Anesthesia for Minimally Invasive Coronary Artery Bypass Surgery
Abstract
1. Introduction
2. Principles of Minimally Invasive Coronary Bypass Surgery
2.1. Minimally Invasive Direct Coronary Artery Bypass Grafting (MIDCAB)
2.2. Multivessel Minimally Invasive Coronary Artery Bypass Grafting (MICS-CABG)
2.3. Total Endoscopic Coronary Artery Bypass (TECAB)
2.4. Cardiopulmonary Bypass-Assisted Minimally Invasive Coronary Artery Bypass Grafting
3. Preoperative Assessment
3.1. Cardiac
3.1.1. Coronary Arteries
3.1.2. Left Ventricular Function
3.1.3. ECG
3.1.4. Valvular Function
3.1.5. Pericardium
3.2. Respiratory
3.2.1. Suitability for One-Lung Ventilation
3.2.2. Pleural Disease
3.2.3. Pulmonary Hypertension
3.3. Vascular
3.4. Gastrointestinal
3.5. Body Habitus
3.5.1. Musculoskeletal
3.5.2. Obesity
4. Intraoperative Management
4.1. Setup
4.1.1. Operating Room Equipment
4.1.2. Airway Equipment
4.1.3. Monitoring
4.1.4. Temperature Management
4.1.5. Medications
5. Induction and Maintenance of Anesthesia
5.1. Positioning
5.2. Desaturation
5.3. Transesophageal Echocardiography
6. Conduct of Surgery
6.1. Conduit Harvesting
6.2. Grafting
6.3. Hemodynamic Management During Grafting
6.4. Postprocedure Intraoperative Management
6.5. Analgesia
7. Postoperative Management
7.1. Extubation
7.2. ICU Management
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.M.; Abu-Omar, Y. Minimally invasive cardiac surgery—A Fad or the Future? J. Thorac. Dis. 2021, 13, 1882–1885. [Google Scholar] [CrossRef] [PubMed]
- Schmitto, J.D.; Mokashi, S.A.; Cohn, L.H. Minimally-invasive valve surgery. J. Am. Coll. Cardiol. 2010, 56, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Rosengart, T.K.; Feldman, T.; Borger, M.A.; Vassiliades, T.A.; Gillinov, A.M.; Hoercher, K.J.; Vahanian, A.; Bonow, R.O.; O’Neill, W. Percutaneous and minimally invasive valve procedures: A scientific statement from the American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2008, 117, 1750–1767. [Google Scholar]
- Misfeld, M.; Brereton, R.J.L.; Sweetman, E.A.; Doig, G.S. Neurologic complications after off-pump coronary artery bypass grafting with and without aortic manipulation: Meta-analysis of 11,398 cases from 8 studies. J. Thorac. Cardiovasc. Surg. 2011, 142, 11. [Google Scholar] [CrossRef]
- Sellke, F.W.; Chu, L.M.; Cohn, W.E. Current state of surgical myocardial revascularization. Circ. J. 2010, 74, 1031–1037. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Shafti, T.Z.N.; El Al, A.A.; van Kampen, A.; Amabile, A.; Torregrossa, G.; Kempfert, J.; Falk, V.; Balkhy, H.H.; et al. Minimally Invasive Coronary Revascularisation Surgery: A Focused Review of the Available Literature. Interv. Cardiol. Rev. 2021, 16, e08. [Google Scholar] [CrossRef]
- Garg, S.; Raja, S.G. Minimally invasive direct coronary artery bypass (MIDCAB) grafting. AME Med. J. 2020, 5, 19. [Google Scholar] [CrossRef]
- Weymann, A.; Amanov, L.; Beltsios, E.; Arjomandi Rad, A.; Szczechowicz, M.; Merzah, A.S.; Ali-Hasan-Al-Saegh, S.; Schmack, B.; Ismail, I.; Popov, A.; et al. Minimally Invasive Direct Coronary Artery Bypass Grafting: Sixteen Years of Single-Center Experience. J. Clin. Med. 2024, 13, 3338. [Google Scholar] [CrossRef]
- Cremer, J.; Schoettler, J.; Thiem, A.; Grothusen, C.; Hoffmann, G. The MIDCAB approach in its various dimensions. HSR Proc. Intensive Care Cardiovasc. Anesth. 2011, 3, 249–253. [Google Scholar]
- Fortunato, G.A.; Davierwala, P.M. The current role and future perspectives of minimally invasive coronary artery bypass grafting. J. Vis. Surg. 2023, 9, 40. [Google Scholar] [CrossRef]
- Wertan, M.C.; Sicouri, S.; Yamashita, Y.; Baudo, M.; Senss, T.A.; Spragan, D.; Torregrossa, G.; Sutter, F.P. Step-by-step technique of robotic-assisted minimally invasive direct coronary artery bypass. Ann. Cardiothorac. Surg. 2024, 13, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Kettering, K.; Dapunt, O.; Baer, F.M. Minimally invasive direct coronary artery bypass grafting: A systematic review. J. Cardiovasc. Surg. 2004, 45, 255–264. [Google Scholar]
- Repossini, A.; Di Bacco, L.; Nicoli, F.; Passaretti, B.; Stara, A.; Jonida, B.; Muneretto, C. Minimally invasive coronary artery bypass: Twenty-year experience. J. Thorac. Cardiovasc. Surg. 2019, 158, 127–138.e1. [Google Scholar] [CrossRef]
- McGinn, J.T.; Usman, S.; Lapierre, H.; Pothula, V.R.; Mesana, T.G.; Ruel, M. Minimally Invasive Coronary Artery Bypass Grafting. Circulation 2009, 120, S78–S84. [Google Scholar] [CrossRef]
- Babliak, O.; Demianenko, V.; Melnyk, Y.; Revenko, K.; Pidgayna, L.; Stohov, O. Complete Coronary Revascularization via Left Anterior Thoracotomy. Innovations 2019, 14, 330–341. [Google Scholar] [CrossRef]
- Davierwala, P.M.; Verevkin, A.; Sgouropoulou, S.; Hasheminejad, E.; von Aspern, K.; Misfeld, M.; Borger, M.A. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: Early outcomes and angiographic patency. J. Thorac. Cardiovasc. Surg. 2021, 162, 1109–1119.e4. [Google Scholar] [CrossRef]
- Algoet, M.; Melvin, T.; Cerny, S.; Bonatti, J.; Singh, S.; Folliguet, T.; Modi, P.; Franke, U.; Gianoli, M.; Agnino, A.; et al. How to advance from minimally invasive coronary artery bypass grafting to totally endoscopic coronary bypass grafting: Challenges in Europe versus United States of America. Ann. Cardiothorac. Surg. 2024, 13, 39708. [Google Scholar] [CrossRef]
- Balkhy, H.H.; Nisivaco, S.M.; Hashimoto, M.; Torregrossa, G.; Grady, K. Robotic Total Endoscopic Coronary Bypass in 570 Patients: Impact of Anastomotic Technique in Two Eras. Ann. Thorac. Surg. 2022, 114, 476–482. [Google Scholar] [CrossRef]
- Lee, J.D.; Srivastava, M.; Bonatti, J. History and Current Status of Robotic Totally Endoscopic Coronary Artery Bypass. Circ. J. 2012, 76, 2058. [Google Scholar] [CrossRef]
- Davierwala, P.M.; Verevkin, A.; Bergien, L.; von Aspern, K.; Deo, S.V.; Misfeld, M.; Holzhey, D.; Borger, M.A. Twenty-year outcomes of minimally invasive direct coronary artery bypass surgery: The Leipzig experience. J. Thorac. Cardiovasc. Surg. 2023, 165, 115–127.e4. [Google Scholar] [CrossRef] [PubMed]
- Diegeler, A.; Matin, M.; Falk, V.; Binner, C.; Walther, T.; Autschbach, R.; Mohr, F.W. Indication and patient selection in minimally invasive and ‘off-pump’ coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 1999, 16 (Suppl. S1), 79. [Google Scholar] [CrossRef] [PubMed]
- Hemmerling, T.M.; Romano, G.; Terrasini, N.; Noiseux, N. Anesthesia for off-pump coronary artery bypass surgery. Ann. Card. Anaesth. 2013, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Ushioda, R.; Hirofuji, A.; Yoongtong, D.; Sakboon, B.; Cheewinmethasiri, J.; Kamiya, H.; Arayawudhikul, N. Off-pump minimally invasive coronary artery bypass grafting in patients with left ventricular dysfunction: The lampang experience. Front. Surg. 2024, 11, 1324343. [Google Scholar] [CrossRef]
- Verevkin, A.; Dashkevich, A.; Gadelkarim, I.; Shaqu, R.; Otto, W.; Sgouropoulou, S.; Ender, J.; Kiefer, P.; Borger, M.A. Minimally invasive coronary artery bypass grafting via left anterior minithoracotomy: Setup, results, and evolution of a new surgical procedure. JTCVS Tech. 2025, 29, 28–39. [Google Scholar] [CrossRef]
- Mcginn, J.; Ruel, M. MICS CABG Single-Vessel Plus Multi-Vessel Techniques. Available online: https://www.medtronic.com/content/dam/medtronic-com/c/right-procedure-cad/mics-cabg-procedure-guide.pdf (accessed on 4 March 2025).
- Alozie, A.; Öner, A.; Löser, B.; Dohmen, P.M. Minimally Invasive Direct Coronary Artery Bypass and Percutaneous Coronary Intervention Followed by Transcatheter Aortic Valve Implantation: A Promising Concept in High-risk Octogenarians. Ann. Card. Anaesth. 2023, 26, 143–148. [Google Scholar] [CrossRef]
- Kayatta, M.O.; Halkos, M.E.; Narayan, P. Minimally invasive coronary artery bypass grafting. Indian J. Thorac. Cardiovasc. Surg. 2018, 34, 302–309. [Google Scholar] [CrossRef]
- Elbadawi, A.; Hamed, M.; Elgendy, I.Y.; Omer, M.A.; Ogunbayo, G.O.; Megaly, M.; Denktas, A.; Ghanta, R.; Jimenez, E.; Brilakis, E.; et al. Outcomes of Reoperative Coronary Artery Bypass Graft Surgery in the United States. J. Am. Heart Assoc. 2020, 9, e016282. [Google Scholar] [CrossRef]
- White, A.; Patvardhan, C.; Falter, F. Anesthesia for minimally invasive cardiac surgery. J. Thorac. Dis. 2021, 13, 1886–1898. [Google Scholar] [CrossRef]
- Shum, S.; Huang, A.; Slinger, P. Hypoxaemia during one lung ventilation. BJA Educ. 2023, 23, 328–336. [Google Scholar] [CrossRef]
- Watanabe, S.; Noguchi, E.; Yamada, S.; Hamada, N.; Kano, T. Sequential Changes of Arterial Oxygen Tension in the Supine Position During One-Lung Ventilation. Anesth. Analg. 2000, 90, 28. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Bainbridge, D. Anesthetic Management in Robotic Hybrid Coronary Artery Bypass Surgery; Cheng, D.C.H., Martin, J., David, T., Eds.; Evidence-Based Practice in Perioperative Cardiac Anesthesia and Surgery; Springer International Publishing: Cham, Switzerland, 2021; pp. 41–49. [Google Scholar]
- Cao, C.; Harris, C.; Croce, B.; Cao, C. Robotic coronary artery bypass graft surgery. Ann. Cardiothorac. Surg. 2016, 5, 594. [Google Scholar] [CrossRef] [PubMed]
- Karzai, W.; Schwarzkopf, K. Hypoxemia during one-lung ventilation: Prediction, prevention, and treatment. Anesthesiology 2009, 110, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Santana, O.; Reyna, J.; Benjo, A.M.; Lamas, G.A.; Lamelas, J. Outcomes of minimally invasive valve surgery in patients with chronic obstructive pulmonary disease. Eur. J. Cardiothorac. Surg. 2012, 42, 648–652. [Google Scholar] [CrossRef]
- Li, S.; Zhou, K.; Wu, Y.; Wang, M.; Shen, C.; Wang, Z.; Che, G.; Liu, L. Presence of pleural adhesions can predict conversion to thoracotomy and postoperative surgical complications in patients undergoing video-assisted thoracoscopic lung cancer lobectomy. J. Thorac. Dis. 2018, 10, 416–431. [Google Scholar] [CrossRef]
- Sintek, M.; Coverstone, E.; Singh, J. Coronary subclavian steal syndrome. Curr. Opin. Cardiol. 2014, 29, 506–513. [Google Scholar] [CrossRef]
- Parnell, A.; Prince, M. Anaesthesia for minimally invasive cardiac surgery. BJA Educ. 2018, 18, 323–330. [Google Scholar] [CrossRef]
- Malik, V.; Jha, A.K.; Kapoor, P.M. Anesthetic challenges in minimally invasive cardiac surgery: Are we moving in a right direction? Ann. Card. Anaesth. 2016, 19, 489–497. [Google Scholar]
- Ramchandani, M.; Al Jabbari, O.; Abu Saleh, W.K.; Ramlawi, B. Cannulation Strategies and Pitfalls in Minimally Invasive Cardiac Surgery. Methodist Debakey Cardiovasc. J. 2016, 12, 10–13. [Google Scholar] [CrossRef]
- Spitaleri, A.; Barbero, C.; Parrella, B.; Marchetto, G.; Salizzoni, S.; La Torre, M.W.; Rinaldi, M.; Pocar, M. Hybrid Setting for Minimally Invasive Mitral Surgery in Patients with Inferior Vena Caval Filters. Ann. Thorac. Surg. Short Rep. 2024, 2, 779–782. [Google Scholar] [CrossRef]
- Ren, S.; Longfellow, E.; Geubelle, G.F.; Fabbro, M.; Lamelas, J.; Alnajar, A.; Bermudez-Velez, R.; Augoustides, J.G.; Shapeton, A.D.; Ortoleva, J.; et al. Femoral Venous Cannulation for Cardiopulmonary Bypass with a Concomitant Inferior Vena Cava Filter. J. Cardiothorac. Vasc. Anesth. 2024, 38, 309–315. [Google Scholar] [CrossRef]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W.; et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.Y.; Wittwer, T.; Haverich, A.; Cremer, J.T. Coronary revascularization without cardiopulmonary bypass in patients with pectus excavatum. Ann. Thorac. Surg. 1999, 68, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Aston, D.; Zeloof, D.; Falter, F. Anaesthesia for Minimally Invasive Cardiac Surgery. J. Cardiovasc. Dev. Dis. 2023, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Höfer, D.; Holfeld, J.; Hangler, H.; Bonaros, N.; Grimm, M. Indications and contra-indications for minimally invasive mitral valve surgery. J. Vis. Surg. 2018, 4, 255. [Google Scholar] [CrossRef]
- Liu, J.; Liang, L.; Kong, Q.; Ma, X.; Chi, L.; Lai, Y. A study on the perioperative effects of obesity on minimally invasive coronary artery bypass grafting and its surgical techniques. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad092. [Google Scholar] [CrossRef]
- Hoogma, D.F.; Oosterlinck, W.; Rex, S. Small incisions still require great anesthesia: Anesthesiology techniques to enhance recovery in robotic coronary bypass grafting. Ann. Cardiothorac. Surg. 2024, 13, 409–416. [Google Scholar] [CrossRef]
- Zhao, Z.; Lau, R.W.H.; Ng, C.S.H. Anaesthesiology for uniportal VATS: Double lumen, single lumen and tubeless. J. Vis. Surg. 2017, 3, 108. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Nersesian, G.; Montagner, M.; Akansel, S.; Eggert-Doktor, D.; Kofler, M.; Sündermann, S.; Falk, V.; Kempfert, J. Endoaortic balloon occlusion in minimally invasive mitral valve surgery. Innovations 2022, 17, 83–87. [Google Scholar] [CrossRef]
- Buhre, W.; Weyland, A.; Kazmaier, S.; Hanekop, G.G.; Baryalei, M.M.; Sydow, M.; Sonntag, H. Comparison of cardiac output assessed by pulse-contour analysis and thermodilution in patients undergoing minimally invasive direct coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 1999, 13, 437–440. [Google Scholar] [CrossRef]
- Kanda, H.; Kunisawa, T.; Kitahara, H.; Iida, T.; Toyama, Y.; Kanao-Kanda, M.; Mori, C.; Kamiya, H. Cerebral Hypoxia Caused by Flow Confliction During Minimally Invasive Cardiac Surgery with Retrograde Perfusion: A Word of Caution. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1838–1840. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Sukesan, S. Anesthesia for robotic cardiac surgery: An amalgam of technology and skill. Ann. Card. Anaesth. 2010, 13, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Leivaditis, V.; Grapatsas, K.; Papaporfyriou, A.; Galanis, M.; Koletsis, E.; Charokopos, N.; Haussmann, E.; Kaplunov, V.; Papatriantafyllou, A.; Dahm, M. The Perioperative Use of Levosimendan as a Means of Optimizing the Surgical Outcome in Patients with Severe Heart Insufficiency Undergoing Cardiac Surgery. J. Cardiovasc. Dev. Dis. 2023, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Goecke, S.; Pitts, L.; Dini, M.; Montagner, M.; Wert, L.; Akansel, S.; Kofler, M.; Stoppe, C.; Ott, S.; Jacobs, S.; et al. Enhanced Recovery After Cardiac Surgery for Minimally Invasive Valve Surgery: A Systematic Review of Key Elements and Advancements. Medicina 2025, 61, 495. [Google Scholar] [CrossRef]
- Mueller, X.M.; Chassot, P.; Zhou, J.; Eisa, K.M.; Chappuis, C.; Tevaearai, H.T.; von Segesser, L.K. Hemodynamics optimization during off-pump coronary artery bypass: The ‘no compression’ technique. Eur. J. Cardio-Thorac. Surg. 2002, 22, 249–254. [Google Scholar] [CrossRef]
- Repossini, A.; Baudo, M.; D’Alonzo, M.; Petruccelli, R.; Rosati, F. MIDCAB Tips and tricks for a successful procedure. Multimed. Man. Cardiothorac. Surg. 2020. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.; Ruel, M.; Bourque, P.R.; Warman-Chardon, J.; Kontolemos, M.; Zwicker, J. Brachial Plexopathy Following Minimally Invasive Coronary Artery Bypass Grafting. Can. J. Neurol. Sci. 2024, 51, 463–465. [Google Scholar] [CrossRef]
- Ozaki, T.; Kawamura, M.; Iwahashi, T.; Miyagawa, S. A case of superior trunk brachial plexus injury after right mini-thoracotomy mitral valve repair. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae190. [Google Scholar] [CrossRef]
- Deshpande, S.P.; Lehr, E.; Odonkor, P.; Bonatti, J.O.; Kalangie, M.; Zimrin, D.A.; Grigore, A.M. Anesthetic management of robotically assisted totally endoscopic coronary artery bypass surgery (TECAB). J. Cardiothorac. Vasc. Anesth. 2013, 27, 586–599. [Google Scholar] [CrossRef]
- Nicoara, A.; Skubas, N.; Ad, N.; Finley, A.; Hahn, R.T.; Mahmood, F.; Mankad, S.; Nyman, C.B.; Pagani, F.; Porter, T.R.; et al. Guidelines for the Use of Transesophageal Echocardiography to Assist with Surgical Decision-Making in the Operating Room: A Surgery-Based Approach: From the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Anesthesiologists and the Society of Thoracic Surgeons. J. Am. Soc. Echocardiogr. 2020, 33, 692–734. [Google Scholar]
- Bhatt, H.V.; Schuessler, M.E.; Torregrossa, G.; Fitzgerald, M.M.; Evans, A.S.; Narasimhan, S.; Ramakrishna, H. Robotic Cardiac Surgery Part II: Anesthetic Considerations for Robotic Coronary Artery Bypass Grafting. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Mathison, M.; Edgerton, J.R.; Horswell, J.L.; Akin, J.J.; Mack, M.J. Analysis of hemodynamic changes during beating heart surgical procedures. Ann. Thorac. Surg. 2000, 70, 1355–1360. [Google Scholar] [CrossRef]
- Karthekeyan, B.R.; Kamalakkannan, G.; Shruthi, S.; Saranya, N.; Kiran, M.; Ashok, G. Hemodynamic comparison between minimally invasive and conventional approaches in off-pump coronary artery bypass grafting: A randomized controlled trial. Indian J. Clin. Anaesth. 2024, 11, 174–180. [Google Scholar]
- Nierich, A.P.; Diephuis, J.; Jansen, E.W.; Borst, C.; Knape, J.T. Heart displacement during off-pump CABG: How well is it tolerated? Ann. Thorac. Surg. 2000, 70, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Kim, K.; Couture, P.; Denault, A.; Kwak, Y.; Yoo, K.; Youn, Y. Hemodynamic management during off-pump coronary artery bypass surgery: A narrative review of proper targets for safe execution and troubleshooting. Korean J. Anesth. 2023, 76, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Kabata, D.; Kakuta, T.; Fukushima, S.; Fujita, T.; Yoshitani, K.; Ohnishi, Y. Association Between Sternotomy Versus Thoracotomy and the Prevalence and Severity of Chronic Postsurgical Pain After Mitral Valve Repair: An Observational Cohort Study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 2937–2944. [Google Scholar] [CrossRef]
- Hoogma, D.F.; Van den Eynde, R.; Oosterlinck, W.; Al Tmimi, L.; Verbrugghe, P.; Tournoy, J.; Fieuws, S.; Coppens, S.; Rex, S. Erector spinae plane block for postoperative analgesia in robotically-assisted coronary artery bypass surgery: Results of a randomized placebo-controlled trial. J. Clin. Anesth. 2023, 87, 111088. [Google Scholar] [CrossRef]
- Ochroch, E.A.; Gottschalk, A. Impact of acute pain and its management for thoracic surgical patients. Thorac. Surg. Clin. 2005, 15, 105–121. [Google Scholar] [CrossRef]
- Bignami, E.; Castella, A.; Pota, V.; Saglietti, F.; Scognamiglio, A.; Trumello, C.; Pace, M.C.; Allegri, M. Perioperative pain management in cardiac surgery: A systematic review. Minerva Anestesiol. 2018, 84, 488–503. [Google Scholar] [CrossRef]
- Xin, L.; Wang, L.; Feng, Y. Ultrasound-guided erector spinae plane block for postoperative analgesia in patients undergoing minimally invasive direct coronary artery bypass surgery: A double-blinded randomized controlled trial. Can. J. Anaesth. 2024, 71, 784–792. [Google Scholar] [CrossRef]
- Ibañez, J.; Riera, M.; Amezaga, R.; Herrero, J.; Colomar, A.; Campillo-Artero, C.; de Ibarra, J.I.S.; Bonnin, O. Long-Term Mortality After Pneumonia in Cardiac Surgery Patients: A Propensity-Matched Analysis. J. Intensive Care Med. 2016, 31, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S.; et al. Perioperative Care in Cardiac Surgery: A Joint Consensus Statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and the Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Cui, B.; Lin, D.; Sun, H.; Ma, J. Advances in Anesthesia Techniques for Postoperative Pain Management in Minimally Invasive Cardiac Surgery: An Expert Opinion. J. Cardiothorac. Vasc. Anesth. 2025, 39, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Kurtoğlu, M.; Ateş, S.; Bakkaloğlu, B.; Beşbaş, S.; Duvan, I.; Akdaş, H.; Aybek, T.; Karagöz, H. Epidural anesthesia versus general anesthesia in patients undergoing minimally invasive direct coronary artery bypass surgery. Anadolu Kardiyol. Derg. 2009, 9, 54–58. [Google Scholar]
- Makkad, B.; Heinke, T.L.; Sheriffdeen, R.; Meng, M.; Kachulis, B.; Grant, M.C.; Popescu, W.M.; Brodt, J.L.; Khatib, D.; Wu, C.L.; et al. Practice Advisory for Postoperative Pain Management of Cardiac Surgical Patients: Executive Summary. A Report from the Society of Cardiovascular Anesthesiologists. J. Cardiothorac. Vasc. Anesth. 2025, 39, 40–48. [Google Scholar] [CrossRef]
- Kessler, P.; Aybek, T.; Neidhart, G.; Dogan, S.; Lischke, V.; Bremerich, D.H.; Byhahn, C. Comparison of three anesthetic techniques for off-pump coronary artery bypass grafting: General anesthesia, combined general and high thoracic epidural anesthesia, or high thoracic epidural anesthesia alone. J. Cardiothorac. Vasc. Anesth. 2005, 19, 32–39. [Google Scholar] [CrossRef]
- Dhole, S.; Mehta, Y.; Saxena, H.; Juneja, R.; Trehan, N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2001, 15, 288–292. [Google Scholar] [CrossRef]
- Mehta, Y.; Arora, D.; Sharma, K.K.; Mishra, Y.; Wasir, H.; Trehan, N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Ann. Card. Anaesth. 2008, 11, 91–96. [Google Scholar] [CrossRef]
- Gautam, S.; Pande, S.; Agarwal, A.; Agarwal, S.K.; Rastogi, A.; Shamshery, C.; Singh, A. Evaluation of Serratus Anterior Plane Block for Pain Relief in Patients Undergoing MIDCAB Surgery. Innovations 2020, 15, 148–154. [Google Scholar] [CrossRef]
- Saikat, S.; Shweta, S.; Somalia, M.; Dibyendu, K.; Sushan, M. Comparative Efficacy of Serratus Anterior Plane Block (SAPB) and Fentanyl for Postoperative Pain Management and Stress Response in Patients Undergoing Minimally Invasive Cardiac Surgery (MICS). Ann. Card. Anaesth. 2023, 26, 268. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, M. The effect of continuous intercostal nerve block vs. single shot on analgesic outcomes and hospital stays in minimally invasive direct coronary artery bypass surgery: A retrospective cohort study. BMC Anesth. 2022, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; DeBose-Scarlett, A.; Irlmeier, R.; Ye, F.; Siegrist, K.; Shah, A.S.; Kingeter, M. Safe Landing: Feasibility and Safety of Operating Room Extubation in Minimally Invasive Cardiac Valve Surgery. J. Cardiothorac. Vasc. Anesth. 2024, 38, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
- Teman, N.R.; Strobel, R.J.; Bonnell, L.N.; Preventza, O.; Yarboro, L.T.; Badhwar, V.; Kaneko, T.; Habib, R.H.; Mehaffey, J.H.; Beller, J.P. Operating Room Extubation for Patients Undergoing Cardiac Surgery: A National Society of Thoracic Surgeons Database Analysis. Ann. Thorac. Surg. 2024, 118, 692–699. [Google Scholar] [CrossRef]
- Werner, A.S.; Foltan, M.; Creutzenberg, M.; Graf, B.M.; Stadlbauer, A.; Tafelmeier, M.; Arzt, M.; Floerchinger, B.; Schmid, C.; Bitzinger, D. Does an Enhanced Recovery After Cardiac Surgery Protocol with On-Table Extubation Improve Patient Outcome and Satisfaction? J. Cardiothorac. Vasc. Anesth. 2025, 39, 328–329. [Google Scholar] [CrossRef]
- Subramaniam, K.; DeAndrade, D.S.; Mandell, D.R.; Althouse, A.D.; Manmohan, R.; Esper, S.A.; Varga, J.M.; Badhwar, V. Predictors of operating room extubation in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2017, 154, 1656–1665.e2. [Google Scholar] [CrossRef]
- Ortoleva, J.P.; Pisano, D.V.; Tull, C.M.; Shapeton, A.D. Operating Room Extubation After Cardiac Surgery: Routine for Some or Routine for None? J. Cardiothorac. Vasc. Anesth. 2025, 39, 1–3. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Hu, Z.; Mao, H.; Xu, Q.; Wu, Q. Clinical Evaluation of on-Table Extubation in Patients Aged over 60 Years Undergoing Minimally Invasive Mitral or Aortic Valve Replacement Surgery. Front. Surg. 2022, 9, 934044. [Google Scholar] [CrossRef]
- Helwani, M.A.; Copeland, C.; Ridley, C.H.; Kaiser, H.A.; De Wet, C.J. A 3-hour fast-track extubation protocol for early extubation after cardiac surgery. JTCVS Open 2022, 12, 299–305. [Google Scholar] [CrossRef]
- Piekarski, F.; Rohner, M.; Monsefi, N.; Bakhtiary, F.; Velten, M. Anesthesia for Minimal Invasive Cardiac Surgery: The Bonn Heart Center Protocol. J. Clin. Med. 2024, 13, 3939. [Google Scholar] [CrossRef]
- Salenger, R.; Ad, N.; Grant, M.C.; Bakaeen, F.; Balkhy, H.H.; Mick, S.L.; Sardari Nia, P.; Kempfert, J.; Bonaros, N.; Bapat, V.; et al. Maximizing Minimally Invasive Cardiac Surgery with Enhanced Recovery (ERAS). Innovations 2024, 19, 371–379. [Google Scholar] [CrossRef]
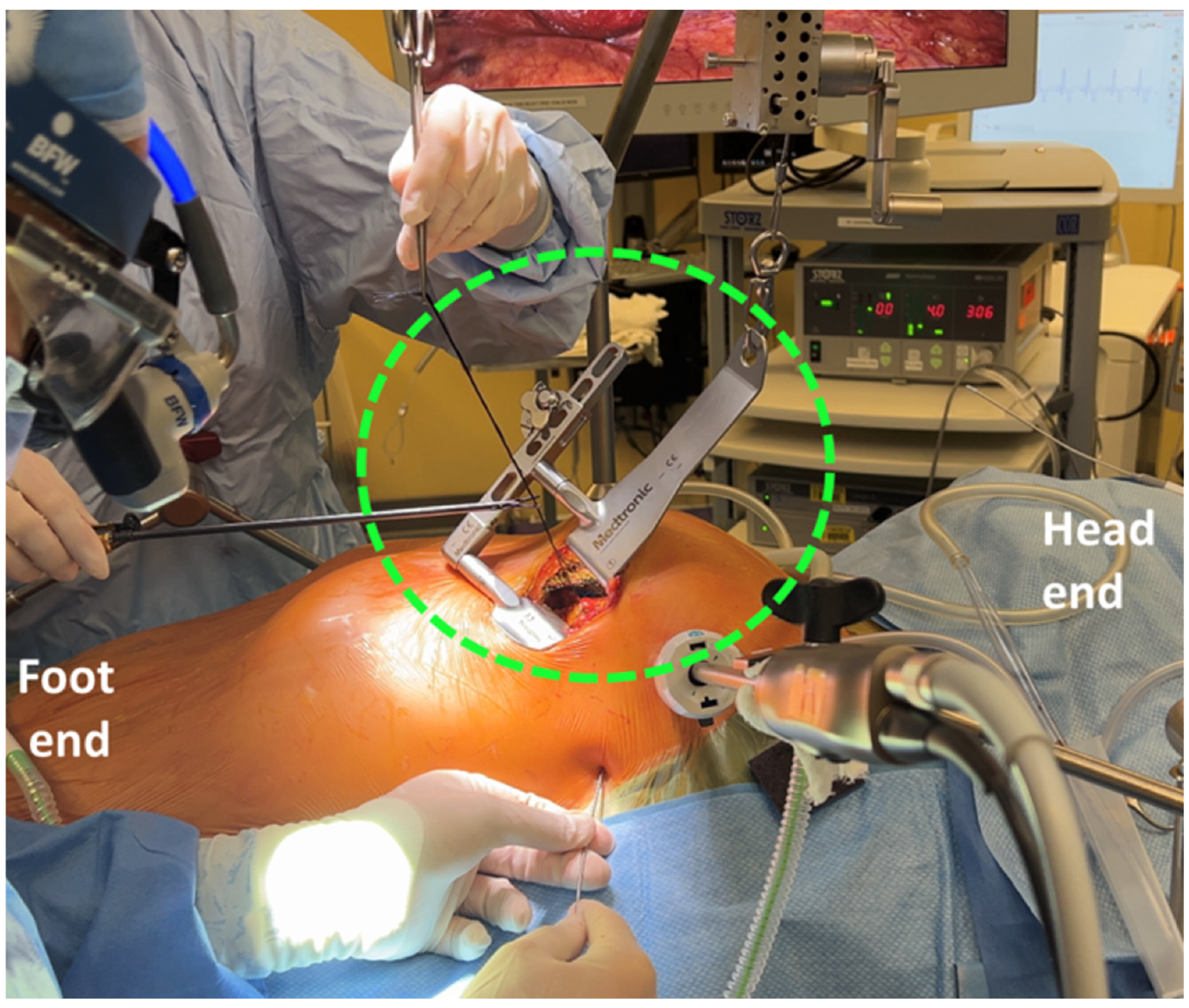
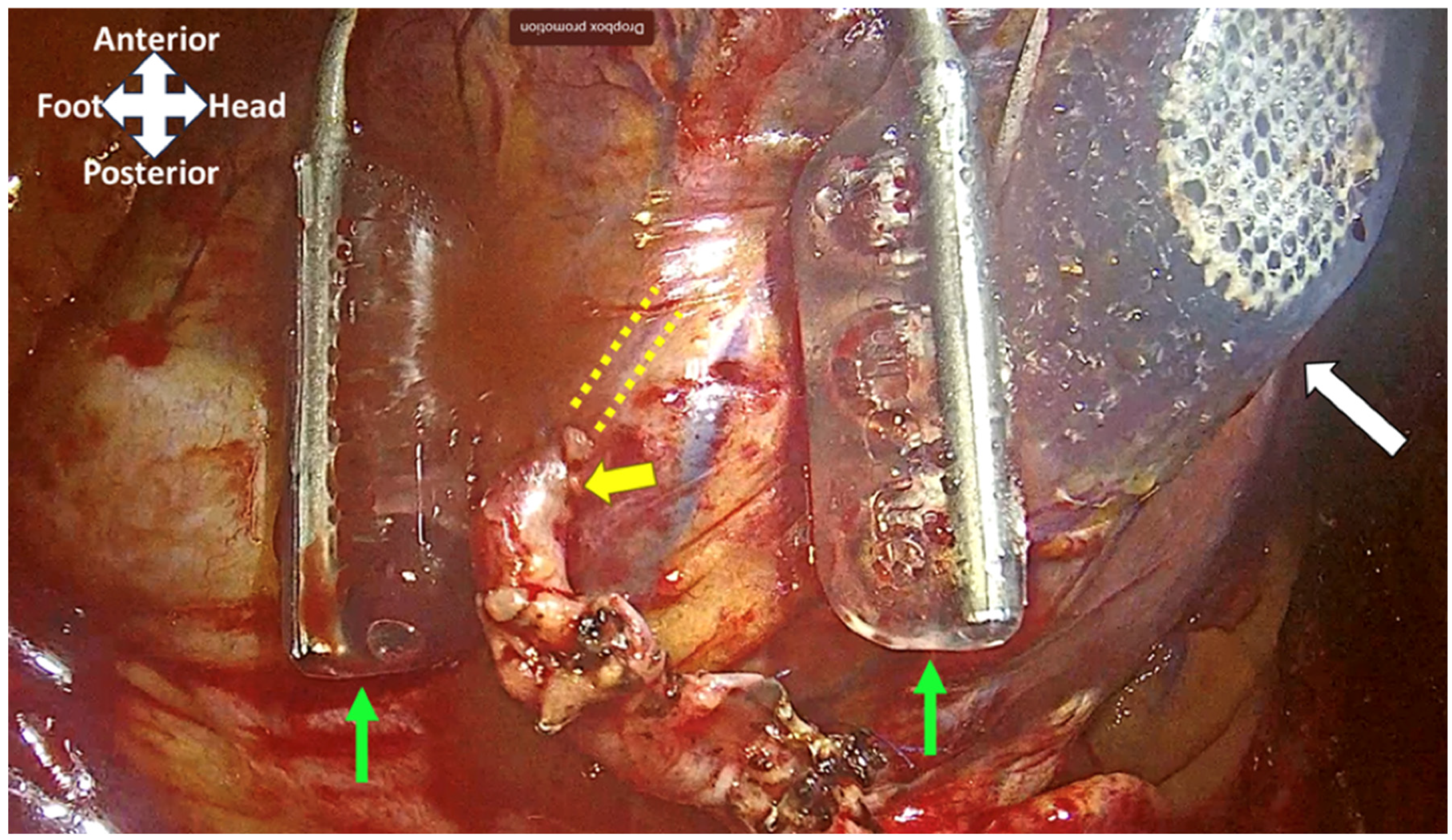
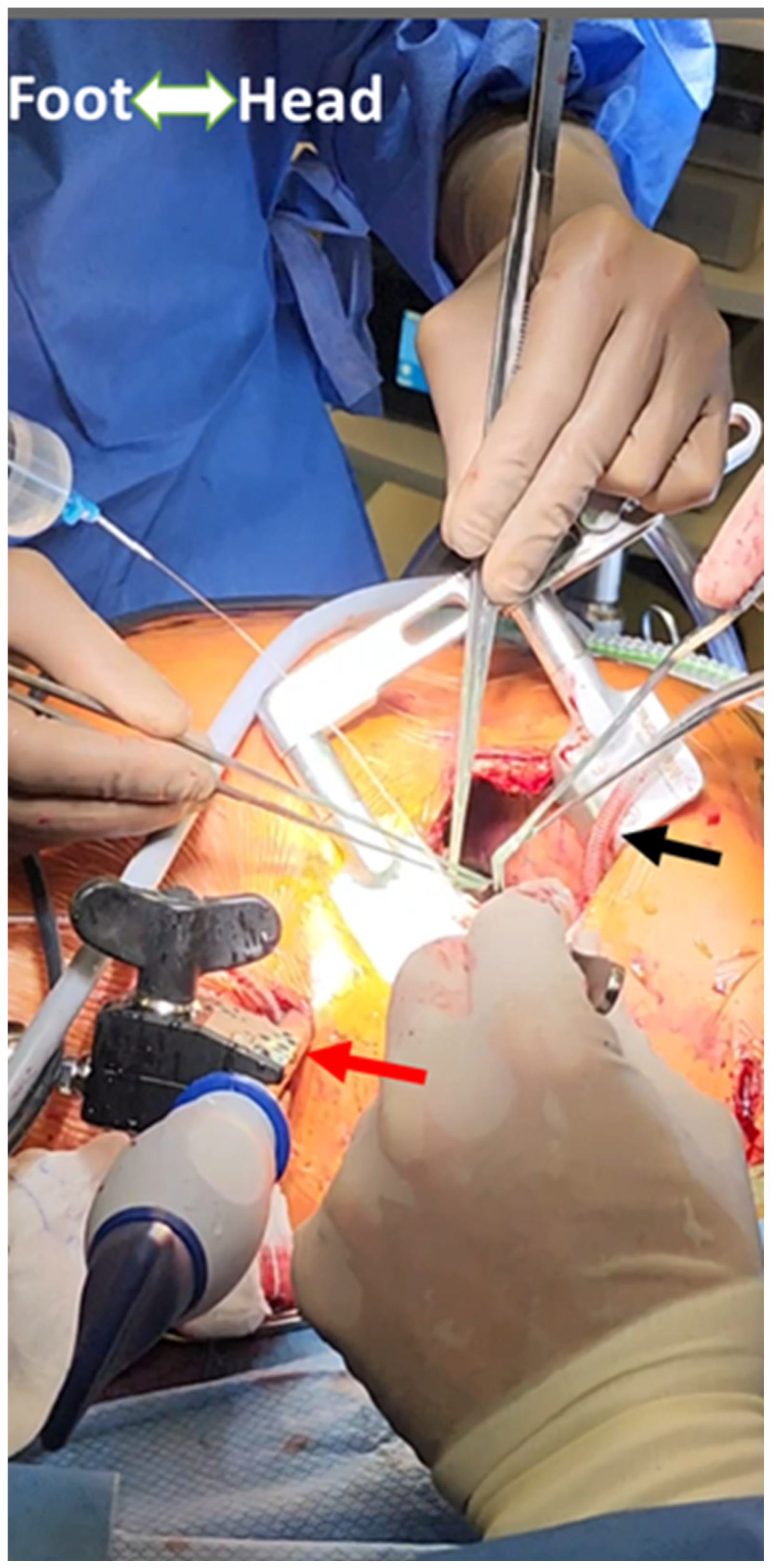
| Technique | Indications | Surgical Approach | Key Components | Advantages | Limitations |
|---|---|---|---|---|---|
| MIDCAB | Isolated proximal LAD disease, hybrid approach for multivessel disease | Small thoracotomy in the 4th or 5th intercostal space | Rib retractor, epicardial stabilizer | Relatively short duration, minimal hemodynamic instability | Limited to proximal LAD disease |
| MICS-CABG | Multivessel coronary artery disease | Slightly more lateral thoracotomy compared to MIDCAB | Rib retractor, epicardial stabilizer, heart positioner | Useful when multivessel grafting is needed | Longer procedural time, potential for hemodynamic instability |
| TECAB | Single or multivessel coronary disease | Entirely robotic with multiple endoscopic port sites, no thoracotomy. | Capnothorax, robotic harvest and anastomosis | No thoracotomy incision | Longer procedural time, requires specialized robotic equipment |
| CPB-assisted MI-CABG | Cases with significant risk of hemodynamic disturbance | Any of the above | Peripheral cannulation, Chitwood clamp or endoballoon if cardioplegia is used | Smooth hemodynamics | Bleeding and coagulopathy |
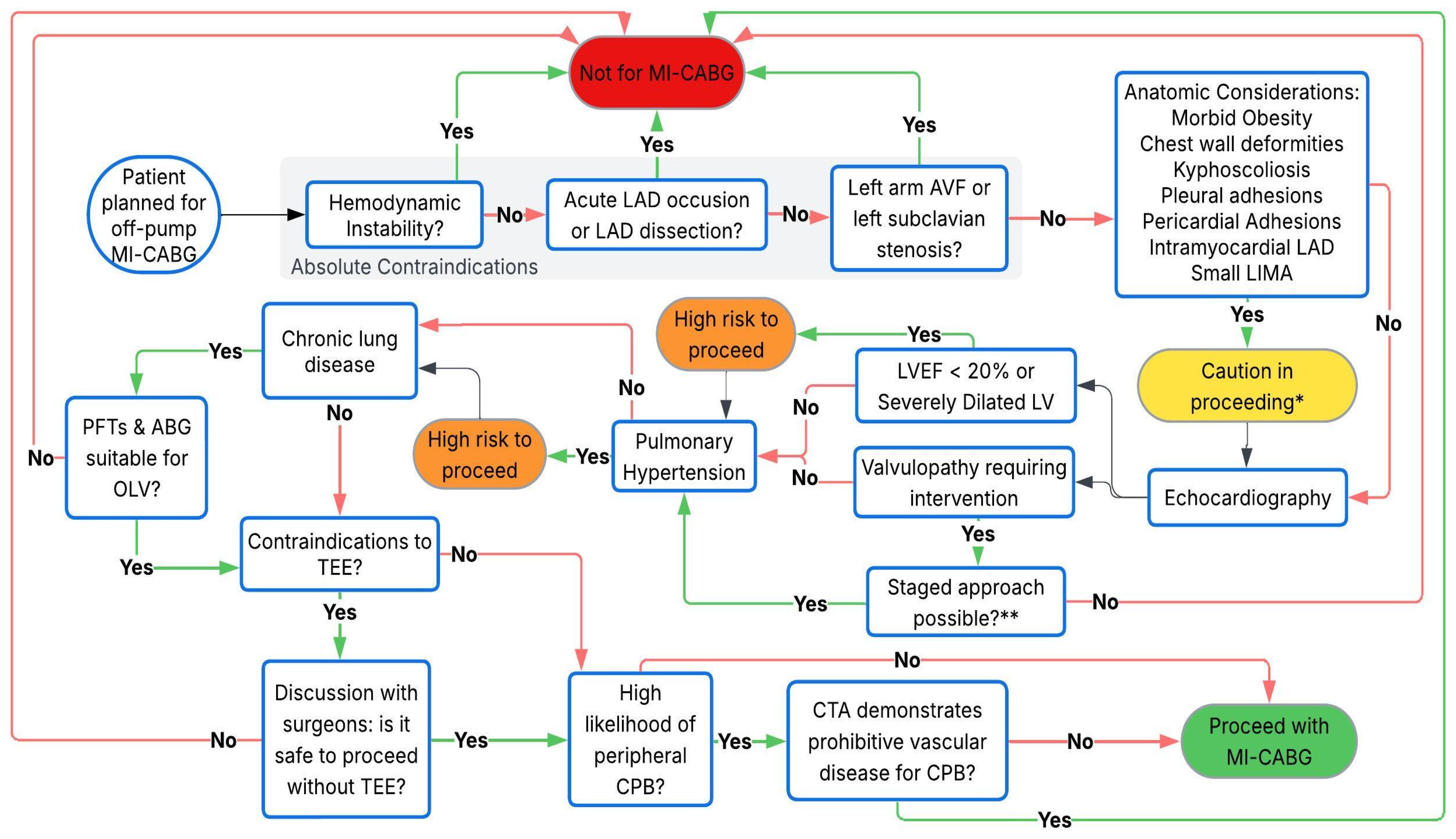
| Category | Condition | Contraindication | Considerations |
|---|---|---|---|
| Cardiac | |||
| Coronary Arteries | Acute LAD occlusion or LAD dissection | Absolute | Unsuitable due to acute nature and complexity. |
| ACS with hemodynamic instability | Absolute | High risk due to instability; urgent cases (e.g., unstable angina) may still be candidates. | |
| Small LIMA (<1.5 mm) or intramyocardial LAD | Relative | Feasibility depends on surgical skill. | |
| RCA or LCx grafting without suitable targets | Relative | Heart subluxation increases hemodynamic instability risk. | |
| Echocardiography | LVEF <20% | Relative | Can be carried out safely with experience but high risk in unstable patients. |
| Severe LV dilation (LVEDD >55 mm) | Relative | Same as above. | |
| Aortic insufficiency | Relative | May preclude CPB-assisted MI-CABG due to cardioplegia delivery issues. | |
| Significant valvular disease requiring intervention | Relative | May necessitate sternotomy/CPB, though staged TAVI feasible in aortic stenosis. | |
| Cardiomegaly | Cardiothoracic ratio >50% | Relative | Complicates surgical access. |
| Pericardium | Inflammatory pericardial disease or adhesions | Relative | Complicates surgical access. |
| Respiratory | |||
| OLV Suitability | Severe chronic lung disease | Relative | High risk of hypoxemia on OLV. |
| Pleural Disease | Pleural adhesions | Relative | May increase bleeding and risk of conversion to sternotomy. |
| Pulmonary Hypertension | Severe pulmonary hypertension/RV dysfunction | Relative | OLV and heart manipulation may precipitate RV failure. |
| Vascular | |||
| Upper Extremity Circulation | Left subclavian artery stenosis | Absolute | Risk of subclavian steal syndrome; critical due to LIMA-LAD graft reliance. |
| Left arm AV fistula | Absolute | Same as above. | |
| Peripheral Vasculature | Peripheral vascular disease | Relative | Impacts peripheral CPB feasibility. |
| Gastrointestinal | |||
| TEE Use | Esophageal webs, strictures, tumors | Relative | Contraindicates TEE; surgery may proceed without TEE in select cases (e.g., single-vessel MIDCAB). |
| Varices, peptic ulcers, hiatal hernia | Relative | TEE risks; discussion if it is safe to proceed without TEE. | |
| Body Habitus | |||
| Musculoskeletal | Chest wall deformities (e.g., pectus excavatum) | Relative | Complicates access; procedures feasible but challenging. |
| Kyphoscoliosis | Relative | Limits positioning and surgical access. | |
| Obesity | BMI >30 with surgical access challenges | Relative | Difficult surgical visualization, poor OLV tolerance. |
| Technique | Advantages | Disadvantages | Use During Heparinization |
|---|---|---|---|
| Deep | |||
| Thoracic Epidural Analgesia (TEA) | Effective analgesia; potential benefit in recovery | Technical complexity, risk of hypotension, hematoma, respiratory depression, possible catheter placement failure | Caution |
| Thoracic Paravertebral Block (TPVB) | Fewer complications than TEA; effective analgesia | Same as TEA, but to a lesser degree | Caution |
| Superficial | |||
| Erector Spinae Plane Block (ESPB) | Simpler to perform with lower risk compared to neuraxial techniques | Limited analgesic efficacy compared to multimodal analgesia alone | Safe |
| Serratus Anterior Plane Block (SAPB) | Simpler to perform with lower risk compared to neuraxial techniques, reduced opioid consumption compared to multimodal analgesia | Catheter placement required, which increases complexity | Safe |
| PECS II Block | Simpler to perform with lower risk compared to neuraxial techniques, can be performed as a rescue block | Efficacy only demonstrated when combined with other blocks | Safe |
| Extrapleural Intercostal Nerve Block (ICNB) | Can be performed under direct vision by surgical team after heparin reversal | Single shot less effective than catheter technique | Safe |
| Category | Criterion | Threshold |
|---|---|---|
| Respiratory | Arterial oxygenation | PaO2 > 75 mmHg with FiO2 < 0.4 |
| Ventilation | PaCO2 < 50 mmHg with spontaneous, unlabored ventilation; PEEP < 7.5 cmH2O | |
| Breathing pattern | Spontaneous, unlabored ventilation | |
| Cardiovascular | Inotropic/vasopressor support | Small and non-escalating doses |
| ST segment stability | No ST elevation or significant depression | |
| Neurological | Level of consciousness | Awake and able to follow commands |
| Neurologic status | No focal neurological deficits | |
| Residual neuromuscular blockade | Train-of-four ratio > 0.9 | |
| Analgesia | Adequate analgesia | |
| Metabolic | Body temperature | >36 °C |
| Arterial pH | >7.25 | |
| Urine output | Adequate | |
| Surgical | Additional surgical concerns | None |
| Chest tube output | <100 mL/h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, M.; White, A.N.J.; Rogers, L.J.; Davierwala, P.M. Anesthesia for Minimally Invasive Coronary Artery Bypass Surgery. J. Cardiovasc. Dev. Dis. 2025, 12, 232. https://doi.org/10.3390/jcdd12060232
Holmes M, White ANJ, Rogers LJ, Davierwala PM. Anesthesia for Minimally Invasive Coronary Artery Bypass Surgery. Journal of Cardiovascular Development and Disease. 2025; 12(6):232. https://doi.org/10.3390/jcdd12060232
Chicago/Turabian StyleHolmes, Miranda, Alexander N. J. White, Luke J. Rogers, and Piroze M. Davierwala. 2025. "Anesthesia for Minimally Invasive Coronary Artery Bypass Surgery" Journal of Cardiovascular Development and Disease 12, no. 6: 232. https://doi.org/10.3390/jcdd12060232
APA StyleHolmes, M., White, A. N. J., Rogers, L. J., & Davierwala, P. M. (2025). Anesthesia for Minimally Invasive Coronary Artery Bypass Surgery. Journal of Cardiovascular Development and Disease, 12(6), 232. https://doi.org/10.3390/jcdd12060232






