Outcomes of Patients with and without Malignancy Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion
Abstract
:1. Introduction
2. Methods
2.1. Patient Population
2.2. Percutaneous Pericardiocentesis Procedures
2.3. Definition
2.4. Study Endpoint
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Patients
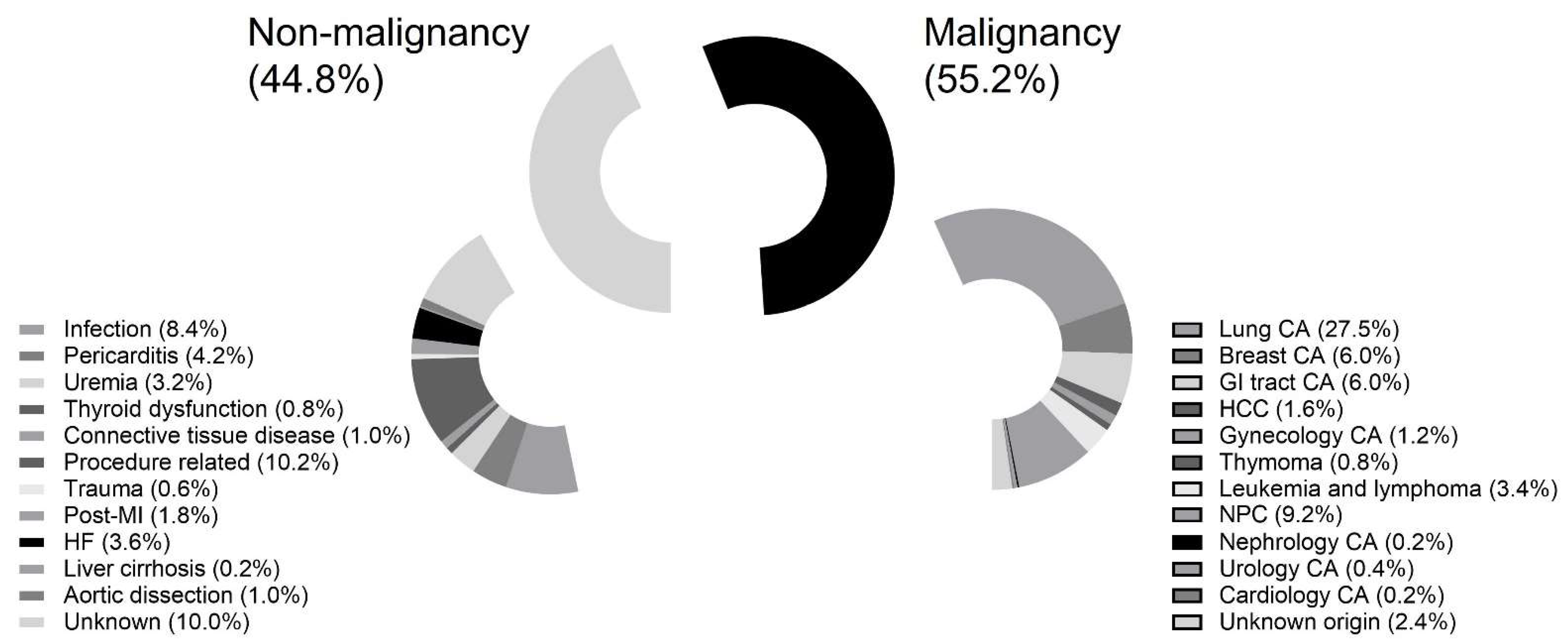
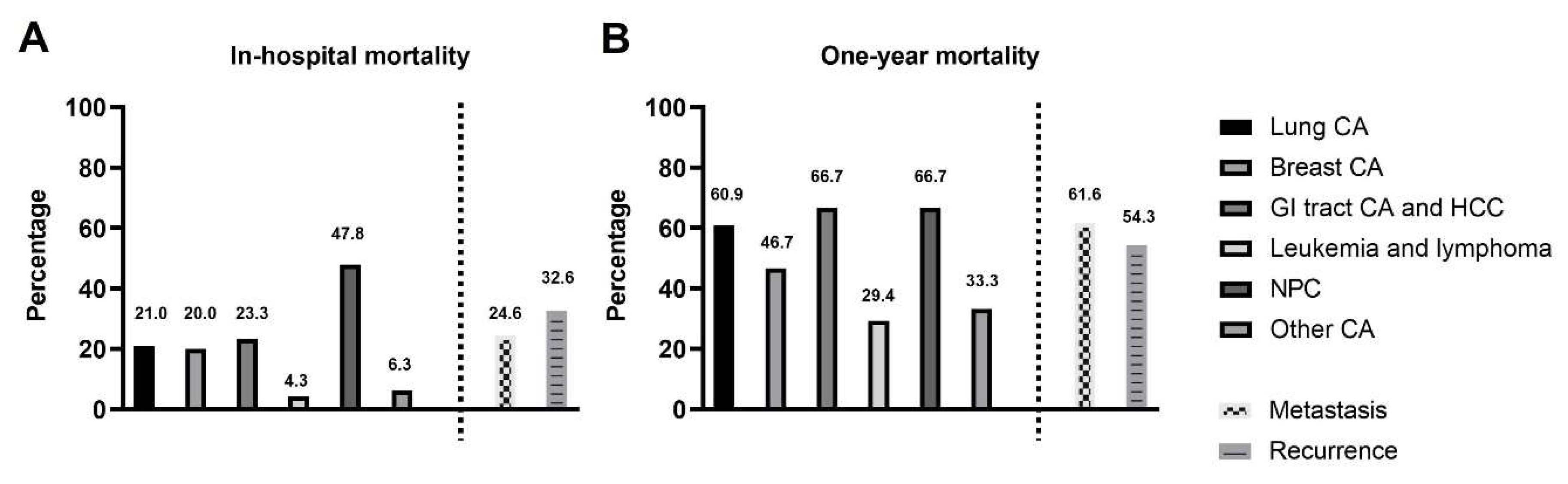
3.2. The Type of Malignancy and Associated Mortality Rate
3.3. Univariable and Multivariable Cox Regression Analyses of Predictors of One-Year Mortality
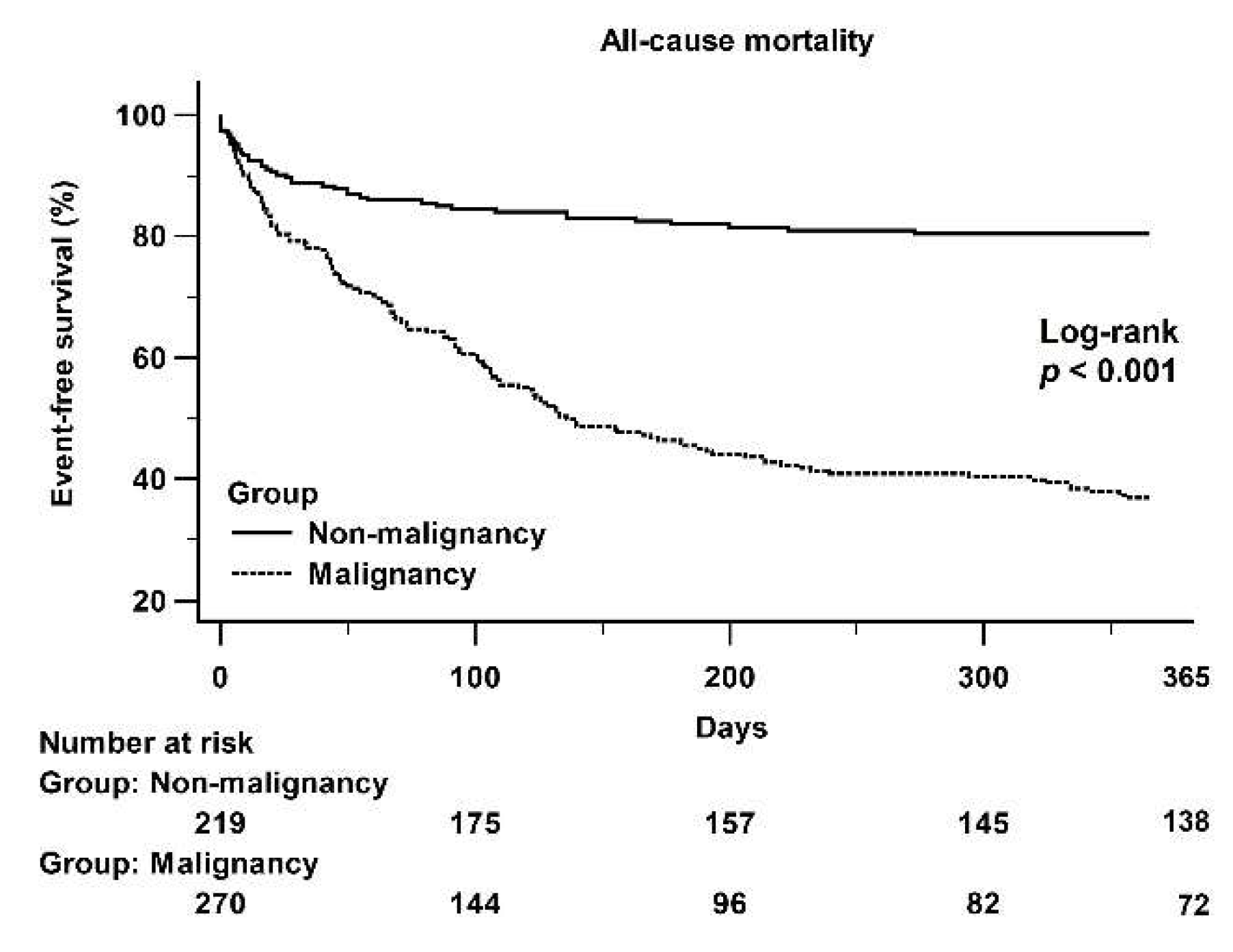
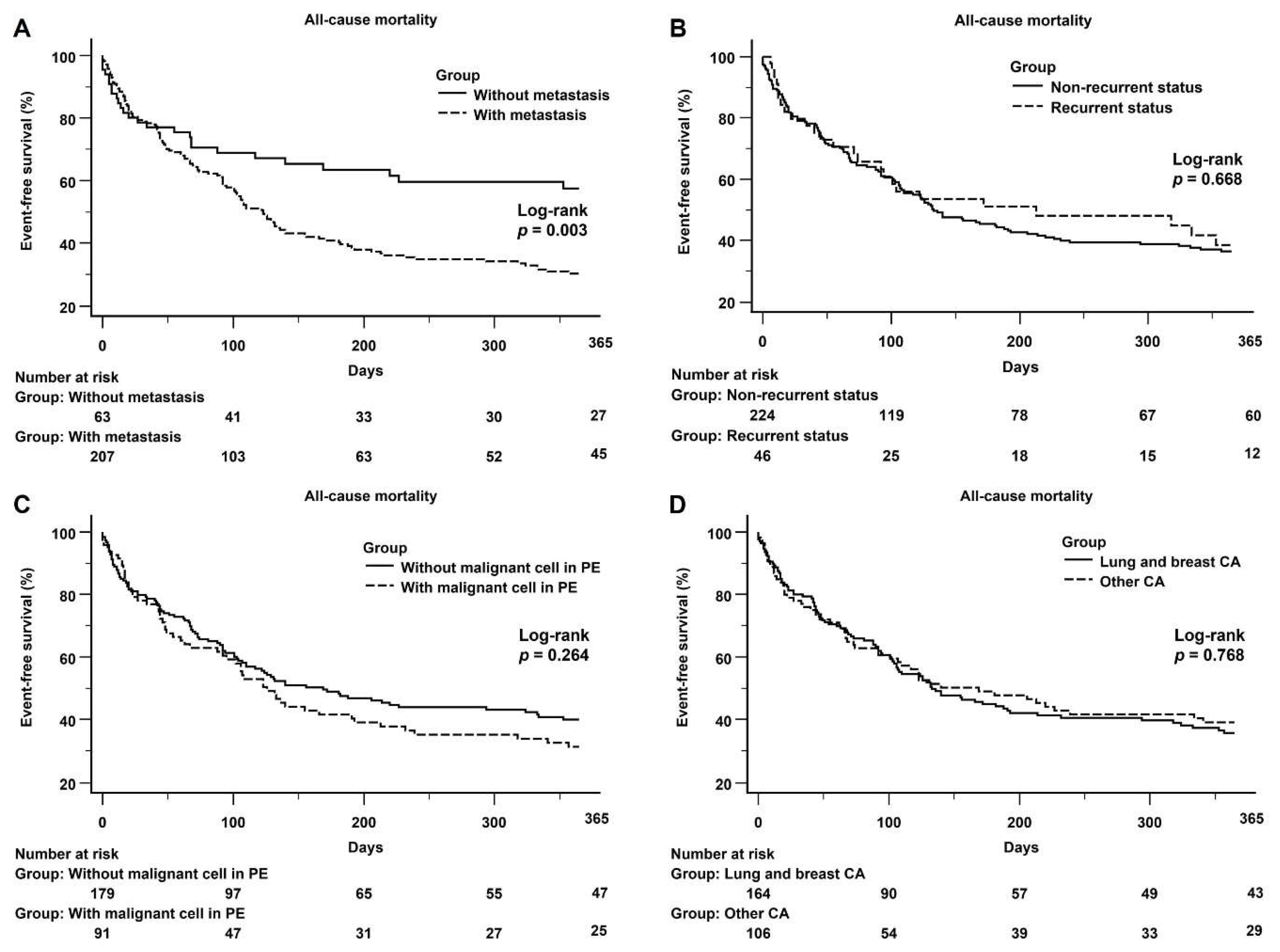
3.4. Kaplan–Meier Curves Showing 1-Year All-Cause Mortality Data of the Two Groups and Subgroups of the Malignancy Population
4. Discussion
4.1. Poor Prognosis in Malignant PE
4.2. The Predictors of Mortality in the Cancer Population with Malignant PE
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsang, T.S.; Enriquez-Sarano, M.; Freeman, W.K.; Barnes, M.E.; Sinak, L.J.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin. Proc. 2002, 77, 429–436. [Google Scholar] [CrossRef]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur. Heart J. 2013, 34, 1186–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercé, J.; Sagristà-Sauleda, J.; Permanyer-Miralda, G.; Soler-Soler, J. Should pericardial drainage be performed routinely in patients who have a large pericardial effusion without tamponade? Am. J. Med. 1998, 105, 106–109. [Google Scholar] [CrossRef]
- Kil, U.H.; Jung, H.O.; Koh, Y.S.; Park, H.J.; Park, C.S.; Kim, P.J.; Baek, S.H.; Seung, K.B.; Choi, K.B. Prognosis of large, symptomatic pericardial effusion treated by echo-guided percutaneous pericardiocentesis. Clin. Cardiol. 2008, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Flint, N.; Siegel, R.J. Echo-Guided Pericardiocentesis: When and How Should It Be Performed? Curr. Cardiol. Rep. 2020, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, A.; Lin, M.J.; Uchino, R.; Butryn, T.; O’Mara, M.S.; Nanda, S.; Shirani, J.; Stawicki, S.P. Complications of pericardiocentesis: A clinical synopsis. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 206–212. [Google Scholar] [PubMed] [Green Version]
- Kabukcu, M.; Demircioglu, F.; Yanik, E.; Basarici, I.; Ersel, F. Pericardial tamponade and large pericardial effusions: Causal factors and efficacy of percutaneous catheter drainage in 50 patients. Tex. Heart Inst. J. 2004, 31, 398–403. [Google Scholar] [PubMed]
- Strobbe, A.; Adriaenssens, T.; Bennett, J.; Dubois, C.; Desmet, W.; McCutcheon, K.; Van Cleemput, J.; Sinnaeve, P.R. Etiology and Long-Term Outcome of Patients Undergoing Pericardiocentesis. J. Am. Heart Assoc. 2017, 6, e007598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jama, G.M.; Scarci, M.; Bowden, J.; Marciniak, S.J. Palliative treatment for symptomatic malignant pericardial effusion . Interact. Cardiovasc. Thorac. Surg. 2014, 19, 1019−1026. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, D.; Iliescu, C.; Yusuf, S.W.; William, W.N., Jr.; Khair, T.H.; Song, J.; Mouhayar, E.N. Outcomes of Cancer Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. J. Am. Coll. Cardiol. 2015, 66, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, A.J.; Paz, Y.E.; Rene, A.G.; Green, P.; Hassanin, A.; Argenziano, M.G.; Rabbani, L.; Dangas, G. Comparison of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J. Invasive Cardiol. 2012, 24, 590–593. [Google Scholar] [PubMed]
- Fowler, N.O. Cardiac tamponade. A clinical or an echocardiographic diagnosis? Circulation 1993, 87, 1738–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, P.J.; Schuck, J. Echocardiographic Assessment of Pericardial Effusion: A Brief Review. J. Diagn. Med. Sonography 2007, 23, 189–197. [Google Scholar] [CrossRef]
- Lekhakul, A.; Assawakawintip, C.; Fenstad, E.R.; Pislaru, S.V.; Thaden, J.J.; Sinak, L.J.; Kane, G.C. Safety and Outcome of Percutaneous Drainage of Pericardial Effusions in Patients with Cancer. Am. J. Cardiol. 2018, 122, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.W.; Cho, D.G.; Park, J.K.; Hyun, K.Y.; Choi, S.Y.; Suh, J.H.; Kim, Y.D. Prognostic factors affecting survival of patients with cancer-related pericardial effusion managed by surgery. World J. Surg. Oncol. 2014, 12, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | All Patients (N = 502) | Non-Malignancy (N = 225) | Malignancy (N = 277) | p Value |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 63 ± 13.4 | 67 ± 15.3 | 59 ± 12.3 | <0.001 |
| Male gender (%) | 309 (61.6) | 150 (66.7) | 159 (57.4) | 0.034 |
| Medical history | ||||
| Diabetes mellitus (%) | 126 (25.1) | 74 (32.9) | 52 (18.8) | <0.001 |
| Hypertension (%) | 213 (42.4) | 140 (62.2) | 73 (26.4) | <0.001 |
| Connective disease (%) | 10 (2.0) | 7 (3.1) | 3 (1.1) | 0.121 |
| Liver cirrhosis (%) | 28 (5.6) | 12 (5.3) | 16 (5.8) | 0.848 |
| CAD (%) | 88 (17.5) | 74 (32.9) | 14 (5.1) | <0.001 |
| Heart failure (%) | 15 (3.0) | 11 (5.2) | 4 (1.7) | 0.062 |
| ESRD (%) | 48 (9.6) | 37 (16.4) | 11 (4.0) | <0.001 |
| Thyroid dysfunction (%) | 72 (26.5) | 33 (14.7) | 39 (14.1) | 0.898 |
| Prior history of pericardiocentesis (%) | 36 (7.2) | 9 (4.0) | 27 (9.7) | 0.014 |
| Lab data | ||||
| WBC (1000/μL) | 10.3 ± 5.7 | 9.6 ± 4.0 | 10.8 ± 6.8 | 0.010 |
| Hgb (g/dL) | 11.1 ± 2.1 | 11.0 ± 2.2 | 11.2 ± 2.1 | 0.153 |
| PLT (1000/μL) | 227 ± 111 | 220 ± 108 | 233 ± 112 | 0.221 |
| Creatinine (excluded ESRD) (mg/dL) | 1.36 ± 1.24 | 1.64 ± 0.66 | 1.16 ± 0.76 | <0.001 |
| LDH (mg/dL) | 267 ± 267 | 242 ± 246 | 284 ± 288 | 0.016 |
| Glucose (mg/dL) | 147.3 ± 57.7 | 141.7 ± 57.5 | 151.7 ± 57.6 | 0.158 |
| Clinical symptoms | ||||
| asystematic | 69 (13.7) | 41 (18.2) | 28 (10.1) | 0.009 |
| dyspnea | 345 (68.7) | 136 (60.4) | 209 (75.5) | <0.001 |
| tachycardia | 408 (81.3) | 163 (72.4) | 245 (88.4) | <0.001 |
| shock | 191 (38.0) | 92 (40.9) | 99 (35.7) | 0.267 |
| Pleural effusion | ||||
| Combined | 354 (70.5) | 136 (60.4) | 218 (78.7) | <0.001 |
| Bilateral side | 177 (35.3) | 63 (28.0) | 114 (41.2) | 0.001 |
| Only left side | 133 (26.5) | 64 (87.7) | 69 (66.3) | |
| Only right side | 44 (8.8) | 9 (12.3) | 35 (33.7) | |
| Ascites | 69 (13.7) | 37 (16.4) | 32 (11.6) | 0.120 |
| Echo | ||||
| Cardiac tamponade (%) | 416 (82.9) | 170 (75.6) | 246 (88.8) | <0.001 |
| Low voltage on ECG | 173 (34.5) | 57 (25.4) | 116 (42.0) | <0.001 |
| Pericardial effusion | ||||
| The color | <0.001 | |||
| Fresh red | 143 (28.5) | 66 (29.3) | 77 (27.8) | |
| Dark red | 178 (35.5) | 66 (29.3) | 112 (40.4) | |
| Yellow | 139 (27.7) | 60 (26.7) | 79 (28.5) | |
| Turbid | 42 (8.4) | 33 (14.7) | 9 (3.2) | |
| Lab data | ||||
| WBC (/μL) | 1470 ± 1476 | 1435 ± 1322 | 1530 ± 1745 | 0.442 |
| LDH (mg/dL) | 566 ± 572 | 462 ± 474 | 666 ± 674 | 0.758 |
| Glucose (mg/dL) | 108 ± 108 | 115 ± 117 | 96 ± 98 | 0.082 |
| The mean of drainage amount (mL) | 376.1 ± 253.1 | 356.9 ± 274.8 | 389.0 ± 237.0 | 0.194 |
| Surgery of pericardial periotoneal window (%) | 30 (6.0) | 9 (4.1) | 21 (7.7) | 0.129 |
| Complications of pericardiocentesis (%) | 5 (1.0) | 4 (1.8) | 1 (0.4) | 0.179 |
| Clinical outcomes | ||||
| In-hospital mortality (%) | 163 (32.5) | 17 (7.6) | 66 (23.8) | <0.001 |
| One-year mortality (%) | 198 (39.4) | 42 (18.7) | 156 (56.3) | <0.001 |
| Median of F/U duration (days) | 206 ± 222 | 630 ± 636 | 106 ± 107 | <0.001 |
| Variables | All Patients (N = 277) |
|---|---|
| Original site | |
| Lung (%) | 138 (49.8) |
| Breast (%) | 30 (10.8) |
| GI tract (%) | 30 (10.8) |
| Hepatology (%) | 8 (2.9) |
| Gynecology (%) | 6 (2.2) |
| Thymoma (%) | 4 (1.4) |
| Leukemia and lymphoma (%) | 17 (6.1) |
| Nasopharyngeal and oropharyngeal (%) | 46 (16.6) |
| Nephrology (%) | 1 (0.4) |
| Urology (%) | 2 (0.7) |
| Cardiology (%) | 1 (0.4) |
| Unknown (%) | 12 (4.3) |
| Double cancer (%) | 18 (6.5) |
| Metastasis | 211 (76.2) |
| Lung/pleural | 181 (65.3) |
| Pericardial | 110 (39.7) |
| Extrathoracic | 125 (45.1) |
| Recurrence | 46 (16.6) |
| Prior history of chemotherapy and/or radiotherapy | 164 (59.2) |
| Malignancy in pericardial effusion | 98 (35.4) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value |
| Age | 0.994 | 0.984−1.003 | 0.188 | |||
| Male gender | 0.884 | 0.666−1.173 | 0.393 | |||
| Diabetes mellitus | 0.781 | 0.559−1.092 | 0.149 | |||
| Hypertension | 0.497 | 0.368−0.673 | <0.001 | |||
| Connective disease | 0.429 | 0.107−1.729 | 0.234 | |||
| Liver cirrhosis | 1.797 | 1.107−2.917 | 0.018 | |||
| CAD | 0.405 | 0.252−0.651 | <0.001 | |||
| Heart failure | 1.140 | 0.505−2.577 | 0.752 | |||
| ESRD | 0.578 | 0.330−1.015 | 0.057 | |||
| Thyroid dysfunction | 0.976 | 0.651−1.465 | 0.908 | |||
| Procedure related | 0.445 | 0.235−0.841 | 0.013 | |||
| Malignancy | 4.055 | 2.879−5.712 | <0.001 | 2.084 | 1.246–3.488 | 0.005 |
| Lung CA | 2.348 | 1.769−3.117 | <0.001 | |||
| Breast CA | 1.250 | 0.726−2.152 | 0.421 | |||
| GI tract CA and HCC | 1.818 | 1.166−2.832 | 0.008 | |||
| Leukemia and lymphoma | 0.917 | 0.407−2.066 | 0.834 | |||
| NPC | 2.490 | 1.685−3.680 | <0.001 | 1.801 | 1.194–2.717 | 0.005 |
| Metastasis | 3.565 | 2.651−4.794 | <0.001 | 2.088 | 1.352–3.223 | 0.001 |
| Recurrence | 1.593 | 1.047−2.423 | 0.030 | |||
| Prior history of pericardiocentesis | 1.388 | 0.865−2.227 | 0.174 | |||
| WBC of PE (1000/μL) | 1.000 | 0.997−1.002 | 0.572 | |||
| LDH of PE (mg/dL) | 1.000 | 0.998−1.003 | 0.865 | |||
| Glucose of PE (mg/dL) | 1.001 | 1.000−1.001 | 0.206 | |||
| Malignant cells in PE | 2.184 | 1.606−2.969 | <0.001 | |||
| Dyspnea | 1.484 | 1.076−2.045 | 0.016 | |||
| Tachycardia | 2.029 | 1.313−3.135 | 0.001 | |||
| Shock | 0.952 | 0.713−1.271 | 0.739 | |||
| Pleural effusion | 2.223 | 1.548−3.192 | <0.001 | |||
| Ascites | 0.984 | 0.660−1.468 | 0.937 | |||
| Cardiac tamponade | 1.397 | 0.931−2.098 | 0.106 | |||
| Drainage amount | 1.000 | 0.999−1.000 | 0.287 | |||
| Complications of pericardiocentesis | 0.049 | 0−19.601 | 0.324 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.-T.; Lee, W.-C.; Fang, H.-Y.; Wu, P.-J.; Fang, Y.-N.; Chong, S.-Z. Outcomes of Patients with and without Malignancy Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. J. Cardiovasc. Dev. Dis. 2021, 8, 150. https://doi.org/10.3390/jcdd8110150
Shih C-T, Lee W-C, Fang H-Y, Wu P-J, Fang Y-N, Chong S-Z. Outcomes of Patients with and without Malignancy Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. Journal of Cardiovascular Development and Disease. 2021; 8(11):150. https://doi.org/10.3390/jcdd8110150
Chicago/Turabian StyleShih, Chun-Ting, Wei-Chieh Lee, Hsiu-Yu Fang, Po-Jui Wu, Yen-Nan Fang, and Shaur-Zheng Chong. 2021. "Outcomes of Patients with and without Malignancy Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion" Journal of Cardiovascular Development and Disease 8, no. 11: 150. https://doi.org/10.3390/jcdd8110150
APA StyleShih, C.-T., Lee, W.-C., Fang, H.-Y., Wu, P.-J., Fang, Y.-N., & Chong, S.-Z. (2021). Outcomes of Patients with and without Malignancy Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. Journal of Cardiovascular Development and Disease, 8(11), 150. https://doi.org/10.3390/jcdd8110150





