma, H.; Batres, K.; Arnaout, R.; Qian, L.; Liu, J. Neuregulin-1 is essential for nerve plexus formation during cardiac maturation. J. Cell. Mol. Med. 2017, 22, 2007–2017. [Google Scholar] [CrossRef]
- Steele, S.L.; Lo, K.H.A.; Li, V.W.T.; Cheng, S.H.; Ekker, M.; Perry, S.F. Loss of M2 muscarinic receptor function inhibits development of hypoxic bradycardia and alters cardiac β-adrenergic sensitivity in larval zebrafish (Danio rerio). Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R412–R420. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.L.; Yang, X.; Debiais-Thibaud, M.; Schwerte, T.; Pelster, B.; Ekker, M.; Tiberi, M.; Perry, S.F. In vivo and in vitro assessment of cardiac β-adrenergic receptors in larval zebrafish (Danio rerio). J. Exp. Biol. 2011, 214, 1445–1457. [Google Scholar] [CrossRef]
- Schwerte, T.; Prem, C.; Mairosl, A.; Pelster, B. Development of the sympatho-vagal balance in the cardiovascular system in zebrafish (Danio rerio) characterized by power spectrum and classical signal analysis. J. Exp. Biol. 2006, 209, 1093–1100. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Jonz, M.G.; Smith, F.M.; Croll, R.P. Distribution and chronotropic effects of serotonin in the zebrafish heart. Auton. Neurosci. 2017, 206, 43–50. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhang, H.; Guo, S.Z.; Wurster, R.; Gozal, D. Differential control over postganglionic neurons in rat cardiac ganglia by NA and DmnX neurons: Anatomical evidence. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R625–R633. [Google Scholar] [CrossRef]
- Shvilkin, A.; Danilo, J.P.; Chevalier, P.; Chang, F.; Cohen, I.S.; Rosen, M.R. Vagal release of vasoactive intestinal peptide can promote vagotonic tachycardia in the isolated innervated rat heart. Cardiovasc. Res. 1994, 28, 1769–1773. [Google Scholar] [CrossRef]
- Shibata, N.; Inada, S.; Mitsui, K.; Honjo, H.; Yamamoto, M.; Niwa, R.; Boyett, M.R.; Kodama, I. Pacemaker shift in the rabbit sinoatrial node in response to vagal nerve stimulation. Exp. Physiol. 2001, 86, 177–184. [Google Scholar] [CrossRef]
- Hucker, W.J.; Nikolski, V.P.; Efimov, I. Autonomic control and innervation of the atrioventricular junctional pacemaker. Heart Rhythm. 2007, 4, 1326–1335. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Rog-Zielinska, E.A.; Quinn, T.A. Age-associated changes in electrical function of the zebrafish heart. Prog. Biophys. Mol. Biol. 2018, 138, 91–104. [Google Scholar] [CrossRef]
- Hu, N.; Yost, H.J.; Clark, E.B. Cardiac morphology and blood pressure in the adult zebrafish. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2001, 264, 1–12. [Google Scholar] [CrossRef]
- Lin, E.; Ribeiro, A.; Ding, W.; Hove-Madsen, L.; Sarunic, M.V.; Beg, M.F.; Tibbits, G.F. Optical mapping of the electrical activity of isolated adult zebrafish hearts: Acute effects of temperature. Am. J. Physiol. Integr. Comp. Physiol. 2014, 306, R823–R836. [Google Scholar] [CrossRef][Green Version]
- Baillie, J.S.; Stoyek, M.R.; Quinn, T.A. Seeing the light: The use of zebrafish for optogenetic studies of the heart. Front. Physiol. 2021, in press. [Google Scholar]
- Rafferty, S.A.; Quinn, T.A. A beginner’s guide to understanding and implementing the genetic modification of zebrafish. Prog. Biophys. Mol. Biol. 2018, 138, 3–19. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Schmidt, M.K.; Wilfart, F.M.; Croll, R.P.; Smith, F.M. The in vitro zebrafish heart as a model to investigate the chronotropic effects of vapor anesthetics. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R669–R679. [Google Scholar] [CrossRef]
- Furshpan, E.J.; MacLeish, P.R.; O’Lague, P.H.; Potter, D.D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: Evidence for cholinergic, adrenergic, and dual-function neurons. Proc. Natl. Acad. Sci. USA 1976, 73, 4225–4229. [Google Scholar] [CrossRef]
- Horackova, M.; Huang, M.H.; Armour, A.J.; Hopkins, A.D.; Mapplebeck, C. Cocultures of adult ventricular myocytes with stellate ganglia or intrinsic cardiac neurones from guinea pigs: Spontaneous activity and pharmacological properties. Cardiovasc. Res. 1993, 27, 1101–1108. [Google Scholar] [CrossRef]
- Burton, R.-A.B.; Tomek, J.; Ambrosi, C.M.; Larsen, H.E.; Sharkey, A.R.; Capel, R.; Corbett, A.D.; Bilton, S.; Klimas, A.; Stephens, G.; et al. Optical interrogation of sympathetic neuronal effects on macroscopic cardiomyocyte network dynamics. iScience 2020, 23, 101334. [Google Scholar] [CrossRef]
- Entcheva, E.; Bien, H. Macroscopic optical mapping of excitation in cardiac cell networks with ultra-high spatiotemporal resolution. Prog. Biophys. Mol. Biol. 2006, 92, 232–257. [Google Scholar] [CrossRef]
- Winbo, A.; Ramanan, S.; Eugster, E.; Jovinge, S.; Skinner, J.R.; Montgomery, J.M. Functional coculture of sympathetic neurons and cardiomyocytes derived from human-induced pluripotent stem cells. Am. J. Physiol. Circ. Physiol. 2020, 319, H927–H937. [Google Scholar] [CrossRef]
- Kember, G.; Armour, J.A.; Zamir, M. Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol. Genom. 2013, 45, 638–644. [Google Scholar] [CrossRef]
- Kember, G.; Armour, J.; Zamir, M. Dynamic neural networking as a basis for plasticity in the control of heart rate. J. Theor. Biol. 2013, 317, 39–46. [Google Scholar] [CrossRef]
- Kember, G.; Ardell, J.L.; Shivkumar, K.; Armour, J.A. Recurrent myocardial infarction: Mechanisms of free-floating adaptation and autonomic derangement in networked cardiac neural control. PLoS ONE 2017, 12, e0180194. [Google Scholar] [CrossRef]
- Quinn, T.A.; Kohl, P. Systems biology of the heart: Hype or hope? Ann. N. Y. Acad. Sci. 2011, 1245, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.A.; Kohl, P. Combining wet and dry research: Experience with model development for cardiac mechano-electric structure-function studies. Cardiovasc. Res. 2013, 97, 601–611. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyek, M.R.; Hortells, L.; Quinn, T.A. From Mice to Mainframes: Experimental Models for Investigation of the Intracardiac Nervous System. J. Cardiovasc. Dev. Dis. 2021, 8, 149. https://doi.org/10.3390/jcdd8110149
Stoyek MR, Hortells L, Quinn TA. From Mice to Mainframes: Experimental Models for Investigation of the Intracardiac Nervous System. Journal of Cardiovascular Development and Disease. 2021; 8(11):149. https://doi.org/10.3390/jcdd8110149
Chicago/Turabian StyleStoyek, Matthew R., Luis Hortells, and T. Alexander Quinn. 2021. "From Mice to Mainframes: Experimental Models for Investigation of the Intracardiac Nervous System" Journal of Cardiovascular Development and Disease 8, no. 11: 149. https://doi.org/10.3390/jcdd8110149
APA StyleStoyek, M. R., Hortells, L., & Quinn, T. A. (2021). From Mice to Mainframes: Experimental Models for Investigation of the Intracardiac Nervous System. Journal of Cardiovascular Development and Disease, 8(11), 149. https://doi.org/10.3390/jcdd8110149





