Predictors of Five-Year Outcomes in Patients with Acute Coronary Syndromes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Residual Risk and Clinical Endpoints
2.3. Coronary Angiographic Analysis
2.4. Biohumoral Data
2.5. Statistical Analysis
3. Results
3.1. Clinical and Angiographic Features Associated with the Four Residual Risk Determinants
3.1.1. Sex-Related Differences
3.1.2. Clinical Presentation
3.1.3. Diabetes Mellitus Presence
3.1.4. Chronic Kidney Disease Presence
3.2. Laboratory Results Associated with the 4 Residual Risk Determinants
3.2.1. Sex-Related Differences
3.2.2. Clinical Presentation
3.2.3. Diabetes Mellitus Presence
3.2.4. Chronic Kidney Disease Presence
3.2.5. LDL-Cholesterol Reduction in the 4 Residual Risk Determinant Groups
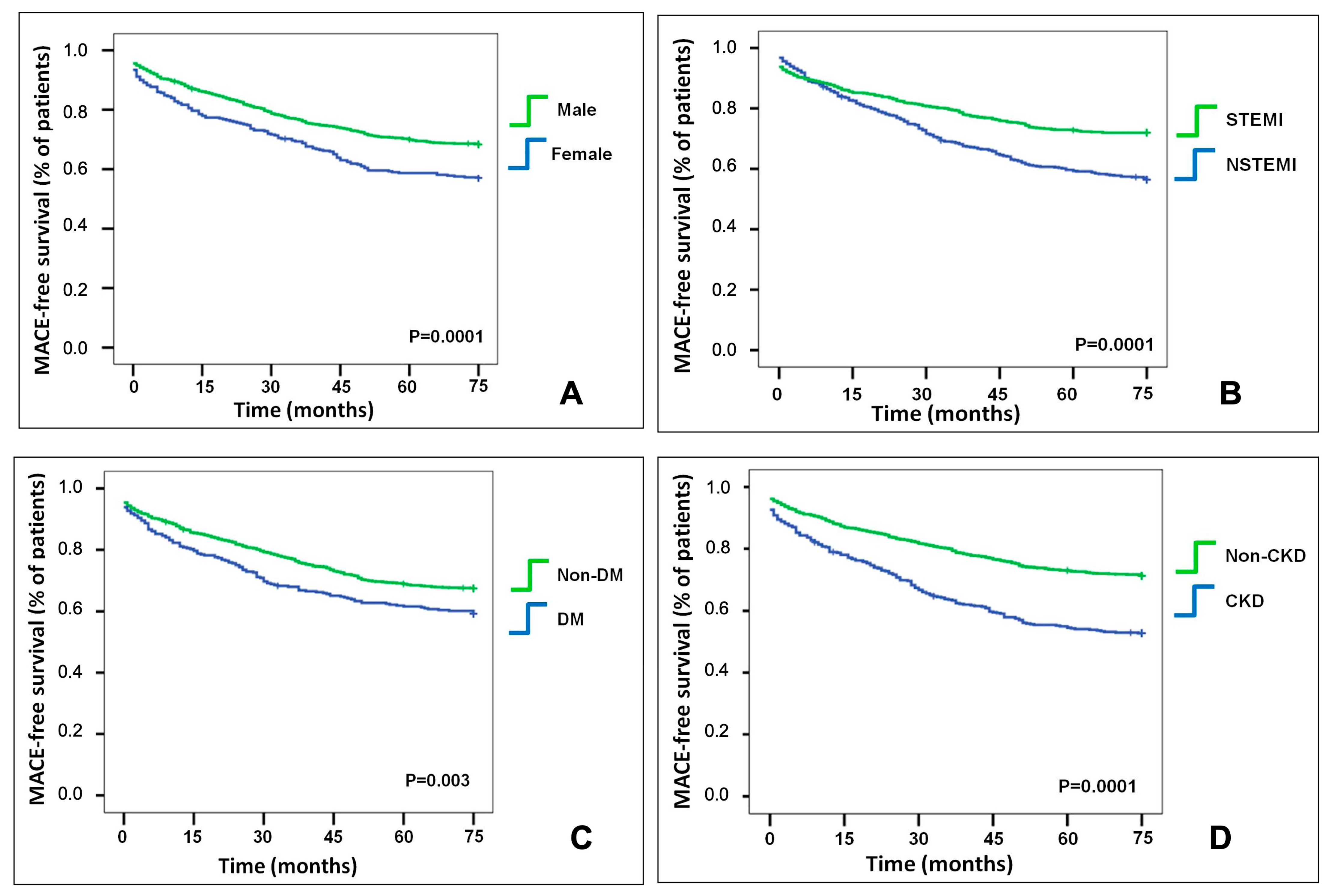
3.3. MACE Association with the Determinants of the Residual Risk
3.3.1. Sex-Related Differences
3.3.2. Clinical Presentation
3.3.3. Diabetes Mellitus Presence
3.3.4. Chronic Kidney Disease Presence
3.4. Predictors of MACE
4. Discussion
- The four residual risk determinants (female sex, NSTEMI, DM, and CKD) clustered and were associated with advanced age.
- NSTEMI, DM, and creatinine levels were independent predictors of MACE, while female sex was not. A lower hemoglobin level at admission was an independent predictor of MACE.
4.1. Clustering of Risk Factors and Sex Differences in LDL-Cholesterol Reduction and MACEs
4.2. Angiographic and Biohumoral Data Associated with the Four Determinants of Residual Risk and Clinical Outcomes
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| ANOVA | analysis of variance |
| CKD | chronic kidney disease |
| DM | diabetes mellitus |
| DS | diameter stenosis |
| eGFR | estimated glomerular filtration rate |
| KDIGO | kidney disease improving global outcomes |
| LM | left main |
| MACE | major adverse cardiovascular events |
| MDRD | modification of diet in renal disease |
| MLD | minimum lumen diameter |
| NSTEMI | non-ST-segment-elevation myocardial infarction |
| OCT | optical coherence tomography |
| PCI | percutaneous coronary intervention |
| RCE | recurrent coronary event |
| RVD | reference vessel diameter |
| SAGER | Sex and Gender Equity in Research |
| STEMI | ST-segment–elevation myocardial infarction |
References
- Mach, F.; Visseren, F.L.J.; Cater, N.B.; Salhi, N.; Soronen, J.; Ray, K.K.; Delgado, V.; Jukema, J.W.; Laufs, U.; Zamorano, J.-L.; et al. Addressing residual risk beyond statin therapy: New targets in the management of Dyslipidaemias-A report from the European Society of Cardiology Cardiovascular Round Table. J. Clin. Lipidol. 2024, 18, e685–e700. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, L.; Di Giusto, F.; Bruscoli, F.; Scalone, G.; Silenzi, S.; Mariani, L.; Selimi, A.; Delfino, D.; Grossi, P. Recurrent events after acute ST-Segment elevation myocardial infarction: Predictors and features of plaque progression and stent failure. Coron. Artery Dis. 2024, 35, 277–285. [Google Scholar] [CrossRef]
- Gouda, P.; Savu, A.; Bainey, K.R.; Kaul, P.; Welsh, R.C. Long-Term risk of death and recurrent cardiovascular events following acute coronary syndromes. PLoS ONE 2021, 16, e0254008. [Google Scholar] [CrossRef]
- Nohara, A. The Worst Combination for Coronary Artery Disease Under Strong-Statin Therapy Is Chronic Kidney Disease and Diabetes—Non-Lipid Residual Risk Assessment From the REAL-CAD Study. Circ. J. 2024, 88, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Collins, B.L.; Singh, A.; Biery, D.W.; Fatima, A.; Qamar, A.; Berman, A.N.; Gupta, A.; Cawley, M.; Wood, M.J.; et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: The Mass General Brigham YOUNG-MI registry. Eur. Heart J. 2020, 41, 4127–4137. [Google Scholar] [CrossRef]
- Tweet, M.S. Sex differences among young individuals with myocardial infarction. Eur. Heart J. 2020, 41, 4138–4140. [Google Scholar] [CrossRef]
- Mora, S.; Wenger, N.K.; Demicco, D.A.; Breazna, A.; Boekholdt, S.M.; Arsenault, B.J.; Deedwania, P.; Kastelein, J.J.P.; Waters, D.D. Determinants of residual risk in secondary prevention patients treated with High-Versus Low-Dose statin therapy: The Treating to New Targets (TNT) study. Circulation 2012, 125, 1979–1987. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kufner, S.; Cassese, S.; Joner, M.; Sager, H.B.; Xhepa, E.; Laugwitz, K.L.; Schunkert, H.; Kastrati, A. Impaired Kidney Function and 10-Year Outcome After Percutaneous Coronary Intervention-Interaction with Age, Sex, Diabetic Status and Clinical Presentation. J. Clin. Med. 2024, 13, 6833. [Google Scholar] [CrossRef] [PubMed]
- Bittner, V.A.; Schwartz, G.G.; Bhatt, D.L.; Chua, T.; De Silva, H.A.; Diaz, R.; Goodman, S.G.; Harrington, R.A.; Jukema, J.W.; McGinniss, J.; et al. Alirocumab and cardiovascular outcomes according to sex and lipoprotein(a) after acute coronary syndrome: A report from the ODYSSEY OUTCOMES study. J. Clin. Lipidol. 2024, 18, e548–e561. [Google Scholar] [CrossRef]
- Ostadal, P.; Steg, P.G.; Poulouin, Y.; Bhatt, D.L.; Bittner, V.A.; Chua, T.; Diaz, R.; Goodman, S.G.; Huo, Y.; Jukema, J.W.; et al. Metabolic risk factors and effect of alirocumab on cardiovascular events after acute coronary syndrome: A Post-Hoc analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2022, 10, 330–340. [Google Scholar] [CrossRef]
- Crea, F. New challenges in the management of acute coronary syndromes: Residual risk and Sex-Related inequalities. Eur. Heart J. 2020, 41, 4075–4078. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, J.; Liu, J.; Yang, N.; Smith, S.C.; Huo, Y.; Fonarow, G.C.; Ge, J.; Taubert, K.A.; Morgan, L.; et al. Sex Differences in In-Hospital Management and Outcomes of Patients with Acute Coronary Syndrome. Circulation 2019, 139, 1776–1785. [Google Scholar] [CrossRef]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; Wanner, C.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Pana, T.A.; Mamas, M.A.; Myint, P.K.; Dawson, D.K. Sex differences in myocardial infarction care and outcomes: A longitudinal Scottish National Data-Linkage Study. Eur. J. Prev. Cardiol. 2024, 32, 696–707. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, 485–510. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Fuchs, A.; Kuhl, J.T.; Sigvardsen, P.E.; Afzal, S.; Knudsen, A.D.; Moller, M.B.; de Knegt, C.; Sørgaard, M.H.; Nordestgaard, B.G.; Køber, L.V.; et al. Subclinical Coronary Atherosclerosis and Risk for Myocardial Infarction in a Danish Cohort: A Prospective Observational Cohort Study. Ann. Intern Med. 2023, 176, 433–442. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Lente, F.V.; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, C.J.; Kurihara, O.; Thondapu, V.; Russo, M.; Yamamoto, E.; Sugiyama, T.; Fracassi, F.; Lee, H.; Yonetsu, T.; et al. Angiographic features of patients with coronary plaque erosion. Int. J. Cardiol. 2019, 288, 12–16. [Google Scholar] [CrossRef]
- De Luca, G.; Gibson, C.M.; Gyongyosi, M.; Zeymer, U.; Dudek, D.; Arntz, H.R.; Bellandi, F.; Maioli, M.; Noc, M.; Zorman, S.; et al. Gender-Related differences in outcome after ST-Segment elevation myocardial infarction treated by primary angioplasty and glycoprotein IIb-IIIa inhibitors: Insights from the EGYPT cooperation. J. Thromb. Thrombolysis 2010, 30, 342–346. [Google Scholar] [CrossRef]
- Diercks, D.B.; Owen, K.P.; Kontos, M.C.; Blomkalns, A.; Chen, A.Y.; Miller, C.; Wiviott, S.; Peterson, E.D. Gender differences in time to presentation for myocardial infarction before and after a national women’s cardiovascular awareness campaign: A temporal analysis from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation (CRUSADE) and the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network-Get with the Guidelines (NCDR ACTION Registry-GWTG). Am. Heart J. 2010, 160, 80–87.e3. [Google Scholar]
- Gavina, C.; Araujo, F.; Teixeira, C.; Ruivo, J.A.; Corte-Real, A.L.; Luz-Duarte, L.; Canelas-Pais, M.; Taveira-Gomes, T. Sex differences in LDL-C control in a primary care population: The PORTRAIT-DYS study. Atherosclerosis 2023, 384, 117148. [Google Scholar] [CrossRef] [PubMed]
- Kotseva, K.; Wood, D.; De Backer, G.; De Bacquer, D.; Pyorala, K.; Keil, U. EUROASPIRE III: A survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Romiti, G.F.; Campolongo, G.; Ruscio, E.; Sciomer, S.; Gianfrilli, D.; Raparelli, V. Gender related differences in treatment and response to statins in primary and secondary cardiovascular prevention: The Never-Ending debate. Pharmacol. Res. 2017, 117, 148–155. [Google Scholar] [CrossRef]
- Mombelli, G.; Bosisio, R.; Calabresi, L.; Magni, P.; Pavanello, C.; Pazzucconi, F.; Sirtori, C.R. Gender-Related lipid and/or lipoprotein responses to statins in subjects in primary and secondary prevention. J. Clin. Lipidol. 2015, 9, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.F.; Tamis-Holland, J.E.; Lu, J.; Bittner, V.A.; Brooks, M.M.; Lopes, N.; Jacobs, A.K. Sex, Prescribing Practices and Guideline Recommended, Blood Pressure, and LDL Cholesterol Targets at Baseline in the BARI 2D Trial. Int. J. Endocrinol. 2015, 2015, 610239. [Google Scholar] [CrossRef]
- Petretta, M.; Costanzo, P.; Perrone-Filardi, P.; Chiariello, M. Impact of gender in primary prevention of coronary heart disease with statin therapy: A Meta-Analysis. Int. J. Cardiol. 2010, 138, 25–31. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Sugiyama, T.; Yamamoto, E.; Bryniarski, K.; Xing, L.; Fracassi, F.; Lee, H.; Jang, I. Coronary Plaque Characteristics in Patients with Diabetes Mellitus Who Presented with Acute Coronary Syndromes. J. Am. Heart Assoc. 2018, 7, e009245. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Stone, G.W.; Weisz, G.; Moreno, P.; Dangas, G.; Maehara, A.; Mintz, G.S.; Cristea, E.; Fahy, M.; Xu, K.; et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc. Imaging 2012, 5, S53–S61. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yonetsu, T.; Jia, H.; Abtahian, F.; Vergallo, R.; Hu, S.; Tian, J.; Kim, S.-J.; Lee, H.; McNulty, I.; et al. Nonculprit coronary plaque characteristics of chronic kidney disease. Circ. Cardiovasc. Imaging 2013, 6, 448–456. [Google Scholar] [CrossRef]
- Seegers, L.M.; Araki, M.; Nakajima, A.; Yonetsu, T.; Minami, Y.; Ako, J.; Soeda, T.; Kurihara, O.; Higuma, T.; Kimura, S.; et al. Sex Differences in Culprit Plaque Characteristics Among Different Age Groups in Patients with Acute Coronary Syndromes. Circ. Cardiovasc. Interv. 2022, 15, e011612. [Google Scholar] [CrossRef]
- Burke, A.P.; Farb, A.; Malcom, G.; Virmani, R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am. Heart J. 2001, 141, S58–S62. [Google Scholar] [CrossRef]
- Wester, A.; Attar, R.; Mohammad, M.A.; Andell, P.; Hofmann, R.; Jensen, J.; Szummer, K.; Erlinge, D.; Koul, S. Impact of Baseline Anemia in Patients with Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: A Prespecified Analysis From the VALIDATE-SWEDEHEART Trial. J. Am. Heart Assoc. 2019, 8, e012741. [Google Scholar] [CrossRef]
- Lawler, P.R.; Filion, K.B.; Dourian, T.; Atallah, R.; Garfinkle, M.; Eisenberg, M.J. Anemia and mortality in acute coronary syndromes: A systematic review and Meta-Analysis. Am. Heart J. 2013, 165, 143–153.e5. [Google Scholar] [CrossRef] [PubMed]
- Wischmann, P.; Kuhn, V.; Suvorava, T.; Muessig, J.M.; Fischer, J.W.; Isakson, B.E.; Haberkorn, S.M.; Flögel, U.; Schrader, J.; Jung, C.; et al. Anaemia is associated with severe RBC dysfunction and a reduced circulating NO pool: Vascular and cardiac eNOS are crucial for the adaptation to anaemia. Basic Res. Cardiol. 2020, 115, 43. [Google Scholar] [CrossRef]
- Jung, C.; Rezar, R.; Wischmann, P.; Masyuk, M.; Datz, C.; Bruno, R.R.; Kelm, M.; Wernly, B. The role of anemia on admission in acute coronary Syndrome—An umbrella review of systematic reviews and Meta-Analyses. Int. J. Cardiol. 2022, 367, 1–10. [Google Scholar] [CrossRef]
- Yamamoto, M.H.; Yamashita, K.; Matsumura, M.; Fujino, A.; Ishida, M.; Ebara, S.; Okabe, T.; Saito, S.; Hoshimoto, K.; Amemiya, K.; et al. Serial 3-Vessel Optical Coherence Tomography and Intravascular Ultrasound Analysis of Changing Morphologies Associated with Lesion Progression in Patients with Stable Angina Pectoris. Circ. Cardiovasc. Imaging 2017, 10, e006347. [Google Scholar] [CrossRef]
- Uemura, S.; Ishigami, K.; Soeda, T.; Okayama, S.; Sung, J.H.; Nakagawa, H.; Somekawa, S.; Takeda, Y.; Kawata, H.; Horii, M.; et al. Thin-Cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur. Heart J. 2012, 33, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K. Plaque Progression: Slow Linear or Rapid Stepwise? Circ. Cardiovasc. Imaging 2017, 10, e006964. [Google Scholar] [CrossRef] [PubMed]

| Male Sex, n (%) | 1056 (75.2) |
| Age, years | 68.6 (12.3) |
| STEMI, n (%) | 816 (58.1) |
| NSTEMI, n (%) | 589 (41.9) |
| CKD, n (%) | 446 (31.7) |
| DM, n (%) | 344 (24.5) |
| Dyslipidemia, n (%) | 677 (48.2) |
| Smoking status | |
| Never, n (%) | 866 (61.6) |
| Present, n (%) | 296 (21.1) |
| Past, n (%) | 243 (17.3) |
| BMI, Kg/m2 | 27.3 (4.4) |
| Weight, Kg | 78.1 (15.3) |
| Height, cm | 168.8 (8.7) |
| Treatment | |
| Aspirin, n (%) | 1177 (83.8) |
| Clopidogrel, n (%) | 565 (40.2) |
| Prasugrel, n (%) | 424 (30.2) |
| Ticagrelor, n (%) | 348 (24.8) |
| High-intensity statin, n (%) | 1311 (93.3) |
| Ezetimibe, n (%) | 373 (26.5) |
| Insulin, n (%) | 123 (8.8) |
| OAD drug, n (%) | 200 (14.2) |
| Total N° of Coronary Stenoses | 3.2 (1.6) |
| N° of Culprit stenoses | 1.5 (0.7) |
| Culprit lesion stenosis | 93.2 (8.6) |
| Culprit RVD, mm | 3.2 (0.5) |
| Culprit MLD, mm | 0.26 (0.26) |
| Culprit stenosis length, mm | 27.6 (14.8) |
| Culprit vessel | |
| LM, n (%) | 29 (2.1) |
| LAD, n (%) | 637 (45.3) |
| LCx, n (%) | 303 (21.5) |
| RCA, n (%) | 436 (31) |
| Bifurcation, n (%) | 406 (28.9%) |
| N° of deployed stent | 1.3 (0.7) |
| Stent diameter, mm | 3.2 (0.4) |
| Non-culprit lesion stenosis, % | 51.6 (18.0) |
| N° of non-culprit stenosis | 1.9 (1.5) |
| LDL-Cholesterol, (mg/dL) | 124.4 (36.8) |
| HDL-cholesterol, (mg/dL) | 43.4 (15.8) |
| Triglycerides, (mg/dL) | 132.5 (76.2) |
| Creatinine, (mg/dL) | 1.2 (4.0) |
| Glycemia, (mg/dL) | 135.8 (58.7) |
| HbA1c, % | 7.1 (1.6) |
| Platelets, (1000/µL) | 227.6 (71.4) |
| Hemoglobin, (g/dL) | 13.8 (1.9) |
| White blood cells, 1000/mm3 | 10.1 (4.1) |
| C-reactive protein, (mg/dL) | 3.3 (5.5) |
| Fibrinogen, (mg/dL) | 476.4 (163.5) |
| Uric acid, (mg/dL) | 6.1 (1.7) |
| Troponin I, (ng/L) | 2422.3 (2773) |
| Parameter | At 1 Month | At 12 Months |
| LDL-cholesterol, (mg/dL) | 79.2 (27.6) | 75.9 (25.1) |
| HDL-cholesterol, (mg/dL) | 44.0 (11.5) | 44.5 (10.9) |
| Creatinine, (mg/dL) | 1.1 (0.8) | 1.2 (2.2) |
| Glycemia, (mg/dL) | 111.8 (35.1) | 114.6 (36.5) |
| HBA1c, % | 6.6 (1.2) | 6.6 (1.1) |
| Platelets, (1000/µL) | 225.5 (68.9) | 222.3 (72.4) |
| Triglycerides, (mg/dL) | 127.5 (73.2) | 124.2 (61.8) |
| Hemoglobin, (mg/dL) | 13.7 (1.7) | 13.7 (1.7) |
| Entire Cohort (1405) | Female (349) | Male (1056) | p | STEMI (816) | NSTEMI (589) | p | DM (344) | Non-DM (1061) | p | CKD (446) | Non-CKD (959) | p | |
| MACE, n (%) | 485 (34.5) | 150 (43) | 335 (31.7) | 0.0001 | 229 (28.1) | 256 (43.5) | 0.0001 | 140 (40.7) | 345 (32.5) | 0.006 | 210 (47.1) | 275 (28.7) | 0.0001 |
| Death from any cause, n (%) | 372 (26.5) | 119 (34.1) | 253 (24) | 0.001 | 188 (23) | 184 (31.2) | 0.001 | 105 (28.2) | 267 (25.2) | 0.057 | 185 (41.5) | 187 (19.5) | 0.0001 |
| RCE, n (%) | 152 (10.8) | 41 (11.7) | 111 (10.5) | 0.551 | 50 (6.1) | 102 (17.3) | 0.0001 | 46 (13.4) | 106 (10.0) | 0.089 | 49 (11) | 103 (10.7) | 0.927 |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||||
| Beta | p Value | OR | 95% CI | Beta | p Value | OR | 95% CI | |
| Female gender | −0.48 | 0.0001 | 0.61 | 0.48–0.79 | 0.32 | 0.894 | 1.38 | 0.62–3.07 |
| NSTEMI presentation | 0.67 | 0.0001 | 1.97 | 1.57–2.46 | 0.91 | 0.0001 | 2.48 | 1.29–4.79 |
| Age | 0.07 | 0.0001 | 1.07 | 1.06–1.08 | 0.01 | 0.221 | 1.01 | 0.97–1.05 |
| CKD | 0.79 | 0.0001 | 2.21 | 1.75–2.79 | 0.14 | 0.769 | 1.19 | 0.799–1.98 |
| Total N° of stenoses | 0.10 | 0.001 | 1.11 | 1.04–1.19 | 0.09 | 0.848 | 1.09 | 0.74–1.62 |
| N° of culprit stenoses | 0.15 | 0.029 | 1.17 | 1.01–1.34 | 0.21 | 0.646 | 1.23 | 0.74–2.07 |
| Stent diameter | −0.24 | 0.047 | 0.78 | 0.61–0.99 | 0.00 | 0.935 | 1.00 | 0.63–1.58 |
| NC lesion stenosis | 0.01 | 0.018 | 1.008 | 1.001–1.015 | 0.00 | 0.844 | 1.00 | 0.99–1.02 |
| Diabetes mellitus | 0.35 | 0.006 | 1.42 | 1.10–1.83 | 0.89 | 0.0001 | 2.44 | 1.66–3.56 |
| N° of NC stenoses | 0.10 | 0.004 | 1.11 | 1.03–1.19 | 0.05 | 0.797 | 1.05 | 0.72–1.53 |
| Hb at admission | −0.34 | 0.0001 | 0.70 | 0.66–0.75 | −0.116 | 0.020 | 0.89 | 0.73–0.97 |
| Weight | −0.1 | 0.0001 | 0.98 | 0.97–0.99 | 0.001 | 0.965 | 1.00 | 0.99–1.02 |
| Height | −0.4 | 0.0001 | 0.95 | 0.94–0.96 | 0.001 | 0.889 | 1.00 | 0.97–1.04 |
| Creatinine at admission | 0.01 | 0.362 | 1.01 | 0.98–1.05 | 0.43 | 0.028 | 1.54 | 1.09–2.17 |
| Glycemia at admission | 0.003 | 0.016 | 1.003 | 1.001–1.005 | 0.001 | 0.685 | 1.00 | 1.0–1.01 |
| LDL-cholesterol at admission | −0.01 | 0.0001 | 0.99 | 0.98–0.99 | −0.001 | 0.681 | 1.00 | 0.99–1.00 |
| HDL-cholesterol at admision | −0.01 | 0.024 | 0.98 | 0.97–0.99 | −0.002 | 0.898 | 0.99 | 0.97–1.01 |
| High-sensitivity troponin I | 0.001 | 0.013 | 1.000 | 1.00–1.00 | 0.001 | 0.933 | 1.00 | 1.00–1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vito, L.; Scalone, G.; Di Giusto, F.; Bruscoli, F.; Silenzi, S.; Selimi, A.; Massari, A.; Delfino, D.; Guerra, F.; Grossi, P. Predictors of Five-Year Outcomes in Patients with Acute Coronary Syndromes. J. Cardiovasc. Dev. Dis. 2025, 12, 234. https://doi.org/10.3390/jcdd12060234
Di Vito L, Scalone G, Di Giusto F, Bruscoli F, Silenzi S, Selimi A, Massari A, Delfino D, Guerra F, Grossi P. Predictors of Five-Year Outcomes in Patients with Acute Coronary Syndromes. Journal of Cardiovascular Development and Disease. 2025; 12(6):234. https://doi.org/10.3390/jcdd12060234
Chicago/Turabian StyleDi Vito, Luca, Giancarla Scalone, Federico Di Giusto, Filippo Bruscoli, Simona Silenzi, Adelina Selimi, Arianna Massari, Domenico Delfino, Federico Guerra, and Pierfrancesco Grossi. 2025. "Predictors of Five-Year Outcomes in Patients with Acute Coronary Syndromes" Journal of Cardiovascular Development and Disease 12, no. 6: 234. https://doi.org/10.3390/jcdd12060234
APA StyleDi Vito, L., Scalone, G., Di Giusto, F., Bruscoli, F., Silenzi, S., Selimi, A., Massari, A., Delfino, D., Guerra, F., & Grossi, P. (2025). Predictors of Five-Year Outcomes in Patients with Acute Coronary Syndromes. Journal of Cardiovascular Development and Disease, 12(6), 234. https://doi.org/10.3390/jcdd12060234







