New Insights into the Development and Morphogenesis of the Cardiac Purkinje Fiber Network: Linking Architecture and Function
Abstract
1. Introduction
2. Developmental Origin of the Purkinje Fiber Network
2.1. Clonal and Genetic Tracing Analyses Reveal the Myogenic Origin of the VCS
2.2. A model of VCS Morphogenesis Based on the Expression of Conductive Markers
2.3. Identification of Conductive Progenitors and Early Commitment of Purkinje Fibers
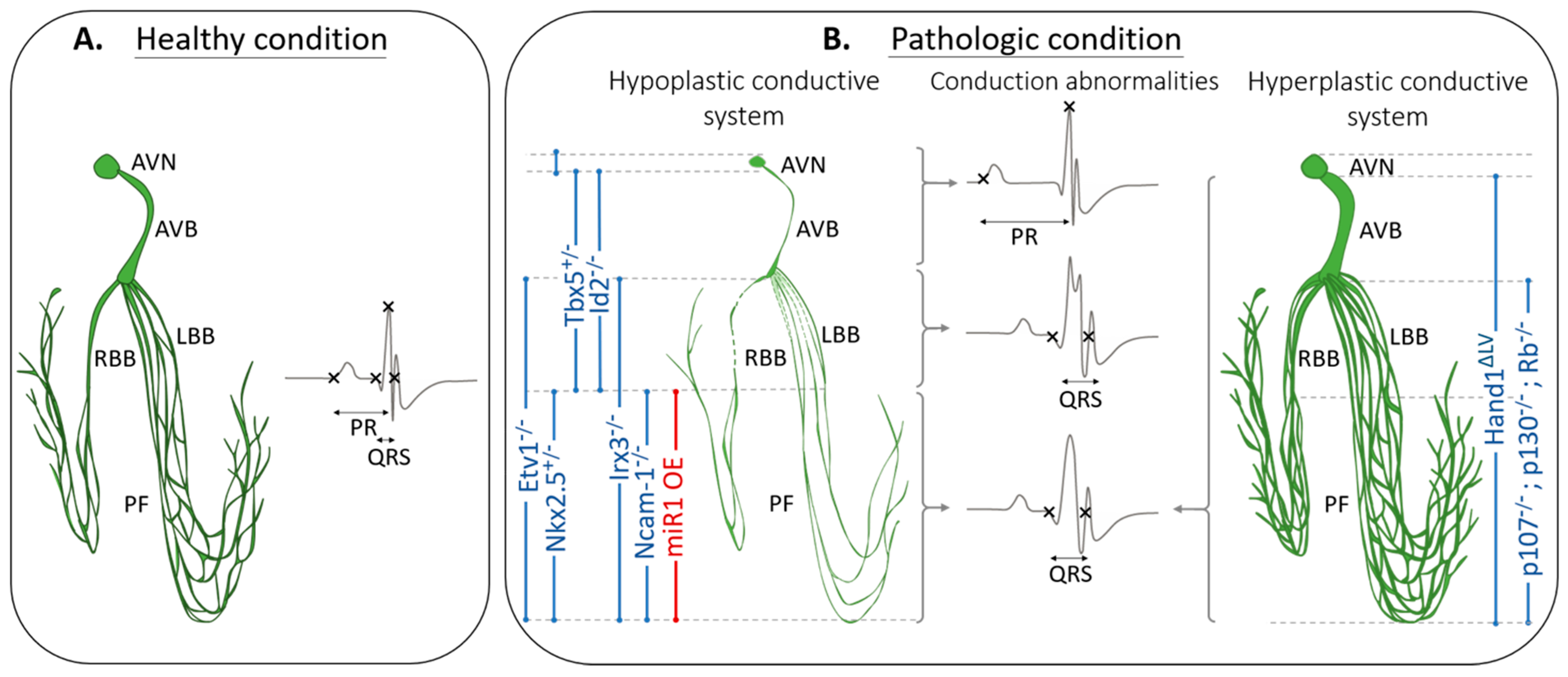
3. Defective VCS Morphogenesis Causes Conduction Defects in Mouse Models
3.1. Structure–Function Relationship between VCS Architecture and Cardiac Conduction
3.2. Mouse Models with a Hypoplastic VCS
3.3. Mouse Models with Hyperplastic VCS
4. A Two-Step Model of PF Network Morphogenesis Involving Scaffold and Recruitment Phases
4.1. The PF Network Grows by Two Phases of Recruitment
4.2. Cell-Autonomous Specification and Recruitment of the PF Network
4.3. PF Network Complexity Is Linked to Ventricular Trabeculae Compaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silverman, M.E.; Grove, D.; Upshaw, C.B., Jr. Why does the heart beat? The discovery of the electrical system of the heart. Circulation 2006, 113, 2775–2781. [Google Scholar] [CrossRef]
- Mazurak, M.; Kusa, J. Jan Evangelista Purkinje: A Passion for Discovery. Tex. Heart Inst. J. 2018, 45, 23–26. [Google Scholar] [CrossRef]
- Dobrzynski, H.; Anderson, R.H.; Atkinson, A.; Borbas, Z.; D’Souza, A.; Fraser, J.F.; Inada, S.; Logantha, S.J.; Monfredi, O.; Morris, G.; et al. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol. Ther. 2013, 139, 260–288. [Google Scholar] [CrossRef]
- Boyden, P.A. Purkinje physiology and pathophysiology. J. Interv. Card. Electrophysiol. 2018, 52, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T. Sunao Tawara: Discoverer of the atrioventricular conduction system of the heart. Cardiol. J. 2010, 17, 428–434. [Google Scholar]
- Boyett, M.R. ‘And the beat goes on’ The cardiac conduction system: The wiring system of the heart. Exp. Physiol. 2009, 94, 1035–1049. [Google Scholar] [CrossRef]
- Miquerol, L.; Beyer, S.; Kelly, R.G. Establishment of the mouse ventricular conduction system. Cardiovasc. Res. 2011, 91, 232–242. [Google Scholar] [CrossRef]
- Gros, D.B.; Jongsma, H.J. Connexins in mammalian heart function. BioEssays 1996, 18, 719–730. [Google Scholar] [CrossRef]
- Miquerol, L.; Moreno-Rascon, N.; Beyer, S.; Dupays, L.; Meilhac, S.; Buckingham, M.E.; Franco, D.; Kelly, R. Biphasic Development of the Mammalian Ventricular Conduction System. Circ. Res. 2010, 107, 153–161. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Vigmond, E.; Stuyvers, B.; Hocini, M.; Bernus, O. Ventricular arrhythmias and the His–Purkinje system. Nat. Rev. Cardiol. 2016, 13, 155–166. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Hocini, M.; Cheniti, G.; Duchateau, J.; Sacher, F.; Puyo, S.; Cochet, H.; Takigawa, M.; Denis, A.; Martin, R.; et al. Localized Structural Alterations Underlying a Subset of Unexplained Sudden Cardiac Death. Circ. Arrhythmia Electrophysiol. 2018, 11, e006120. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.K.; Boyden, P.A.; Scheinman, M. Cellular Physiology and Clinical Manifestations of Fascicular Arrhythmias in Normal Hearts. JACC Clin. Electrophysiol. 2017, 3, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Meysen, S.; Mangoni, M.; Bois, P.; van Rijen, H.; Abran, P.; Jongsma, H.; Nargeot, J.; Gros, D. Architectural and functional asymmetry of the His–Purkinje system of the murine heart. Cardiovasc. Res. 2004, 63, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Yamaguchi, T.; Ishikawa, H.; Arakawa, M.; Takahashi, N.; Saikawa, T.; Shimada, T. Morphological varieties of the Purkinje fiber network in mammalian hearts, as revealed by light and electron microscopy. Arch. Histol. Cytol. 2009, 72, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Aumentado-Armstrong, T.; Kadivar, A.; Savadjiev, P.; Zucker, S.W.; Siddiqi, K. Conduction in the Heart Wall: Helicoidal Fibers Minimize Diffusion Bias. Sci. Rep. 2018, 8, 7165. [Google Scholar] [CrossRef]
- Liu, B.; Cherry, E.M. Image-Based Structural Modeling of the Cardiac Purkinje Network. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Tusscher, K.T.; Panfilov, A. Modelling of the ventricular conduction system. Prog. Biophys. Mol. Biol. 2008, 96, 152–170. [Google Scholar] [CrossRef]
- Stephenson, R.S.; Boyett, M.R.; Hart, G.; Nikolaidou, T.; Cai, X.; Corno, A.F.; Alphonso, N.; Jeffery, N.; Jarvis, J.C. Contrast enhanced micro-computed tomography resolves the 3-dimensional morphology of the cardiac conduction system in mammalian hearts. PLoS ONE 2012, 7, e35299. [Google Scholar] [CrossRef]
- Yao, X.; Gan, Y.; Marboe, C.C.; Hendon, C.P. Myocardial imaging using ultrahigh-resolution spectral domain optical coherence tomography. J. Biomed. Opt. 2016, 21, 061006. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.; Bueno-Orovio, A.; Zacur, E.; Ariga, R.; Grau, V.; Neubauer, S.; Watkins, H.; Rodriguez, B.; Mincholé, A. Electrocardiogram phenotypes in hypertrophic cardiomyopathy caused by distinct mechanisms: Apico-basal repolarization gradients vs. Purkinje-myocardial coupling abnormalities. Europace 2018, 20, iii102–iii112. [Google Scholar] [CrossRef]
- Jensen, B.; Boukens, B.J.; Postma, A.; Gunst, Q.D.; van den Hoff, M.J.; Moorman, A.F.M.; Wang, T.; Christoffels, V.M. Identifying the Evolutionary Building Blocks of the Cardiac Conduction System. PLoS ONE 2012, 7, e44231. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.-Y.; Cha, M.-J.; Lee, T.-H.; Seo, J.-W.; Oh, S. Unsolved Questions on the Anatomy of the Ventricular Conduction System. Korean Circ. J. 2018, 48, 1081–1096. [Google Scholar] [CrossRef]
- Kondo, R.P.; Anderson, R.H.; Kupershmidt, S.; Roden, D.M.; Evans, S.M. Development of the cardiac conduction system as delineated by mink-lacz. J. Cardiovasc. Electrophysiol. 2003, 14, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Vaidya, D.; Tamaddon, H.; Degenhardt, K.; Sassoon, D.; Morley, G.; Jalife, J.; Fishman, G. Visualization and functional characterization of the developing murine cardiac conduction system. Development 2001, 128, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Pallante, B.A.; Giovannone, S.; Fang-Yu, L.; Zhang, J.; Liu, N.; Kang, G.; Dun, W.; Boyden, P.A.; Fishman, G.I. Contactin-2 Expression in the Cardiac Purkinje Fiber Network. Circ. Arrhythmia Electrophysiol. 2010, 3, 186–194. [Google Scholar] [CrossRef]
- Romero, D.; Camara, O.; Sachse, F.; Sebastian, R. Analysis of Microstructure of the Cardiac Conduction System Based on Three-Dimensional Confocal Microscopy. PLoS ONE 2016, 11, e0164093. [Google Scholar] [CrossRef]
- Gurjarpadhye, A.; Hewett, K.W.; Justus, C.; Wen, X.; Stadt, H.; Kirby, M.L.; Sedmera, D.; Gourdie, R.G. Cardiac neural crest ablation inhibits compaction and electrical function of conduction system bundles. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1291–H1300. [Google Scholar] [CrossRef]
- Nakamura, T.; Colbert, M.C.; Robbins, J. Neural Crest Cells Retain Multipotential Characteristics in the Developing Valves and Label the Cardiac Conduction System. Circ. Res. 2006, 98, 1547–1554. [Google Scholar] [CrossRef]
- Cheng, G.; Litchenberg, W.; Cole, G.; Mikawa, T.; Thompson, R.; Gourdie, R. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development 1999, 126, 5041–5049. [Google Scholar] [CrossRef]
- Miquerol, L.; Bellon, A.; Moreno, N.; Beyer, S.; Meilhac, S.; Buckingham, M.; Franco, D.; Kelly, R.G. Resolving cell lineage contributions to the ventricular conduction system with a Cx40-GFP allele: A dual contribution of the first and second heart fields. Dev. Dyn. 2013, 242, 665–677. [Google Scholar] [CrossRef]
- Gourdie, R.; Mima, T.; Thompson, R.; Mikawa, T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development 1995, 121, 1423–1431. [Google Scholar] [CrossRef]
- Hoogaars, W.M.H.; Lansbergen-Engel, A.; Brons, J.F.; Verkerk, A.; De Lange, F.J.; Wong, L.Y.E.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007, 21, 1098–1112. [Google Scholar] [CrossRef]
- Miquerol, L.; Kelly, R. Organogenesis of the vertebrate heart. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, I.P.; Kim, J.B.; Moore, M.L.; Wolf, C.M.; Peterson, M.A.; Shendure, J.; Nobrega, M.A.; Yokota, Y.; Berul, C.; Izumo, S.; et al. A Molecular Pathway Including Id2, Tbx5, and Nkx2-5 Required for Cardiac Conduction System Development. Cell 2007, 129, 1365–1376. [Google Scholar] [CrossRef]
- Sedmera, D.; Reckova, M.; DeAlmeida, A.; Coppen, S.R.; Kubalak, S.W.; Gourdie, R.G.; Thompson, R.P. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2003, 274A, 773–777. [Google Scholar] [CrossRef]
- Kitajima, S.; Miyagawa-Tomita, S.; Inoue, T.; Kanno, J.; Saga, Y. Mesp1-nonexpressing cells contribute to the ventricular cardiac conduction system. Dev. Dyn. 2006, 235, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G.; Buckingham, M.E.; Moorman, A.F. Heart Fields and Cardiac Morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 4, a015750. [Google Scholar] [CrossRef]
- Meilhac, S.M.; Buckingham, M.E. The deployment of cell lineages that form the mammalian heart. Nat. Rev. Cardiol. 2018, 15, 705–724. [Google Scholar] [CrossRef]
- Liang, X.; Wang, G.; Lin, L.; Lowe, J.; Zhang, Q.; Bu, L.; Chen, Y.; Chen, J.; Sun, Y.; Evans, S.M. HCN4 Dynamically Marks the First Heart Field and Conduction System Precursors. Circ. Res. 2013, 113, 399–407. [Google Scholar] [CrossRef]
- Später, D.; Abramczuk, M.K.; Buac, K.; Zangi, L.; Stachel, M.W.; Clarke, J.; Sahara, M.; Ludwig, A.; Chien, K.R. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell Biol. 2013, 15, 1098–1106. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Q.; Cattaneo, P.; Zhuang, S.; Gong, X.; Spann, N.J.; Jiang, C.; Cao, X.; Zhao, X.; Zhang, X.; et al. Transcription factor ISL1 is essential for pacemaker development and function. J. Clin. Investig. 2015, 125, 3256–3268. [Google Scholar] [CrossRef]
- Aanhaanen, W.T.J.; Mommersteeg, M.; Norden, J.; Wakker, V.; de Gier-de Vries, C.; Anderson, R.H.; Kispert, A.; Moorman, A.F.M.; Christoffels, V.M. Developmental Origin, Growth, and Three-Dimensional Architecture of the Atrioventricular Conduction Axis of the Mouse Heart. Circ. Res. 2010, 107, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Vermeulen, J.L.M.; Verbeek, F.J.; Virágh, S.Z.; Kálmán, F.; Lamers, W.H.; Moorman, A.F.M. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1992, 232, 97–111. [Google Scholar] [CrossRef]
- Hoogaars, W.M.; Tessari, A.; Moorman, A.F.; de Boer, P.A.; Hagoort, J.; Soufan, A.T.; Campione, M.; Christoffels, V.M. The transcriptional repressor tbx3 delineates the developing central conduction system of the heart. Cardiovasc. Res. 2004, 62, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.M.; Christoffels, V.M. Development of the cardiac conduction system: A matter of chamber development. Novartis Found. Symp. 2003, 250, 25–43; discussion 34–43, 276–279. [Google Scholar]
- Sankova, B.; Benes, J., Jr.; Krejci, E.; Dupays, L.; Théveniau-Ruissy, M.; Miquerol, L.; Sedmera, D. The effect of connexin40 deficiency on ventricular conduction system function during development. Cardiovasc. Res. 2012, 95, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Delorme, B.; Dahl, E.; Jarry-Guichard, T.; Marics, I.; Briand, J.-P.; Willecke, K.; Gros, D.; Théveniau-Ruissy, M. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev. Dyn. 1995, 204, 358–371. [Google Scholar] [CrossRef]
- Beyer, S.; Kelly, R.G.; Miquerol, L. Inducible cx40-cre expression in the cardiac conduction system and arterial endothelial cells. Genesis 2011, 49, 83–91. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Zhao, H.; He, L.; Zhang, S.; Huang, X.; Zhang, H.; Miquerol, L.; Zhou, B. Genetic targeting of purkinje fibres by sema3a-creert2. Sci. Rep. 2018, 8, 2382. [Google Scholar] [CrossRef]
- Van Eif, V.W.W.; Devalla, H.D.; Boink, G.J.J.; Christoffels, V.M. Transcriptional regulation of the cardiac conduction system. Nat. Rev. Cardiol. 2018, 15, 617–630. [Google Scholar] [CrossRef]
- van Eif, V.W.; Stefanovic, S.; Mohan, R.A.; Christoffels, V.M. Gradual differentiation and confinement of the cardiac conduction system as indicated by marker gene expression. Biochim. Biophys. Acta BBA Mol. Cell Res. 2020, 1867, 118509. [Google Scholar] [CrossRef]
- Shekhar, A.; Lin, X.; Lin, B.; Liu, F.-Y.; Zhang, J.; Khodadadi-Jamayran, A.; Tsirigos, A.; Bu, L.; Fishman, G.I.; Park, D.S. ETV1 activates a rapid conduction transcriptional program in rodent and human cardiomyocytes. Sci. Rep. 2018, 8, 9944. [Google Scholar] [CrossRef]
- Shekhar, A.; Lin, X.; Liu, F.-Y.; Zhang, J.; Mo, H.; Bastarache, L.; Denny, J.; Cox, N.J.; Delmar, M.; Roden, D.M.; et al. Transcription factor ETV1 is essential for rapid conduction in the heart. J. Clin. Investig. 2016, 126, 4444–4459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-S.; Kim, K.-H.; Rosen, A.; Smyth, J.; Sakuma, R.; Delgado-Olguín, P.; Davis, M.; Chi, N.C.; Puviindran, V.; Gaborit, N.; et al. Iroquois homeobox gene 3 establishes fast conduction in the cardiac His-Purkinje network. Proc. Natl. Acad. Sci. USA 2011, 108, 13576–13581. [Google Scholar] [CrossRef] [PubMed]
- Gourdie, R.G.; Wei, Y.; Kim, D.; Klatt, S.C.; Mikawa, T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting Purkinje fibers. Proc. Natl. Acad. Sci. USA 1998, 95, 6815–6818. [Google Scholar] [CrossRef] [PubMed]
- Hyer, J.; Johansen, M.; Prasad, A.; Wessels, A.; Kirby, M.L.; Gourdie, R.G.; Mikawa, T. Induction of Purkinje fiber differentiation by coronary arterialization. Proc. Natl. Acad. Sci. USA 1999, 96, 13214–13218. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, T.; Hurtado, R. Development of the cardiac conduction system. Semin. Cell Dev. Biol. 2007, 18, 90–100. [Google Scholar] [CrossRef]
- Takebayashi-Suzuki, K.; Yanagisawa, M.; Gourdie, R.; Kanzawa, N.; Mikawa, T. In vivo induction of cardiac Purkinje fiber differentiation by coexpression of preproendothelin-1 and endothelin converting enzyme-1. Development 2000, 127, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.L.; Vedantham, V.; Barnes, R.M.; Hu, J.; Robinson, A.S.; Bressan, M.; Srivastava, D.; Black, B.L. Specification of the mouse cardiac conduction system in the absence of Endothelin signaling. Dev. Biol. 2014, 393, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Delorme, B.; Dahl, E.; Jarry-Guichard, T.; Briand, J.-P.; Willecke, K.; Gros, D.; Théveniau-Ruissy, M. Expression Pattern of Connexin Gene Products at the Early Developmental Stages of the Mouse Cardiovascular System. Circ. Res. 1997, 81, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Yanni, J.; Boyett, M.; Anderson, R.H.; Dobrzynski, H. The extent of the specialized atrioventricular ring tissues. Heart Rhythm. 2009, 6, 672–680. [Google Scholar] [CrossRef]
- Goodyer, W.R.; Beyersdorf, B.M.; Paik, D.T.; Tian, L.; Li, G.; Buikema, J.W.; Chirikian, O.; Choi, S.; Venkatraman, S.; Adams, E.L.; et al. Transcriptomic Profiling of the Developing Cardiac Conduction System at Single-Cell Resolution. Circ. Res. 2019, 125, 379–397. [Google Scholar] [CrossRef]
- Choquet, C.; Marcadet, L.; Beyer, S.; Kelly, R.G.; Miquerol, L. Segregation of Central Ventricular Conduction System Lineages in Early SMA+ Cardiomyocytes Occurs Prior to Heart Tube Formation. J. Cardiovasc. Dev. Dis. 2016, 3, 2. [Google Scholar] [CrossRef]
- Mohan, R.A.; Mommersteeg, M.T.M.; Domínguez, J.N.; Choquet, C.; Wakker, V.; de Gier-de Vries, C.; Boink, G.J.J.; Boukens, B.J.; Miquerol, L.; Verkerk, A.; et al. Embryonic Tbx3+ cardiomyocytes form the mature cardiac conduction system by progressive fate restriction. Development 2018, 145, dev.167361. [Google Scholar] [CrossRef]
- Choquet, C.; Kelly, R.G.; Miquerol, L. Nkx2-5 defines distinct scaffold and recruitment phases during formation of the murine cardiac Purkinje fiber network. Nat. Commun. 2020, 11, 530. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Munshi, N.V. Development of the Cardiac Conduction System. Cold Spring Harb. Perspect. Biol. 2020, 12, a037408. [Google Scholar] [CrossRef]
- Park, D.S.; Fishman, G.I. Development and Function of the Cardiac Conduction System in Health and Disease. J. Cardiovasc. Dev. Dis. 2017, 4, 7. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Shah, D.C.; Jaïs, P.; Shoda, M.; Kautzner, J.; Arentz, T.; Kalushe, D.; Kadish, A.; Griffith, M.; Gaita, F.; et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet 2002, 359, 677–678. [Google Scholar] [CrossRef]
- Rentschler, S.; Morley, G.E.; Fishman, G.I. Patterning of the mouse conduction system. Novartis Found. Symp. 2003, 250, 194–205, discussion 205–199, 276–199. [Google Scholar]
- Rentschler, S.; Zander, J.; Meyers, K.; France, D.; Levine, R.; Porter, G.; Rivkees, S.A.; Morley, G.E.; Fishman, G.I. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc. Natl. Acad. Sci. USA 2002, 99, 10464–10469. [Google Scholar] [CrossRef]
- van Veen, T.A.; van Rijen, H.V.; van Kempen, M.J.; Miquerol, L.; Opthof, T.; Gros, D.; Vos, M.A.; Jongsma, H.J.; de Bakker, J.M. Discontinuous conduction in mouse bundle branches is caused by bundle-branch architecture. Circulation 2005, 112, 2235–2244. [Google Scholar] [CrossRef]
- Olejnickova, V.; Kocka, M.; Kvasilova, A.; Kolesova, H.; Dziacky, A.; Gidor, T.; Gidor, L.; Sankova, B.; Gregorovicova, M.; Gourdie, R.; et al. Gap Junctional Communication via Connexin43 between Purkinje Fibers and Working Myocytes Explains the Epicardial Activation Pattern in the Postnatal Mouse Left Ventricle. Int. J. Mol. Sci. 2021, 22, 2475. [Google Scholar] [CrossRef]
- Mohan, R.A.; Boukens, B.J.; Christoffels, V.M. Developmental Origin of the Cardiac Conduction System: Insight from Lineage Tracing. Pediatr. Cardiol. 2018, 39, 1107–1114. [Google Scholar] [CrossRef]
- Jay, P.Y.; Harris, B.S.; Buerger, A.; Rozhitskaya, O.; Maguire, C.T.; Barbosky, L.A.; McCusty, E.; Berul, C.I.; O’Brien, T.X.; Gourdie, R.G.; et al. Function follows form: Cardiac conduction system defects inNkx2-5 mutation. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2004, 280, 966–972. [Google Scholar] [CrossRef]
- Moskowitz, I.P.G.; Pizard, A.; Patel, V.V.; Bruneau, B.; Kim, J.B.; Kupershmidt, S.; Roden, D.; Berul, C.I.; Seidman, C.E.; Seidman, J.G. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development 2004, 131, 4107–4116. [Google Scholar] [CrossRef]
- Meyer, D.; Birchmeier, C. Multiple essential functions of neuregulin in development. Nature 1995, 378, 386–390. [Google Scholar] [CrossRef]
- Del Monte-Nieto, G.; Ramialison, M.; Adam, A.; Wu, B.; Aharonov, A.; D’Uva, G.; Bourke, L.; Pitulescu, M.E.; Chen, H.; De La Pompa, J.L.; et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 2018, 557, 439–445. [Google Scholar] [CrossRef]
- Kim, K.-H.; Rosen, A.; Hussein, S.M.I.; Puviindran, V.; Korogyi, A.S.; Chiarello, C.; Nagy, A.; Hui, C.-C.; Backx, P.H. Irx3 is required for postnatal maturation of the mouse ventricular conduction system. Sci. Rep. 2016, 6, 19197. [Google Scholar] [CrossRef]
- Ellesøe, S.G.; Johansen, M.M.; Bjerre, J.V.; Hjortdal, V.E.; Brunak, S.; Larsen, L.A. Familial Atrial Septal Defect and Sudden Cardiac Death: Identification of a NovelNKX2-5Mutation and a Review of the Literature. Congenit. Heart Dis. 2015, 11, 283–290. [Google Scholar] [CrossRef]
- Schott, J.-J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital Heart Disease Caused by Mutations in the Transcription Factor NKX2-5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef]
- Costa, M.W.; Guo, G.; Wolstein, O.; Vale, M.; Castro, M.L.; Wang, L.; Otway, R.; Riek, P.; Cochrane, N.; Furtado, M.; et al. Functional Characterization of a Novel Mutation in NKX2-5 Associated With Congenital Heart Disease and Adult-Onset Cardiomyopathy. Circ. Cardiovasc. Genet. 2013, 6, 238–247. [Google Scholar] [CrossRef]
- Maury, P.; Gandjbakhch, E.; Baruteau, A.-E.; Bessière, F.; Kyndt, F.; Bouvagnet, P.; Rollin, A.; Bonnet, D.; Probst, V.; Maltret, A. Cardiac Phenotype and Long-Term Follow-Up of Patients With Mutations in NKX2-5 Gene. J. Am. Coll. Cardiol. 2016, 68, 2389–2390. [Google Scholar] [CrossRef]
- Tanaka, M.; Berul, C.; Ishii, M.; Jay, P.; Wakimoto, H.; Douglas, P.; Yamasaki, N.; Kawamoto, T.; Gehrmann, J.; Maguire, C.; et al. A Mouse Model of Congenital Heart Disease: Cardiac Arrhythmias and Atrial Septal Defect Caused by Haploinsufficiency of the Cardiac Transcription Factor Csx/Nkx2.5. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 317–326. [Google Scholar] [CrossRef]
- Meysen, S.; Marger, L.; Hewett, K.W.; Jarry-Guichard, T.; Agarkova, I.; Chauvin, J.P.; Perriard, J.C.; Izumo, S.; Gourdie, R.G.; Mangoni, M.; et al. Nkx2.5 cell-autonomous gene function is required for the postnatal formation of the peripheral ventricular conduction system. Dev. Biol. 2007, 303, 740–753. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ashraf, H.; Melanson, M.; Tanada, Y.; Nguyen, M.; Silberbach, M.; Wakimoto, H.; Benson, D.W.; Anderson, R.H.; Kasahara, H. Mouse model of human congenital heart disease: Progressive atrioventricular block induced by a heterozygous nkx2-5 homeodomain missense mutation. Circ. Arrhythm. Electrophysiol. 2015, 8, 1255–1264. [Google Scholar] [CrossRef]
- Pashmforoush, M.; Lu, J.T.; Chen, H.; Amand, T.S.; Kondo, R.; Pradervand, S.; Evans, S.M.; Clark, B.; Feramisco, J.R.; Giles, W.; et al. Nkx2-5 Pathways and Congenital Heart Disease: Loss of Ventricular Myocyte Lineage Specification Leads to Progressive Cardiomyopathy and Complete Heart Block. Cell 2004, 117, 373–386. [Google Scholar] [CrossRef]
- Jay, P.Y.; Harris, B.S.; Maguire, C.T.; Buerger, A.; Wakimoto, H.; Tanaka, M.; Kupershmidt, S.; Roden, D.M.; Schultheiss, T.M.; O’Brien, T.X.; et al. Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J. Clin. Investig. 2004, 113, 1130–1137. [Google Scholar] [CrossRef]
- Bruneau, B.; Logan, M.; Davis, N.; Levi, T.; Tabin, C.J.; Seidman, J.; Seidman, C.E. Chamber-Specific Cardiac Expression of Tbx5 and Heart Defects in Holt–Oram Syndrome. Dev. Biol. 1999, 211, 100–108. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Habets, P.E.; Franco, D.; Campione, M.; de Jong, F.; Lamers, W.H.; Bao, Z.-Z.; Palmer, S.; Biben, C.; Harvey, R.; et al. Chamber Formation and Morphogenesis in the Developing Mammalian Heart. Dev. Biol. 2000, 223, 266–278. [Google Scholar] [CrossRef]
- Basson, C.T.; Huang, T.; Lin, R.C.; Bachinsky, D.R.; Weremowicz, S.; Vaglio, A.; Bruzzone, R.; Quadrelli, R.; Lerone, M.; Romeo, G.; et al. Different TBX5 interactions in heart and limb defined by Holt-Oram syndrome mutations. Proc. Natl. Acad. Sci. USA 1999, 96, 2919–2924. [Google Scholar] [CrossRef]
- Bruneau, B.; Nemer, G.; Schmitt, J.P.; Charron, F.; Robitaille, L.; Caron, S.; Conner, D.A.; Gessler, M.; Nemer, M.; Seidman, C.E.; et al. A Murine Model of Holt-Oram Syndrome Defines Roles of the T-Box Transcription Factor Tbx5 in Cardiogenesis and Disease. Cell 2001, 106, 709–721. [Google Scholar] [CrossRef]
- Koizumi, A.; Sasano, T.; Kimura, W.; Miyamoto, Y.; Aiba, T.; Ishikawa, T.; Nogami, A.; Fukamizu, S.; Sakurada, H.; Takahashi, Y.; et al. Genetic defects in a His-Purkinje system transcription factor, IRX3, cause lethal cardiac arrhythmias. Eur. Heart J. 2016, 37, 1469–1475. [Google Scholar] [CrossRef]
- Qian, L.; Wythe, J.; Liu, J.; Cartry, J.; Vogler, G.; Mohapatra, B.; Otway, R.T.; Huang, Y.; King, I.N.; Maillet, M.; et al. Tinman/Nkx2-5 acts via miR-1 and upstream of Cdc42 to regulate heart function across species. J. Cell Biol. 2011, 193, 1181–1196. [Google Scholar] [CrossRef]
- Wu, M.; Wu, D.; Wang, C.; Guo, Z.; Li, B.; Zuo, Z. Hexabromocyclododecane exposure induces cardiac hypertrophy and arrhythmia by inhibiting miR-1 expression via up-regulation of the homeobox gene Nkx2.5. J. Hazard. Mater. 2016, 302, 304–313. [Google Scholar] [CrossRef]
- Samal, E.; Evangelista, M.; Galang, G.; Srivastava, D.; Zhao, Y.; Vedantham, V. Premature MicroRNA-1 Expression Causes Hypoplasia of the Cardiac Ventricular Conduction System. Front. Physiol. 2019, 10, 235. [Google Scholar] [CrossRef]
- Su, X.; Liang, H.; Wang, H.; Chen, G.; Jiang, H.; Wu, Q.; Liu, T.; Liu, Q.; Yu, T.; Gu, Y.; et al. Over-expression of microRNA-1 causes arrhythmia by disturbing intracellular trafficking system. Sci. Rep. 2017, 7, 46259. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Zhang, Y.; Liang, H.; Li, X.; Cai, R.; Wang, L.; Du, W.; Zhang, R.; Li, J.; et al. Overexpression of microRNA-1 Causes Atrioventricular Block in Rodents. Int. J. Biol. Sci. 2013, 9, 455–462. [Google Scholar] [CrossRef]
- Liu, C.J.; Ding, B.; Wang, H.; Lengyel, P. The myod-inducible p204 protein overcomes the inhibition of myoblast differentiation by id proteins. Mol. Cell Biol. 2002, 22, 2893–2905. [Google Scholar] [CrossRef][Green Version]
- Delgado, C.; Bu, L.; Zhang, J.; Fang-Yu, L.; Sall, J.; Liang, F.X.; Furley, A.J.; Fishman, G.I. Neural cell adhesion molecule (ncam-1) is required for ventricular conduction system development. Development 2021, 148, dev199431. [Google Scholar] [CrossRef]
- Ismat, F.A.; Zhang, M.; Kook, H.; Huang, B.; Zhou, R.; Ferrari, V.; Epstein, J.A.; Patel, V.V. Homeobox protein Hop functions in the adult cardiac conduction system. Circ. Res. 2005, 96, 898–903. [Google Scholar] [CrossRef]
- Vincentz, J.W.; Firulli, B.A.; Toolan, K.P.; Arking, D.E.; Sotoodehnia, N.; Wan, J.; Chen, P.-S.; de Gier-de Vries, C.; Christoffels, V.M.; Der Lohe, M.R.-V.; et al. Variation in a Left Ventricle–Specific Hand1 Enhancer Impairs GATA Transcription Factor Binding and Disrupts Conduction System Development and Function. Circ. Res. 2019, 125, 575–589. [Google Scholar] [CrossRef]
- Park, D.S.; Tompkins, R.O.; Liu, F.; Zhang, J.; Phoon, C.K.L.; Zavadil, J.; Fishman, G.I. Pocket proteins critically regulate cell cycle exit of the trabecular myocardium and the ventricular conduction system. Biol. Open 2013, 2, 968–978. [Google Scholar] [CrossRef]
- Choquet, C.; Kelly, R.; Miquerol, L. Defects in Trabecular Development Contribute to Left Ventricular Noncompaction. Pediatr. Cardiol. 2019, 40, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.; Nguyen, T.H.M.; Sicard, P.; Buttigieg, E.; Tran, T.T.; Kober, F.; Varlet, I.; Sturny, R.; Costa, M.W.; Harvey, R.P.; et al. Deletion of nkx2-5 in trabecular myocardium reveals the developmental origins of pathological heterogeneity associated with ventricular non-compaction cardiomyopathy. PLoS Genet. 2018, 14, e1007502. [Google Scholar]
- Sedmera, D.; Thomas, P.S. Trabeculation in the embryonic heart. BioEssays 1996, 18, 607. [Google Scholar] [CrossRef]
- Samsa, L.; Yang, B.; Liu, J. Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation. Am. J. Med Genet. Part C Semin. Med Genet. 2013, 163, 157–168. [Google Scholar] [CrossRef]
- Faber, J.W.; D’Silva, A.; Christoffels, V.M.; Jensen, B. Lack of morphometric evidence for ventricular compaction in humans. J. Cardiol. 2021. [Google Scholar] [CrossRef]
- Sedmera, D.; Gourdie, R.G. Why do we have Purkinje fibers deep in our heart? Physiol. Res. 2014, 63 (Suppl. 1), S9–S18. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; He, L.; Zhang, H.; Huang, X.; Liu, Q.; Pu, W.; Zhang, L.; Li, Y.; Zhao, H.; et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat. Commun. 2017, 8, 87. [Google Scholar] [CrossRef]
- Meyer, H.V.; Dawes, T.J.W.; Serrani, M.; Bai, W.; Tokarczuk, P.; Cai, J.; de Marvao, A.; Henry, A.; Lumbers, R.T.; Gierten, J.; et al. Genetic and functional insights into the fractal structure of the heart. Nature 2020, 584, 589–594. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choquet, C.; Boulgakoff, L.; Kelly, R.G.; Miquerol, L. New Insights into the Development and Morphogenesis of the Cardiac Purkinje Fiber Network: Linking Architecture and Function. J. Cardiovasc. Dev. Dis. 2021, 8, 95. https://doi.org/10.3390/jcdd8080095
Choquet C, Boulgakoff L, Kelly RG, Miquerol L. New Insights into the Development and Morphogenesis of the Cardiac Purkinje Fiber Network: Linking Architecture and Function. Journal of Cardiovascular Development and Disease. 2021; 8(8):95. https://doi.org/10.3390/jcdd8080095
Chicago/Turabian StyleChoquet, Caroline, Lucie Boulgakoff, Robert G. Kelly, and Lucile Miquerol. 2021. "New Insights into the Development and Morphogenesis of the Cardiac Purkinje Fiber Network: Linking Architecture and Function" Journal of Cardiovascular Development and Disease 8, no. 8: 95. https://doi.org/10.3390/jcdd8080095
APA StyleChoquet, C., Boulgakoff, L., Kelly, R. G., & Miquerol, L. (2021). New Insights into the Development and Morphogenesis of the Cardiac Purkinje Fiber Network: Linking Architecture and Function. Journal of Cardiovascular Development and Disease, 8(8), 95. https://doi.org/10.3390/jcdd8080095





