Coronary Vasculature in Cardiac Development and Regeneration
Abstract
1. Introduction
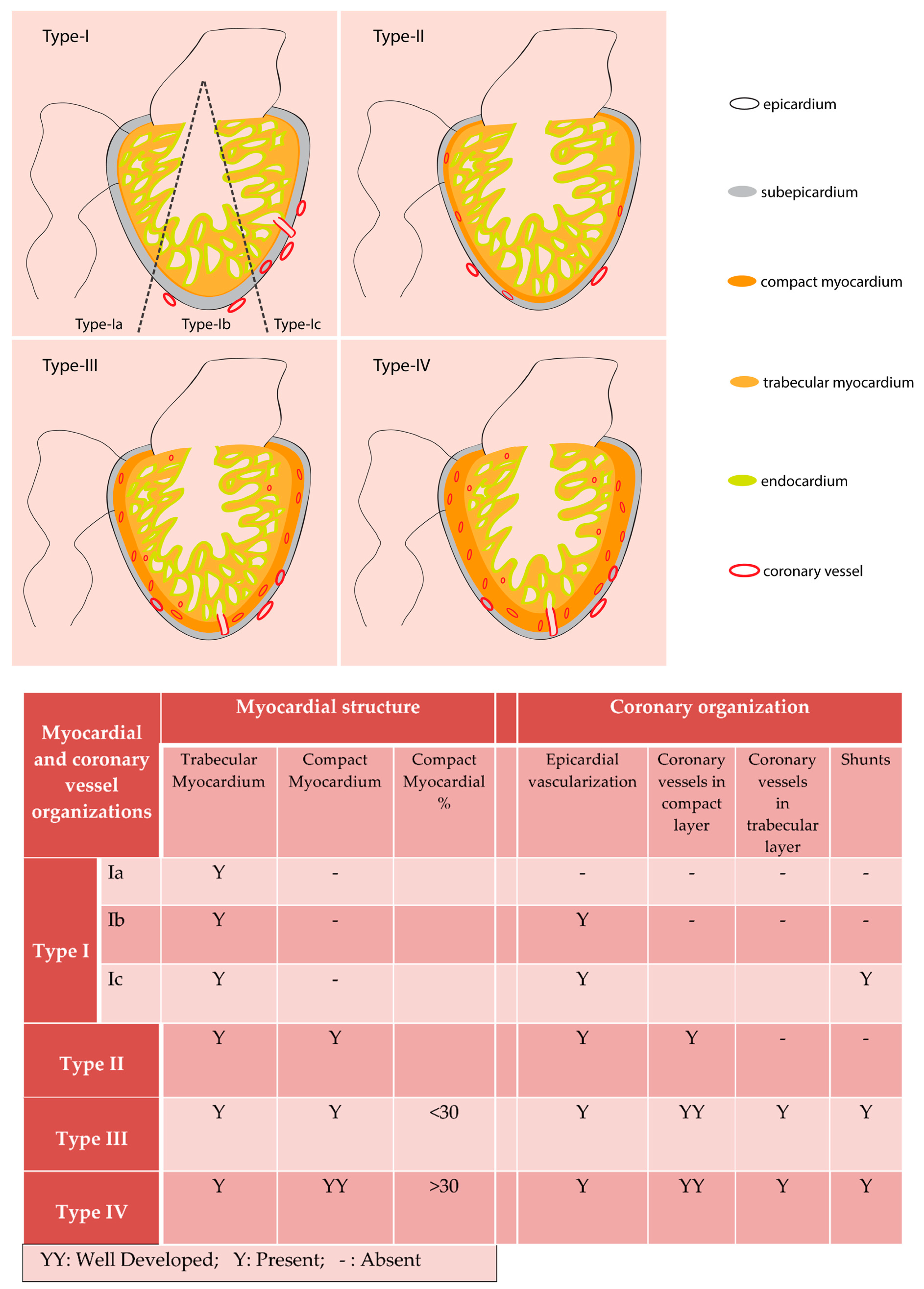
2. Evolutionary Aspects of Coronary Vessels
3. Endothelial Cells: Origins and Developmental Signaling Mechanisms
Signaling Pathways Regulating Coronary Endothelial Cell Development
4. Mural Cells: Origin, Development and Signaling Pathways in Recruitment and Differentiation
Signaling Pathways Regulating Coronary Perivascular Cell Development
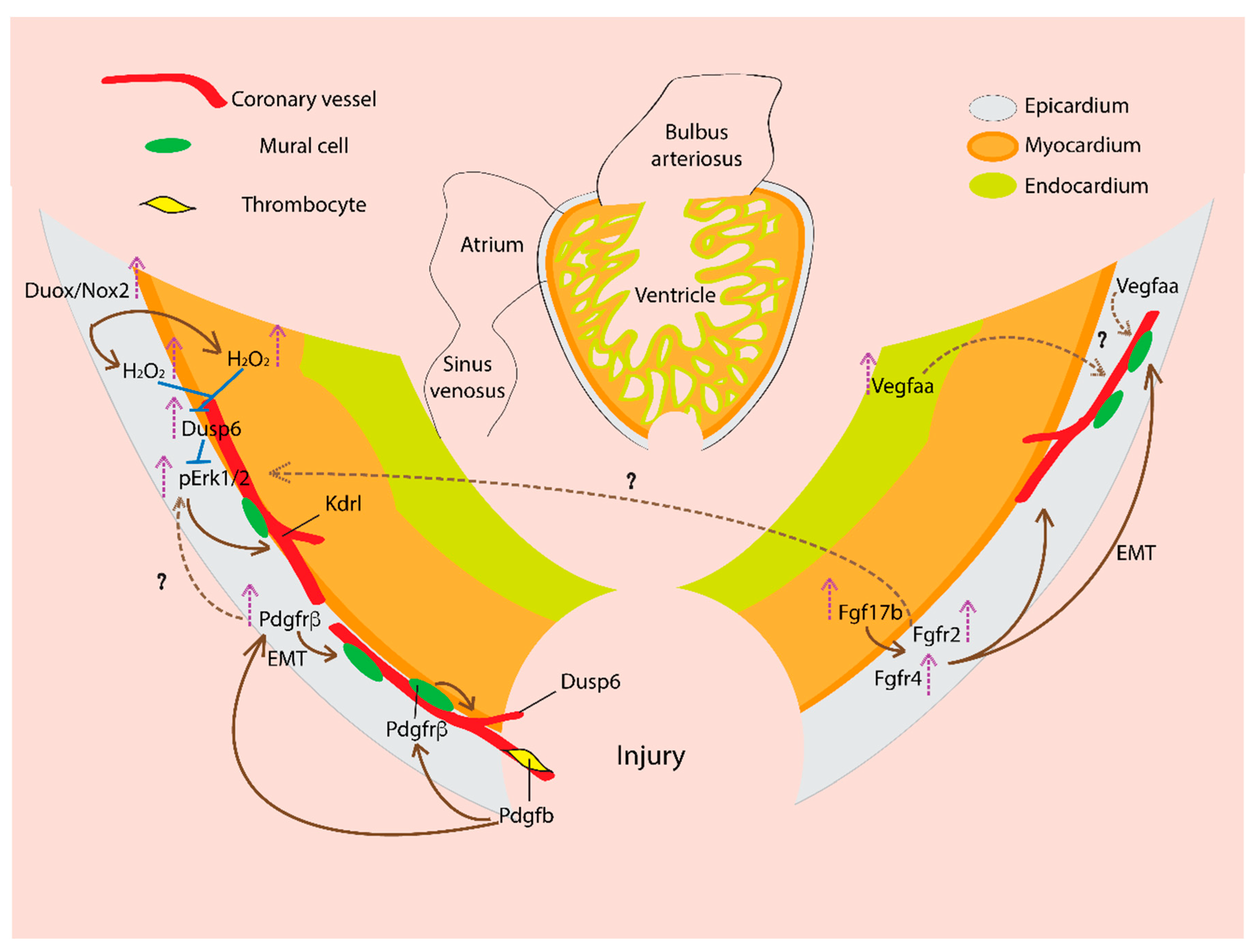
5. Coronary Vasculature and Cardiac Regeneration: Signaling Mechanisms
5.1. Vascular Endothelial Growth Factor (VEGF)
5.2. Fibroblast Growth Factor (FGF)
5.3. Platelet-Derived Growth Factor (PDGF)
5.4. Reactive Oxygen Species (ROS)
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient Regenerative Potential of the Neonatal Mouse Heart. Science (80-) 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Welz, A.; Fleischmann, B.K. Heart regeneration and the cardiomyocyte cell cycle. Pflugers Arch. 2018, 470, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science (80-) 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Oberpriller, J.O.; Oberpriller, J.C. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974, 187, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Raya, A.; Koth, C.M.; Buscher, D.; Kawakami, Y.; Itoh, T.; Raya, R.M.; Sternik, G.; Tsai, H.-J.; Rodriguez-Esteban, C.; Izpisua-Belmonte, J.C. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl. Acad. Sci. USA 2003, 100, 11889–11895. [Google Scholar] [CrossRef] [PubMed]
- Cano-Martínez, A.; Vargas-González, A.; Guarner-Lans, V.; Prado-Zayago, E.; León-Oleda, M.; Nieto-Lima, B. Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation. Arch. Cardiol. Mex. 2010, 80, 79–86. [Google Scholar]
- Liao, S.; Dong, W.; Lv, L.; Guo, H.; Yang, J.; Zhao, H.; Huang, R.; Yuan, Z.; Chen, Y.; Feng, S.; et al. Heart regeneration in adult Xenopus tropicalis after apical resection. Cell Biosci. 2017, 7, 70. [Google Scholar] [CrossRef]
- Marín-Juez, R.; Marass, M.; Gauvrit, S.; Rossi, A.; Lai, S.-L.; Materna, S.C.; Black, B.L.; Stainier, D.Y.R. Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 11237–11242. [Google Scholar] [CrossRef]
- Ingason, A.B.; Goldstone, A.B.; Paulsen, M.J.; Thakore, A.D.; Truong, V.N.; Edwards, B.B.; Eskandari, A.; Bollig, T.; Steele, A.N.; Woo, Y.J. Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts. J. Thorac. Cardiovasc. Surg. 2018, 155, 1118–1127.el. [Google Scholar] [CrossRef]
- Farrell, A.P.; Farrell, N.D.; Jourdan, H.; Cox, G.K. A Perspective on the Evolution of the Coronary Circulation in Fishes and the Transition to Terrestrial Life. In Ontogeny and Phylogeny of the Vertebrate Heart; Springer: New York, NY, USA, 2012; pp. 75–102. [Google Scholar]
- Tota, B. Myoarchitecture and vascularization of the elasmobranch heart ventricle. J. Exp. Zool. 1989, 252, 122–135. [Google Scholar] [CrossRef]
- Santer, R.M. Morphology and Innervation of the Fish Heart; Springer: Berlin, Germany, 1985; ISBN 9783540139959. [Google Scholar]
- Santer, R.M.; Walker, M.G. Morphological studies on the ventricle of teleost and elasmobranch hearts. J. Zool. 2010, 190, 259–272. [Google Scholar] [CrossRef]
- Patra, C.; Kontarakis, Z.; Kaur, H.; Rayrikar, A.; Mukherjee, D.; Stainier, D.Y.R. The zebrafish ventricle: A hub of cardiac endothelial cells for in vitro cell behavior studies. Sci. Rep. 2017, 7, 2687. [Google Scholar] [CrossRef] [PubMed]
- Pombal, M.A.; Carmona, R.; Megías, M.; Ruiz, A.; Pérez-Pomares, J.M.; Muñoz-Chápuli, R. Epicardial development in lamprey supports an evolutionary origin of the vertebrate epicardium from an ancestral pronephric external glomerulus. Evol. Dev. 2008, 10, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Poss, K.D. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 2018, 15, 1–17. [Google Scholar] [CrossRef]
- Simões, F.C.; Riley, P.R. The ontogeny, activation and function of the epicardium during heart development and regeneration. Development 2018, 145, dev155994. [Google Scholar] [CrossRef]
- Männer, J.; Pérez-Pomares, J.M.; Macías, D.; Muñoz-Chápuli, R. The Origin, Formation and Developmental Significance of the Epicardium: A Review. Cells Tissues Organs 2001, 169, 89–103. [Google Scholar] [CrossRef]
- De Oliveira Silva-Junior, G.; da Silva Miranda, S.W.; Mandarim-de-Lacerda, C.A. Origin and Development of the Coronary Arteries. Int. J. Morphol. 2009, 27, 891–898. [Google Scholar] [CrossRef]
- Sharma, B.; Chang, A.; Red-Horse, K. Coronary Artery Development: Progenitor Cells and Differentiation Pathways. Annu Rev Physiol. 2017, 79, 1–19. [Google Scholar] [CrossRef]
- Ito, K.; Morioka, M.; Kimura, S.; Tasaki, M.; Inohaya, K.; Kudo, A. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev. Dyn. 2014, 243, 1106–1115. [Google Scholar] [CrossRef]
- Lai, S.-L.; Marín-Juez, R.; Moura, P.L.; Kuenne, C.; Lai, J.K.H.; Tsedeke, A.T.; Guenther, S.; Looso, M.; Stainier, D.Y. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.S. The development of the blood supply to the heart in the embryo pig. Am. J. Anat. 1936, 60, 27–53. [Google Scholar] [CrossRef]
- Goldsmith, J.B.; Butler, H.W. The development of the cardiac-coronary circulatory system. Am. J. Anat. 1937, 60, 185–201. [Google Scholar] [CrossRef]
- Waldo, K.L.; Willner, W.; Kirby, M.L. Origin of the proximal coronary artery stems and a review of ventricular vascularization in the chick embryo. Am. J. Anat. 1990, 188, 109–120. [Google Scholar] [CrossRef]
- Mikawa, T.; Fischman, D.A. Retroviral analysis of cardiac morphogenesis: Discontinuous formation of coronary vessels. Proc. Natl. Acad. Sci. USA 1992, 89, 9504–9508. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Gittenberger-de Groot, A.C.; Mentink, M.M.; Bökenkamp, R.; Hogers, B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ. Res. 1993, 73, 559–568. [Google Scholar] [CrossRef]
- Männer, J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 1999, 255, 212–226. [Google Scholar] [CrossRef]
- Mikawa, T.; Gourdie, R.G. Pericardial Mesoderm Generates a Population of Coronary Smooth Muscle Cells Migrating into the Heart along with Ingrowth of the Epicardial Organ. Dev. Biol. 1996, 174, 221–232. [Google Scholar] [CrossRef]
- Pérez-Pomares, J.-M.; Carmona, R.; González-Iriarte, M.; Atencia, G.; Wessels, A.; Muñoz-Chápuli, R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 2002, 46, 1005–1013. [Google Scholar]
- Pérez-Pomares, J.M.; Phelps, A.; Sedmerova, M.; Carmona, R.; González-Iriarte, M.; Muñoz-Chápuli, R.; Wessels, A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: A model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs). Dev. Biol. 2002, 247, 307–326. [Google Scholar] [CrossRef]
- Red-Horse, K.; Ueno, H.; Weissman, I.L.; Krasnow, M.A. Coronary arteries form by developmental reprogramming of venous cells. Nature 2010, 464, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, Z.; Lui, W.; Chen, X.; Wang, Y.; Chamberlain, A.A.; Moreno-Rodriguez, R.A.; Markwald, R.R.; O’Rourke, B.P.; Sharp, D.J.; et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 2012, 151, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Hu, T.; Zhang, H.; He, L.; Huang, X.; Liu, Q.; Yu, W.; He, L.; Yang, Z.; Zhang, Z.; et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013, 23, 1075–1090. [Google Scholar] [CrossRef]
- Chen, H.I.; Sharma, B.; Akerberg, B.N.; Numi, H.J.; Kivela, R.; Saharinen, P.; Aghajanian, H.; McKay, A.S.; Bogard, P.E.; Chang, A.H.; et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 2014, 141, 4500–4512. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Weaver, C.; Koyano-Nakagawa, N.; Garry, D.J. Visualizing Vascular Structure in the Adult Newt (Notophthalmus viridescens) Heart1. J. Med. Devices 2015, 9, 20912–20917. [Google Scholar]
- Ghiara, P.; Parente, L.; Piomelli, D. The cyclo-oxygenase pathway in the avascular heart of the frog, Rana esculenta L. Gen. Pharmacol. Vasc. Syst. 1984, 15, 309–313. [Google Scholar] [CrossRef]
- Grant, R.T.; Regnier, M. The comparative anatomy of the cardiac coronary vessels. Heart 1926, 13, 285. [Google Scholar]
- Foxon, G.E.H. Problems of the double circulation in vertebrates. Biol. Rev. 1955, 30, 196–228. [Google Scholar] [CrossRef]
- Sedmera, D.; Wang, T. Ontogeny and Phylogeny of the Vertebrate Heart; Springer: New York, NY, USA, 2012. [Google Scholar]
- De Andrés, A.V.; Muñoz-Chápuli, R.; Sans-Coma, V. Development of the coronary arteries and cardiac veins in the dogfish (Scyliorhinus canicula). Anat. Rec. 1993, 235, 436–442. [Google Scholar] [CrossRef]
- Muñoz-Chápuli, R.; Macías, D.; Ramos, C.; Gallego, A.; De Andrés, V. Development of the subepicardial mesenchyme and the early cardiac vessels in the dogfish (Scyliorhinus canicula). J. Exp. Zool. 1996, 275, 95–111. [Google Scholar] [CrossRef]
- Hu, N.; Sedmera, D.; Yost, H.J.; Clark, E.B. Structure and function of the developing zebrafish heart. Anat. Rec. 2000, 260, 148–157. [Google Scholar] [CrossRef]
- Harrison, M.R.M.; Bussmann, J.; Huang, Y.; Zhao, L.; Osorio, A.; Burns, C.G.; Burns, C.E.; Sucov, H.M.; Siekmann, A.F.; Lien, C.-L. Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish. Dev. Cell 2015, 33, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Shifatu, O.; Glasshagel-Chilson, S.; Nelson, H.; Patel, P.; Tomamichel, W.; Higginbotham, C.; Evans, P.; Lafontant, G.; Burns, A.; Lafontant, P. Heart Development, Coronary Vascularization and Ventricular Maturation in a Giant Danio (Devario malabaricus). J. Dev. Biol. 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Vrancken Peeters, M.-P.F.M.; Gittenberger-de Groot, A.C.; Mentink, M.M.T.; Hungerford, J.E.; Little, C.D.; Poelmann, R.E. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev. Dyn. 1997, 208, 338–348. [Google Scholar] [CrossRef]
- Patterson, A.J.; Zhang, L. Hypoxia and fetal heart development. Curr. Mol. Med. 2010, 10, 653–666. [Google Scholar] [CrossRef]
- Wikenheiser, J.; Doughman, Y.-Q.; Fisher, S.A.; Watanabe, M. Differential levels of tissue hypoxia in the developing chicken heart. Dev. Dyn. 2006, 235, 115–123. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, R.J.; Ratajska, A.; Kitten, G.T.; Yue, X.; Sandra, A. Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev. Dyn. 1999, 215, 54–61. [Google Scholar] [CrossRef]
- Yamashita, J.; Itoh, H.; Hirashima, M.; Ogawa, M.; Nishikawa, S.; Yurugi, T.; Naito, M.; Nakao, K.; Nishikawa, S.-I. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000, 408, 92–96. [Google Scholar] [CrossRef]
- Miquerol, L.; Gertsenstein, M.; Harpal, K.; Rossant, J.; Nagy, A. Multiple Developmental Roles of VEGF Suggested by a LacZ-Tagged Allele. Dev. Biol. 1999, 212, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Langille, B.L.; Nagy, A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 2000, 127, 3941–3946. [Google Scholar] [PubMed]
- Karra, R.; Foglia, M.J.; Choi, W.-Y.; Belliveau, C.; DeBenedittis, P.; Poss, K.D. Vegfaa instructs cardiac muscle hyperplasia in adult zebrafish. Proc. Natl. Acad. Sci. USA 2018, 115, 8805–8810. [Google Scholar] [CrossRef]
- Guadix, J.A.; Carmona, R.; Muñoz-Chápuli, R.; Pérez-Pomares, J.M. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev. Dyn. 2006, 235, 1014–1026. [Google Scholar] [CrossRef]
- Lavine, K.J.; Yu, K.; White, A.C.; Zhang, X.; Smith, C.; Partanen, J.; Ornitz, D.M. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev. Cell 2005, 8, 85–95. [Google Scholar] [CrossRef]
- Merki, E.; Zamora, M.; Raya, A.; Kawakami, Y.; Wang, J.; Zhang, X.; Burch, J.; Kubalak, S.W.; Kaliman, P.; Izpisua Belmonte, J.C.; et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl. Acad. Sci. USA 2005, 102, 18455–18460. [Google Scholar] [CrossRef]
- Lavine, K.J.; White, A.C.; Park, C.; Smith, C.S.; Choi, K.; Long, F.; Hui, C.; Ornitz, D.M. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006, 20, 1651–1666. [Google Scholar] [CrossRef]
- Arita, Y.; Nakaoka, Y.; Matsunaga, T.; Kidoya, H.; Yamamizu, K.; Arima, Y.; Kataoka-Hashimoto, T.; Ikeoka, K.; Yasui, T.; Masaki, T.; et al. Myocardium-derived angiopoietin-1 is essential for coronary vein formation in the developing heart. Nat. Commun. 2014, 5, 4552. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.H.; Raftrey, B.C.; D’Amato, G.; Surya, V.N.; Poduri, A.; Chen, H.I.; Goldstone, A.B.; Woo, J.; Fuller, G.G.; Dunn, A.R.; et al. DACH1 stimulates shear stress-guided endothelial cell migration and coronary artery growth through the CXCL12–CXCR4 signaling axis. Genes Dev. 2017, 31, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Ho, L.; Ford, G.H.; Chen, H.I.; Goldstone, A.B.; Woo, Y.J.; Quertermous, T.; Reversade, B.; Red-Horse, K. Alternative Progenitor Cells Compensate to Rebuild the Coronary Vasculature in Elabela- and Apj-Deficient Hearts. Dev. Cell 2017, 42, 655–666. [Google Scholar] [CrossRef]
- Serluca, F.C. Development of the proepicardial organ in the zebrafish. Dev. Biol. 2008, 315, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Jahr, M.; Schlueter, J.; Brand, T.; Männer, J. Development of the proepicardium in Xenopus laevis. Dev. Dyn. 2008, 237, 3088–3096. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.E.; Lemanski, L.F. Epicardial development in the axolotl, ambystoma mexicanum. Anat. Rec. 1990, 226, 228–236. [Google Scholar] [CrossRef]
- Männer, J. The development of pericardial villi in the chick embryo. Anat. Embryol. 1992, 186, 379–385. [Google Scholar] [CrossRef]
- Viragh, S.; Challice, C.E. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat. Rec. 1981, 201, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Ito, K.; Shimada, Y. Origin and development of the epicardium in the mouse embryo. Anat. Embryol. 1987, 176, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hirakow, R. Epicardial formation in staged human embryos. Kaibogaku Zasshi 1992, 67, 616–622. [Google Scholar]
- Risebro, C.A.; Vieira, J.M.; Klotz, L.; Riley, P.R. Characterisation of the human embryonic and foetal epicardium during heart development. Development 2015, 142, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Schulte, I.; Schlueter, J.; Abu-Issa, R.; Brand, T.; Männer, J. Morphological and molecular left–right asymmetries in the development of the proepicardium: A comparative analysis on mouse and chick embryos. Dev. Dyn. 2007, 236, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.; Steed, E.; Harlepp, S.; González-Rosa, J.M.; Monduc, F.; Ariza-Cosano, A.; Cortés, A.; Rayón, T.; Gómez-Skarmeta, J.-L.; Zapata, A.; et al. Heartbeat-Driven Pericardiac Fluid Forces Contribute to Epicardium Morphogenesis. Curr. Biol. 2013, 23, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Nahirney, P.C.; Mikawa, T.; Fischman, D.A. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev. Dyn. 2003, 227, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Dettman, R.W.; Denetclaw, W.; Ordahl, C.P.; Bristow, J. Common Epicardial Origin of Coronary Vascular Smooth Muscle, Perivascular Fibroblasts, and Intermyocardial Fibroblasts in the Avian Heart. Dev. Biol. 1998, 193, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lie-Venema, H.; van den Akker, N.M.S.; Bax, N.A.M.; Winter, E.M.; Maas, S.; Kekarainen, T.; Hoeben, R.C.; de Ruiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Origin, Fate, and Function of Epicardium-Derived Cells (EPDCs) in Normal and Abnormal Cardiac Development. Sci. World J. 2007, 7, 1777–1798. [Google Scholar] [CrossRef]
- Von Gise, A.; Pu, W.T. Endocardial and Epicardial Epithelial to Mesenchymal Transitions in Heart Development and Disease. Circ. Res. 2012, 110, 1628–1645. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pomares, J.M.; De La Pompa, J.L. Signaling during epicardium and coronary vessel development. Circ. Res. 2011, 109, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, H.; Liu, Y.; Adams, S.; Eilken, H.; Stehling, M.; Corada, M.; Dejana, E.; Zhou, B.; Adams, R.H. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat. Commun. 2016, 7, 12422. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Gupta, V.; Wang, J.; Holdway, J.E.; Wills, A.A.; Fang, Y.; Poss, K.D. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 2011, 138, 2895–2902. [Google Scholar] [CrossRef]
- González-Rosa, J.M.; Peralta, M.; Mercader, N. Pan-epicardial lineage tracing reveals that epicardium derived cells give rise to myofibroblasts and perivascular cells during zebrafish heart regeneration. Dev. Biol. 2012, 370, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.; Johansson, B.R.; Levéen, P.; Betsholtz, C. Pericyte Loss and Microaneurysm Formation in PDGF-B-Deficient Mice. Science (80-) 1997, 277, 242–245. [Google Scholar] [CrossRef]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [PubMed]
- Mellgren, A.M.; Smith, C.L.; Olsen, G.S.; Eskiocak, B.; Zhou, B.; Kazi, M.N.; Ruiz, F.R.; Pu, W.T.; Tallquist, M.D. Platelet-Derived Growth Factor Receptor β Signaling Is Required for Efficient Epicardial Cell Migration and Development of Two Distinct Coronary Vascular Smooth Muscle Cell Populations. Circ. Res. 2008, 103, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Baek, S.T.; Sung, C.Y.; Tallquist, M.D. Epicardial derived cell epithelial to mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 2011, 108, e15–e26. [Google Scholar] [CrossRef] [PubMed]
- Volz, K.S.; Jacobs, A.H.; Chen, H.I.; Poduri, A.; McKay, A.S.; Riordan, D.P.; Kofler, N.; Kitajewski, J.; Weissman, I.; Red-Horse, K. Pericytes are progenitors for coronary artery smooth muscle. Elife 2015, 4, 1–22. [Google Scholar] [CrossRef]
- Del Monte, G.; Casanova, J.C.; Guadix, J.A.; MacGrogan, D.; Burch, J.B.E.; Pérez-Pomares, J.M.; de la Pompa, J.L. Differential Notch Signaling in the Epicardium Is Required for Cardiac Inflow Development and Coronary Vessel Morphogenesis. Circ. Res. 2011, 108, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Grieskamp, T.; Rudat, C.; Lüdtke, T.H.-W.; Norden, J.; Kispert, A. Notch Signaling Regulates Smooth Muscle Differentiation of Epicardium-Derived Cells. Circ. Res. 2011, 108, 813–823. [Google Scholar] [CrossRef]
- Lepilina, A.; Coon, A.N.; Kikuchi, K.; Holdway, J.E.; Roberts, R.W.; Burns, C.G.; Poss, K.D. A Dynamic Epicardial Injury Response Supports Progenitor Cell Activity during Zebrafish Heart Regeneration. Cell 2006, 127, 607–619. [Google Scholar] [CrossRef]
- Lien, C.-L.; Schebesta, M.; Makino, S.; Weber, G.J.; Keating, M.T. Gene expression analysis of zebrafish heart regeneration. PLoS Biol. 2006, 4, e260. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wu, Q.; Zhang, Y.; Wiens, K.M.; Huang, Y.; Rubin, N.; Shimada, H.; Handin, R.I.; Chao, M.Y.; Tuan, T.-L.; et al. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc. Natl. Acad. Sci. USA 2010, 107, 17206–17210. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhou, X.-H.; Chang, N.; Xiao, C.-L.; Yan, S.; Ren, H.; Yang, X.-Z.; Zhang, M.-L.; Wu, Q.; Tang, B.; et al. Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res. 2014, 24, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Missinato, M.A.; Saydmohammed, M.; Zuppo, D.A.; Rao, K.S.; Opie, G.W.; Kühn, B.; Tsang, M. Dusp6 attenuates Ras/MAPK signaling to limit zebrafish heart regeneration. Development 2018, 145, dev157206. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, K.; Wu, C.-C.; Kurth, T.; Weidinger, G. Regeneration of Cryoinjury Induced Necrotic Heart Lesions in Zebrafish Is Associated with Epicardial Activation and Cardiomyocyte Proliferation. PLoS ONE 2011, 6, e18503. [Google Scholar] [CrossRef] [PubMed]
- González-Rosa, J.M.; Martín, V.; Peralta, M.; Torres, M.; Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011, 138, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, J.; Dickson, A.L.; Poss, K.D. Epicardial regeneration is guided by cardiac outflow tract and Hedgehog signalling. Nature 2015, 522, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Karra, R.; Knecht, A.K.; Kikuchi, K.; Poss, K.D. Myocardial NF-κB activation is essential for zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 13255–13260. [Google Scholar] [CrossRef]
- Huang, Y.; Harrison, M.R.; Osorio, A.; Kim, J.; Baugh, A.; Duan, C.; Sucov, H.M.; Lien, C.-L. Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. PLoS ONE 2013, 8, e67266. [Google Scholar] [CrossRef] [PubMed]
- Sallin, P.; Jaźwińska, A. Acute stress is detrimental to heart regeneration in zebrafish. Open Biol. 2016, 6, 160012. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, W.; Castillo, H.A.; Kane, M.A.; Xavier-Neto, J.; Trainor, P.A.; Moise, A.R. Alterations in retinoic acid signaling affect the development of the mouse coronary vasculature. Dev. Dyn. 2018, 247, 976–991. [Google Scholar] [CrossRef]
- Kikuchi, K.; Holdway, J.E.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, G.; Poss, K.D. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
| Organism | Coronary Endothelial Origin | Mechanism | Experimental Approach | Reference | |
|---|---|---|---|---|---|
| Fish | Zebrafish (Danio rerio) | Angiogenic sprouting from the endocardial derived cells at the AV junction | Angiogenesis | Genetic lineage tracing, multispectral clonal analysis using ubb:zebrabow. | [47] |
| Giant danio (Devario malabaricus) | Extension of a hypobranchial vessel from BA; Angiogenic sprouting from endocardium (?) at the AV junction | Angiogenesis? | Whole mount studies; BS lectin histochemistry | [48] | |
| Blue Gourami (Trichogaster tricopterus) | Extension of one or many hypobranchial vessels from BA | Angiogenesis? | Whole mount studies; BS lectin histochemistry | [48] | |
| Dogfish (Scyliorhinus canicula) | Diverticulum from the sinus venosus Epicardium derived subepicardial mesenchyme | Angiogenesis Vasculogenesis | Tissue section based morphological studies; Fibronectin imunohistochemistry | [44,45] | |
| Bird | Chick (Gallus gallus) | Proepicardium and Epicardium derived EPDCs | Vasculogenesis | Ink/retroviral tagging /chick-quail chimera based lineage tracing studies | [28,29,30,31,32,33] |
| Quail (Coturnix coturnix japonica) | Extension of the vessels from the sinus venosus | Angiogenesis | Tissue section based immunohistochemistry using Quail endothelium specific QH1 antibody | [49] | |
| Mammal | Mouse (Mus musculus) | Mostly sinus venosus and endocardium; minor contribution from epicardium | Angiogenesis | Cre-LoxP based genetic lineage tracing studies | [34,35,36,37,38] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapuria, S.; Yoshida, T.; Lien, C.-L. Coronary Vasculature in Cardiac Development and Regeneration. J. Cardiovasc. Dev. Dis. 2018, 5, 59. https://doi.org/10.3390/jcdd5040059
Kapuria S, Yoshida T, Lien C-L. Coronary Vasculature in Cardiac Development and Regeneration. Journal of Cardiovascular Development and Disease. 2018; 5(4):59. https://doi.org/10.3390/jcdd5040059
Chicago/Turabian StyleKapuria, Subir, Tyler Yoshida, and Ching-Ling Lien. 2018. "Coronary Vasculature in Cardiac Development and Regeneration" Journal of Cardiovascular Development and Disease 5, no. 4: 59. https://doi.org/10.3390/jcdd5040059
APA StyleKapuria, S., Yoshida, T., & Lien, C.-L. (2018). Coronary Vasculature in Cardiac Development and Regeneration. Journal of Cardiovascular Development and Disease, 5(4), 59. https://doi.org/10.3390/jcdd5040059





