Navigating the Complexities of Cancer Treatment-Induced Hypertension
Abstract
1. Introduction
2. Paraneoplastic Hypertension
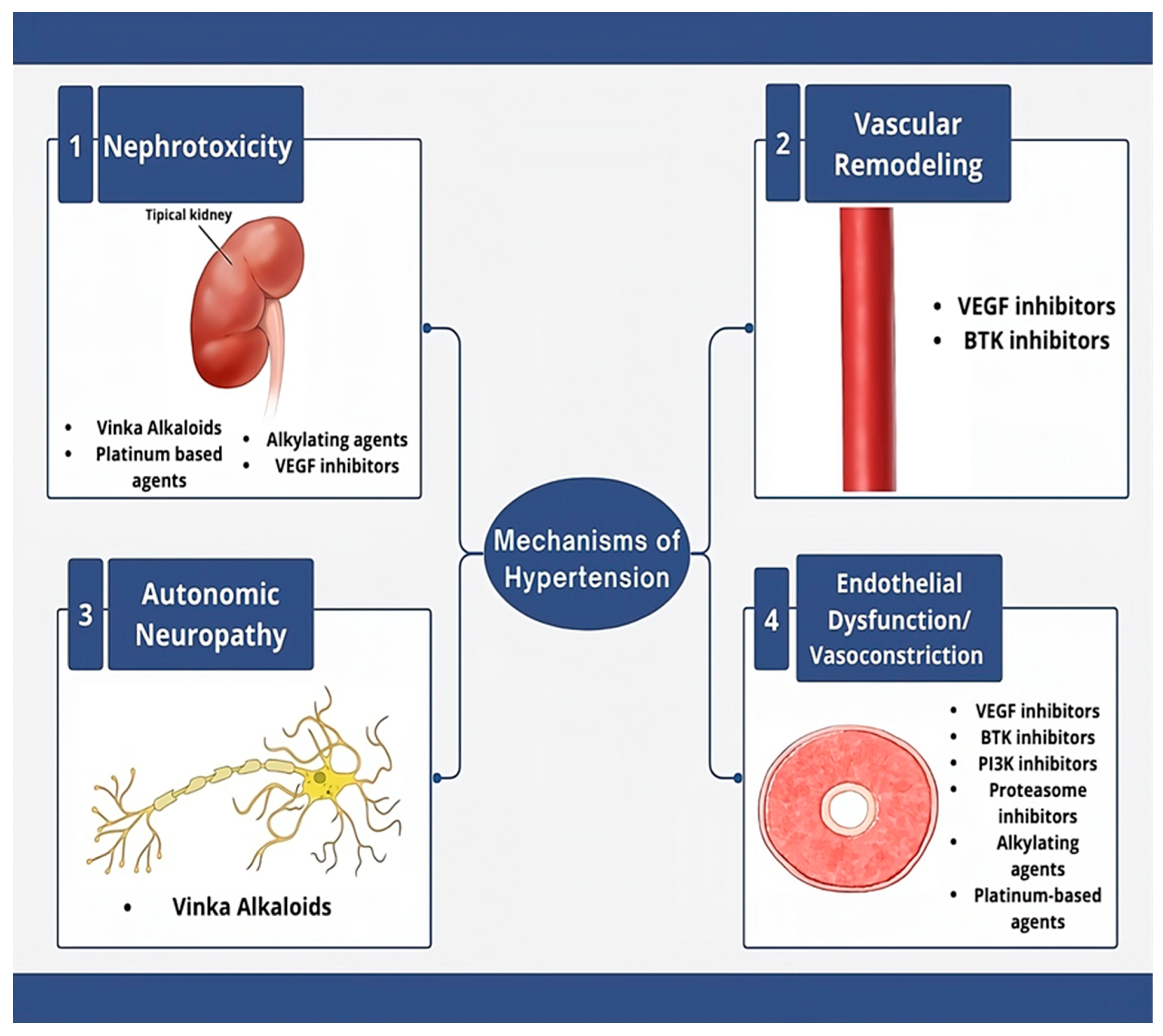
3. Etiologies of Cancer Treatment-Induced Hypertension
3.1. VEGF Signaling Pathway Inhibitors
3.2. Proteasome Inhibitors
3.3. Bruton’s Tyrosine Kinase (BTK) Inhibitors
3.4. Rapidly Accelerated Fibrosarcoma B-Type (BRAF) and Mitogen-Activated Extracellular Signal-Regulated Kinase (MEK) Inhibitors
3.5. Radiation Therapy
3.6. Alkylating Agents
3.7. Platinum-Based Compounds
3.8. BCR-ABL Tyrosine Kinase Inhibitors
3.9. Mammalian Target of Rapamycin Inhibitors
3.10. Anti-Androgen Therapy
3.11. Adjuvant Therapies
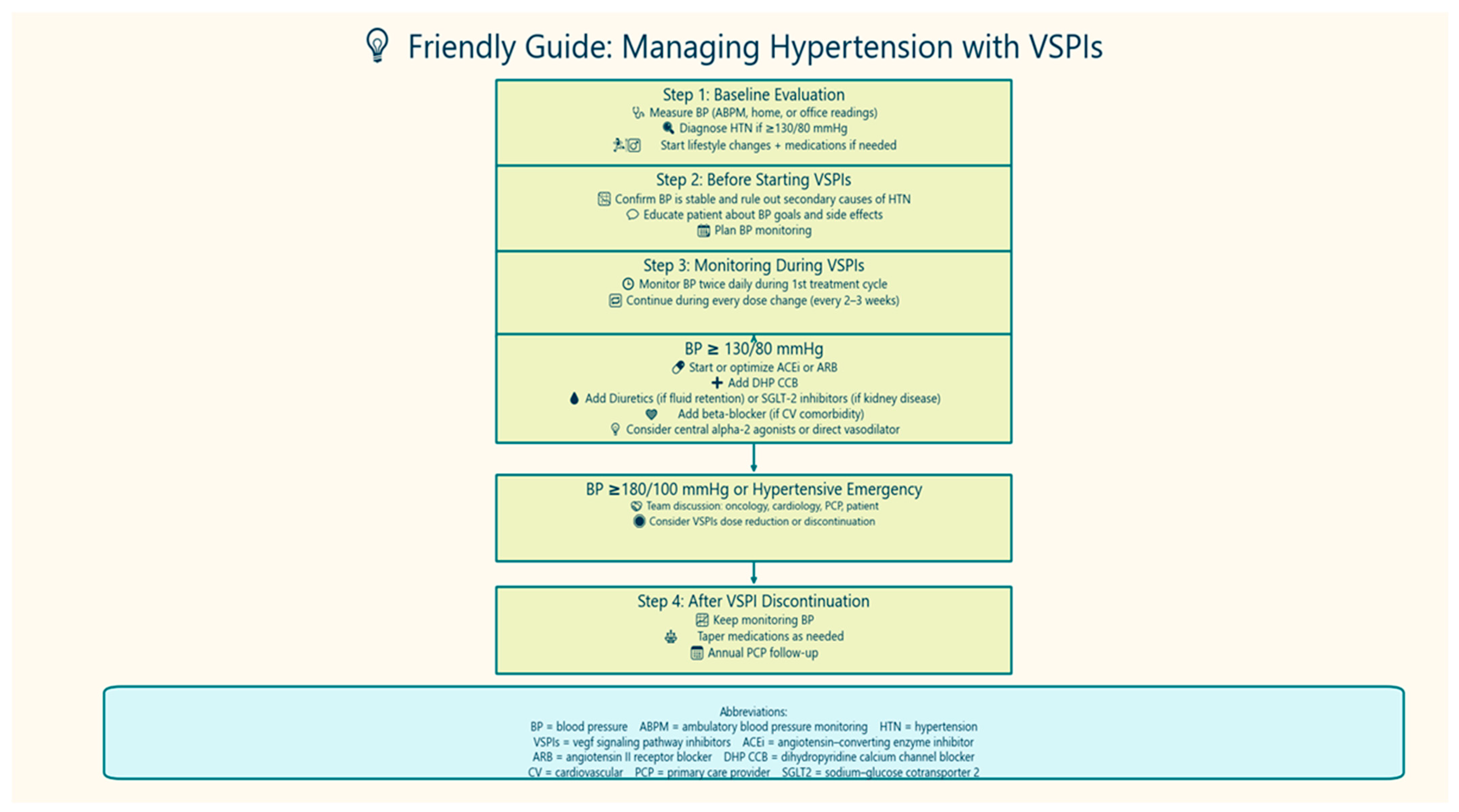
4. Diagnosis of Hypertension in Cancer Patients
5. Blood Pressure Goal in Patients with Cancer
6. Management of Cancer Therapy-Related HTN
6.1. Lifestyle Modifications
6.2. Antihypertensive Medications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef]
- Van Leeuwen, M.T.; Luu, S.; Gurney, H.; Brown, M.R.; Pearson, S.A.; Webber, K.; Hunt, L.; Soojung, H.; Delaney, G.P.; Vajdic, C.M. Cardiovascular Toxicity of Targeted Therapies for Cancer: An Overview of Systematic Reviews. JNCI Cancer Spectr. 2020, 4, pkaa076. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ. Heart Fail. 2016, 9, e002661. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Cohen, J.B.; Brown, N.J.; Brown, S.A.; Dent, S.; van Dorst, D.C.H.; Herrmann, S.M.; Lang, N.N.; Oudit, G.Y.; Touyz, R.M.; American Heart Association Council on Hypertension; et al. Cancer Therapy-Related Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e46–e57. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical Renin-Angiotensin system in kidney physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [CrossRef]
- Carlström, M.; Wilcox, C.S.; Arendshorst, W.J. Renal autoregulation in health and disease. Physiol. Rev. 2015, 95, 405–511. [Google Scholar] [CrossRef]
- Ivy, J.R.; Bailey, M.A. Pressure natriuresis and the renal control of arterial blood pressure. J. Physiol. 2014, 592, 3955–3967. [Google Scholar] [CrossRef]
- Wadei, H.M.; Textor, S.C. The role of the kidney in regulating arterial blood pressure. Nat. Rev. Nephrol. 2012, 8, 602–609. [Google Scholar] [CrossRef]
- Rosner, M.H.; Perazella, M.A. Acute Kidney Injury in Patients with Cancer. N. Engl. J. Med. 2017, 376, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Kozlowska, K.; Kozlowski, L.; Malyszko, J. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transplant. 2017, 32, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Troxell, M.L.; Higgins, J.P.; Kambham, N. Antineoplastic Treatment and Renal Injury: An Update on Renal Pathology Due to Cytotoxic and Targeted Therapies. Adv. Anat. Pathol. 2016, 23, 310–329. [Google Scholar] [CrossRef]
- Bridoux, F.; Cockwell, P.; Glezerman, I.; Gutgarts, V.; Hogan, J.J.; Jhaveri, K.D.; Joly, F.; Nasr, S.H.; Sawinski, D.; Leung, N. Kidney injury and disease in patients with haematological malignancies. Nat. Rev. Nephrol. 2021, 17, 386–401. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.C.; Tofthagen, C.; Morgan, M.A. Relationships among pain, sleep disturbances, and depressive symptoms in outpatients from a comprehensive cancer center. Oncol. Nurs. Forum 2008, 35, 603–611. [Google Scholar] [CrossRef]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef]
- Palagini, L.; Bruno, R.M.; Gemignani, A.; Baglioni, C.; Ghiadoni, L.; Riemann, D. Sleep loss and hypertension: A systematic review. Curr. Pharm. Des. 2013, 19, 2409–2419. [Google Scholar] [CrossRef]
- Johnson, C.B.; Davis, M.K.; Law, A.; Sulpher, J. Shared Risk Factors for Cardiovascular Disease and Cancer: Implications for Preventive Health and Clinical Care in Oncology Patients. Can. J. Cardiol. 2016, 32, 900–907. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Gupta, S.; Nichols, P.; Lohse, C.M.; Kosari, F.; Kattah, A.G.; Harris, F.R.; Karagouga, G.; Mehra, R.; Fine, S.M.; Reuter, V.E.; et al. Renin Production by Juxtaglomerular Cell Tumors and Clear Cell Renal Cell Carcinoma and the Role of Angiotensin Signaling Inhibitors. Mayo Clin. Proc. 2022, 97, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Tun, N.N.; Arunagirinathan, G.; Sodi, R.; Hanna, F.W.F. Pheochromocytomas and Hypertension. Curr. Hypertens. Rep. 2018, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.L.; Caplin, M.E.; Yao, J.C.; et al. The NANETS Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors: Well-Differentiated Neuroendocrine Tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010, 39, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Pelosof, L.C.; Gerber, D.E. Paraneoplastic syndromes: An approach to diagnosis and treatment. Mayo Clin. Proc. 2010, 85, 838–854. [Google Scholar] [CrossRef] [PubMed]
- Haq, N.; Taherian, M.; Rout, P. Adrenal Cortical Nodular Hyperplasia. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kew, M.C.; Leckie, B.J.; Greeff, M.C. Arterial hypertension as a paraneoplastic phenomenon in hepatocellular carcinoma. Arch. Intern. Med. 1989, 149, 2111–2113. [Google Scholar] [CrossRef]
- Pursell, R.N.; Quinlan, P.M. Secondary hypertension due to a renin-producing teratoma. Am. J. Hypertens. 2003, 16, 592–595. [Google Scholar] [CrossRef]
- Alrobaiq, B.M.; Alharbi, R.S.; Alhoshan, F.S.; Alnasyan, M.A.; Alahideb, A.; Omair, A. Hypertension and Ovarian Cancer: A Case-Control Study in Saudi Arabia. Cureus 2023, 15, e35294. [Google Scholar] [CrossRef]
- Sauzeau, V.; Le Mellionnec, E.; Bertoglio, J.; Scalbert, E.; Pacaud, P.; Loirand, G. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ. Res. 2001, 88, 1102–1104. [Google Scholar] [CrossRef]
- Pflug, B.R.; Zheng, H.; Udan, M.S.; D’Antonio, J.M.; Marshall, F.F.; Brooks, J.D.; Nelson, J.B. Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett. 2007, 246, 139–148. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, P.H.; Zhang, X.B.; Xu, C.G.; Wang, W. Plasma concentrations of adrenomedullin and natriuretic peptides in patients with essential hypertension. Exp. Ther. Med. 2015, 9, 1901–1908. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, F.Z.; Chang, R.; Wang, Q.; Liu, S.Y.; Cheng, Z.X.; Gao, Q.; Zhou, H.; Zhou, Y.-B. Adrenomedullin Improves Hypertension and Vascular Remodeling partly through the Receptor-Mediated AMPK Pathway in Rats with Obesity-Related Hypertension. Int. J. Mol. Sci. 2023, 24, 3943. [Google Scholar] [CrossRef] [PubMed]
- Iring, A.; Jin, Y.J.; Albarrán-Juárez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef]
- Mogi, M. Effect of adrenomedullin on obesity-related hypertension. Hypertens. Res. 2024, 47, 2221–2222. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.P.; Black, H.R. A simplified diagnostic approach to pheochromocytoma. A review of the literature and report of one institution’s experience. Medicine 1991, 70, 46–66. [Google Scholar] [CrossRef]
- Manger, W.M. The protean manifestations of pheochromocytoma. Horm. Metab. Res. 2009, 41, 658–663. [Google Scholar] [CrossRef]
- Zelinka, T.; Eisenhofer, G.; Pacak, K. Pheochromocytoma as a catecholamine producing tumor: Implications for clinical practice. Stress 2007, 10, 195–203. [Google Scholar] [CrossRef]
- Zuber, S.M.; Kantorovich, V.; Pacak, K. Hypertension in pheochromocytoma: Characteristics and treatment. Endocrinol. Metab. Clin. N. Am. 2011, 40, 295–311+vii. [Google Scholar] [CrossRef] [PubMed]
- Manger, W.M.; Eisenhofer, G. Pheochromocytoma: Diagnosis and management update. Curr. Hypertens. Rep. 2004, 6, 477–484. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Staessen, J.A.; Vlachopoulos, C.; Duprez, D.; Plante, G.E. Clinical applications of arterial stiffness; definitions and reference values. Am. J. Hypertens. 2002, 15, 426–444. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Hu, Y.F.; Chen, T.C.; Chau, G.Y.; Yang, T.L.; Liu, C.J.; Chen, M.H.; Chang, P.M.-H.; Chen, T.-J.; Hsiao, M.; Huang, C.-Y.F.; et al. Baseline hypertension: New insight into the potential predictors of survival in patients with hepatocellular carcinoma. Int. J. Cardiol. 2013, 168, 2979–2981. [Google Scholar] [CrossRef]
- Zhang, W.S.; Li, X.O.; Zhang, H.; Gao, C.; Fang, L.; Yang, H.Y. Increased Level of Systolic Blood Pressure in Hepatocellular Carcinoma Patients with Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 1979–1988. [Google Scholar] [CrossRef]
- Yoshiji, H.; Noguchi, R.; Ikenaka, Y.; Kaji, K.; Aihara, Y.; Fukui, H. Impact of renin-angiotensin system in hepatocellular carcinoma. Curr. Cancer Drug Targets 2011, 11, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Sternberg, C.N.; Porta, C.; Verzoni, E.; de Braud, F.; Escudier, B.; Procopio, G. Inhibition of the VEGF/VEGFR pathway improves survival in advanced kidney cancer: A systematic review and meta-analysis. Curr. Drug Targets 2015, 16, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Tadokoro, T.; Fujita, K.; Tani, J.; Kobara, H. Immune Microenvironment and the Effect of Vascular Endothelial Growth Factor Inhibition in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 13590. [Google Scholar] [CrossRef]
- Murphy, A.D.; Morgan, R.D.; Clamp, A.R.; Jayson, G.C. The role of vascular endothelial growth factor inhibitors in the treatment of epithelial ovarian cancer. Br. J. Cancer 2022, 126, 851–864. [Google Scholar] [CrossRef]
- Kamat, A.A.; Merritt, W.M.; Coffey, D.; Lin, Y.G.; Patel, P.R.; Broaddus, R.; Nurgent, E.; Han, L.Y.; Landen Jr, C.N.; Spannuth, W.A.; et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin. Cancer Res. 2007, 13, 7487–7495. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szcylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef]
- Versmissen, J.; Mirabito Colafella, K.M.; Koolen, S.L.W.; Danser, A.H.J. Vascular Cardio-Oncology: Vascular Endothelial Growth Factor inhibitors and hypertension. Cardiovasc. Res. 2019, 115, 904–914. [Google Scholar] [CrossRef]
- Izzedine, H.; Massard, C.; Spano, J.P.; Goldwasser, F.; Khayat, D.; Soria, J.C. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance, and management. Eur. J. Cancer 2010, 46, 439–448. [Google Scholar] [CrossRef]
- Liu, B.; Ding, F.; Liu, Y.; Xiong, G.; Lin, T.; He, D.; Zhang, Y.; Zhang, D.; Wei, G. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: A comprehensive network meta-analysis of 72 randomized controlled trials involving 30,013 patients. Oncotarget 2016, 7, 67661–67673. [Google Scholar] [CrossRef]
- van Dorst, D.C.H.; Kabadayi, S.; Oomen-de Hoop, E.; Danser, A.H.J.; Mathijssen, R.H.J.; Versmissen, J. Treatment and Implications of Vascular Endothelial Growth Factor Inhibitor-Induced Blood Pressure Rise: A Clinical Cohort Study. J. Am. Heart Assoc. 2023, 12, e028050. [Google Scholar] [CrossRef]
- Moslehi, J.J. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Hamnvik, O.P.; Choueiri, T.K.; Turchin, A.; McKay, R.R.; Goyal, L.; Davis, M.; Kaymakcalan, D.M.; Williams, J.S. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015, 121, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, J.C.F.; Kelly, W.K.; Innocenti, F. Contribution of plasma levels of VEGF-A and angiopoietin-2 in addition to a genetic variant in KCNAB1 to predict the risk of bevacizumab-induced hypertension. Pharmacogenomics J. 2024, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef]
- Li, J.; Gu, J. Cardiovascular Toxicities with Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: A Meta-Analysis of 77 Randomized Controlled Trials. Clin. Drug Investig. 2018, 38, 1109–1123. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Wang, T.; Liu, L.H.; Guo, H.Q. Risks of proteinuria associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: A systematic review and meta-analysis. PLoS ONE 2014, 9, e90135. [Google Scholar] [CrossRef]
- Kato, T.; Mizuno, R.; Miyake, H. Prevalence and management of proteinuria associated with vascular endothelial growth factor receptor-targeted tyrosine kinase inhibitor treatment in advanced renal cell carcinoma, hepatocellular carcinoma, and thyroid cancer. Int. J. Urol. 2024, 31, 465–474. [Google Scholar] [CrossRef]
- Wu, S.; Kim, C.; Baer, L.; Zhu, X. Bevacizumab increases risk for severe proteinuria in cancer patients. J. Am. Soc. Nephrol. 2010, 21, 1381–1389. [Google Scholar] [CrossRef]
- Eremina, V.; Quaggin, S.E. The role of VEGF-A in glomerular development and function. Curr. Opin. Nephrol. Hypertens. 2004, 13, 9–15. [Google Scholar] [CrossRef]
- Abbas, A.; Mirza, M.M.; Ganti, A.K.; Tendulkar, K. Renal Toxicities of Targeted Therapies. Target. Oncol. 2015, 10, 487–499. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.; Song, E.J. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch. Pharm. Res. 2020, 43, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B.; Patel, D.; Wu, X. Molecular Mechanisms of the Cardiotoxicity of the Proteasomal-Targeted Drugs Bortezomib and Carfilzomib. Cardiovasc. Toxicol. 2017, 17, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, G.; Makris, N.; Laina, A.; Theodorakakou, F.; Briasoulis, A.; Trougakos, I.P.; Dismopoulos, M.-A.; Kastritis, E.; Stametelopoulos, K. Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies: JACC: Cardio Oncology State-of-the-Art Review. JACC Cardio Oncol. 2023, 5, 1–21. [Google Scholar] [CrossRef]
- Mohammed, T.; Singh, M.; Tiu, J.G.; Kim, A.S. Etiology and management of hypertension in patients with cancer. Cardio-oncology 2021, 7, 14. [Google Scholar] [CrossRef]
- Chari, A.; Hajje, D. Case series discussion of cardiac and vascular events following carfilzomib treatment: Possible mechanism, screening, and monitoring. BMC Cancer 2014, 14, 915. [Google Scholar] [CrossRef]
- Wu, P.; Oren, O.; Gertz, M.A.; Yang, E.H. Proteasome Inhibitor-Related Cardiotoxicity: Mechanisms, Diagnosis, and Management. Curr. Oncol. Rep. 2020, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef]
- Siegel, D.; Martin, T.; Nooka, A.; Harvey, R.D.; Vij, R.; Niesvizky, R.; Badros, A.Z.; Jagannath, S.; McCulloch, L.; Rajangam, K.; et al. Integrated safety profile of single-agent carfilzomib: Experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica 2013, 98, 1753–1761. [Google Scholar] [CrossRef]
- Bishnoi, R.; Xie, Z.; Shah, C.; Bian, J.; Murthy, H.S.; Wingard, J.R.; Farhadfar, N. Real-world experience of carfilzomib-associated cardiovascular adverse events: SEER-Medicare data set analysis. Cancer Med. 2021, 10, 70–78. [Google Scholar] [CrossRef]
- Waxman, A.J.; Clasen, S.; Hwang, W.T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, e174519. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Barlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Jurczak, W.; Jerkeman, M.; Silva, R.S.; Rusconi, C.; Trneny, M.; Offner, F.; Caballero, D.; Joao, C.; Witzens-Harig, M.; et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet 2016, 387, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.G.; McCarthy, H.; Rule, S.; D’Sa, S.; Thomas, S.K.; Tournilhac, O.; Forconi, F.; Kersten, M.J.; Zinzani, P.L.; Iyengar, S.; et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: A single-arm, multicentre, phase 2 study. Lancet Haematol. 2020, 7, e112–e121. [Google Scholar] [CrossRef]
- Chen, S.T.; Azali, L.; Rosen, L.; Zhao, Q.; Wiczer, T.; Palettas, M.; Gambril, J.; Kola-Kehinde, O.; Ruz, P.; Kalathoor, S.; et al. Hypertension and incident cardiovascular events after next-generation BTKi therapy initiation. J. Hematol. Oncol. 2022, 15, 92. [Google Scholar] [CrossRef]
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Garcia Sanz, R.; et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef]
- Dickerson, T.; Wiczer, T.; Waller, A.; Philippon, J.; Porter, K.; Haddad, D.; Guha, A.; Rogers, K.A.; Bhat, S.; Byrd, J.C.; et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019, 134, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Ding, N.; Shi, Y.; Feng, L.; Li, J.; Liu, Y.; Lin, Y.; Shi, C.; Wang, X.; Pan, Z.; et al. The Bruton’s tyrosine kinase inhibitor ibrutinib exerts immunomodulatory effects through regulation of tumor-infiltrating macrophages. Oncotarget 2017, 8, 39218–39229. [Google Scholar] [CrossRef]
- Kappers, M.H.; van Esch, J.H.; Sluiter, W.; Sleijfer, S.; Danser, A.H.; van den Meiracker, A.H. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010, 56, 675–681. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.J.; Kudelka, A.; Lu, J.; Zhu, X. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2008, 9, 117–123. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef]
- Bronte, E.; Bronte, G.; Novo, G.; Rinaldi, G.; Bronte, F.; Passiglia, F.; Russo, A. Cardiotoxicity mechanisms of the combination of BRAF-inhibitors and MEK-inhibitors. Pharmacol. Ther. 2018, 192, 65–73. [Google Scholar] [CrossRef]
- Glen, C.; Tan, Y.Y.; Waterston, A.; Evans, T.R.J.; Jones, R.J.; Petrie, M.C.; Lang, N.N. Mechanistic and Clinical Overview Cardiovascular Toxicity of BRAF and MEK Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC Cardio Oncol. 2022, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Kashfi, S.; Hong, S.; Kalaria, A.; Kim, A.S. Onco-Hypertension in Patients with Kidney Disease. Am. J. Nephrol. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mincu, R.I.; Mahabadi, A.A.; Michel, L.; Mrotzek, S.M.; Schadendorf, D.; Rassaf, T.; Totzeck, M. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198890. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Jain, P.; Fradley, M.G.; Lenihan, D.; Gutierrez, J.M.; Jain, C.; de Lima, M.; Barnholtz-Sloan, J.S.; Oliveira, G.H.; Dowlati, A.; et al. Cardiovascular adverse events associated with BRAF versus BRAF/MEK inhibitor: Cross-sectional and longitudinal analysis using two large national registries. Cancer Med. 2021, 10, 3862–3872. [Google Scholar] [CrossRef]
- Larkin, J.; Del Vecchio, M.; Ascierto, P.A.; Krajsova, I.; Schachter, J.; Neyns, B.; Espinosa, E.; Garbe, C.; Chiaron Sileni, V.; Gogas, H.; et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: An open-label, multicentre, safety study. Lancet Oncol. 2014, 15, 436–444. [Google Scholar] [CrossRef]
- Pandey, S.; Kalaria, A.; Jhaveri, K.D.; Herrmann, S.M.; Kim, A.S. Management of hypertension in patients with cancer: Challenges and considerations. Clin. Kidney J. 2023, 16, 2336–2348. [Google Scholar] [CrossRef]
- Sharabi, Y.; Dendi, R.; Holmes, C.; Goldstein, D.S. Baroreflex failure as a late sequela of neck irradiation. Hypertension 2003, 42, 110–116. [Google Scholar] [CrossRef]
- Timmers, H.J.; Wieling, W.; Karemaker, J.M.; Lenders, J.W. Cardiovascular responses to stress after carotid baroreceptor denervation in humans. Ann. N. Y. Acad. Sci. 2004, 1018, 515–519. [Google Scholar] [CrossRef]
- Piani, F.; Landolfo, M.; Fiorini, G.; D’Addato, S.; Mancia, G.; Borghi, C. Severe impaired blood pressure control caused by baroreflex failure as a late sequela of neck irradiation. J. Hypertens. 2020, 38, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef]
- Cohen, E.P.; Lawton, C.A.; Moulder, J.E.; Becker, C.G.; Ash, R.C. Clinical course of late-onset bone marrow transplant nephropathy. Nephron 1993, 64, 626–635. [Google Scholar] [CrossRef]
- Cohen, E.P. Radiation nephropathy after bone marrow transplantation. Kidney Int. 2000, 58, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A.; Cohen, E.P.; Li, N.; Takayama, K.; Farese, A.M.; MacVittie, T.J. Radiation Nephropathy in a Nonhuman Primate Model of Partial-Body Irradiation With Minimal Bone Marrow Sparing-Part 2: Histopathology, Mediators, and Mechanisms. Health Phys. 2019, 116, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Baradaran-Ghahfarokhi, M. Radiation-induced kidney injury. J. Ren. Inj. Prev. 2012, 1, 49–50. [Google Scholar]
- Saka, B.; Bilge, A.K.; Umman, B.; Yilmaz, E.; Nisanci, Y.; Erten, N.; Karan, M.A.; Tascioglu, C. Bilateral renal artery stenosis after abdominal radiotherapy for Hodgkin’s disease. Int. J. Clin. Pract. 2003, 57, 247–248. [Google Scholar] [CrossRef]
- Bali, L.; Silhol, F.; Kateb, A.; Vaisse, B. Renal artery stenosis after abdominal radiotherapy. Ann. Cardiol. Angeiol. 2009, 58, 183–186. [Google Scholar] [CrossRef]
- Karati, D.; Mahadik, K.R.; Trivedi, P.; Kumar, D. Alkylating Agents, the Road Less Traversed, Changing Anticancer Therapy. Anticancer. Agents Med. Chem. 2022, 22, 1478–1495. [Google Scholar]
- Lajous, H.; Lelièvre, B.; Vauléon, E.; Lecomte, P.; Garcion, E. Rethinking Alkylating(-Like) Agents for Solid Tumor Management. Trends Pharmacol. Sci. 2019, 40, 342–357. [Google Scholar] [CrossRef]
- Zhu, X.; Lei, J.; Chen, R.; Chen, Z.; Xiong, Z.; Yang, L.; Jiang, M.; Zhang, H. Cancer Recurrence Fear and Return to Work in Breast Cancer Survivors: The Mediating Effects of Health Literacy. J. Multidiscip. Healthc. 2025, 18, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, E.C.; Bökenkamp, A.; Tjahjadi, N.S.; Tettero, J.M.; van Dulmen-den Broeder, E.; van der Pal, H.J.; Veening, M.A. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst. Rev. 2019, 3, Cd008944. [Google Scholar] [CrossRef] [PubMed]
- Soultati, A.; Mountzios, G.; Avgerinou, C.; Papaxoinis, G.; Pectasides, D.; Dimopoulos, M.A.; Papadimitriou, C. Endothelial vascular toxicity from chemotherapeutic agents: Preclinical evidence and clinical implications. Cancer Treat. Rev. 2012, 38, 473–483. [Google Scholar] [CrossRef]
- Nuver, J.; Smit, A.J.; Sleijfer, D.T.; van Gessel, A.I.; van Roon, A.M.; van der Meer, J.; van den Berg, M.P.; Burgerhof, J.G.M.; Hoekstra, H.J.; Sluiter, W.J.; et al. Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur. J. Cancer 2004, 40, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Valentová, M.; Mladosievicová, B. Coronary heart disease and hypertension as late effects of testicular cancer treatment—A minireview. Klin. Onkol. 2011, 24, 18–22. [Google Scholar]
- Knijnenburg, S.L.; Jaspers, M.W.; van der Pal, H.J.; Schouten-van Meeteren, A.Y.; Bouts, A.H.; Lieverst, J.A.; Bökenkamp, A.; Koning, C.C.E.; Oldenburger, F.; Wilde, J.C.H.; et al. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin. J. Am. Soc. Nephrol. 2012, 7, 1416–1427. [Google Scholar] [CrossRef]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC Cardio Oncol. 2019, 1, 238–251. [Google Scholar] [CrossRef]
- Katsi, V.; Magkas, N.; Georgiopoulos, G.; Athanasiadi, E.; Virdis, A.; Masi, S.; Kliridis, P.; Hatziyanni, A.; Tsioufis, C.; Tousoulis, D. Arterial hypertension in patients under antineoplastic therapy: A systematic review. J. Hypertens. 2019, 37, 884–901. [Google Scholar] [CrossRef]
- Herradón, E.; González, C.; Uranga, J.A.; Abalo, R.; Martín, M.I.; López-Miranda, V. Characterization of Cardiovascular Alterations Induced by Different Chronic Cisplatin Treatments. Front. Pharmacol. 2017, 8, 196. [Google Scholar] [CrossRef]
- Rahman, A.A.; Stojanovska, V.; Pilowsky, P.; Nurgali, K. Platinum accumulation in the brain and alteration in the central regulation of cardiovascular and respiratory functions in oxaliplatin-treated rats. Pflug. Arch. 2021, 473, 107–120. [Google Scholar] [CrossRef]
- Sagstuen, H.; Aass, N.; Fosså, S.D.; Dahl, O.; Klepp, O.; Wist, E.A.; Wilsgaard, T.; Bremnes, R.M. Blood pressure and body mass index in long-term survivors of testicular cancer. J. Clin. Oncol. 2005, 23, 4980–4990. [Google Scholar] [CrossRef] [PubMed]
- Rix, U.; Hantschel, O.; Dürnberger, G.; Remsing Rix, L.L.; Planyavsky, M.; Fernbach, N.V.; Kaupe, I.; Bennett, K.L.; Valent, P.; Colinge, J.; et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007, 110, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Mulas, O.; Caocci, G.; Mola, B.; La Nasa, G. Arterial Hypertension and Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 674748. [Google Scholar] [CrossRef]
- Ai, N.; Chong, C.M.; Chen, W.; Hu, Z.; Su, H.; Chen, G.; Lei Wong, Q.W.; Ge, W. Ponatinib exerts anti-angiogenic effects in the zebrafish and human umbilical vein endothelial cells via blocking VEGFR signaling pathway. Oncotarget 2018, 9, 31958–31970. [Google Scholar] [CrossRef]
- Hadzijusufovic, E.; Albrecht-Schgoer, K.; Huber, K.; Hoermann, G.; Grebien, F.; Eisenwort, G.; Schgoer, W.; Herndlhofer, S.; Kaun, C.; Theurl, M.; et al. Nilotinib-induced vasculopathy: Identification of vascular endothelial cells as a primary target site. Leukemia 2017, 31, 2388–2397. [Google Scholar] [CrossRef]
- Chiarini, F.; Evangelisti, C.; McCubrey, J.A.; Martelli, A.M. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol. Sci. 2015, 36, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Bardhi, E.; Ruscito, I.; Papadia, A.; Farooqi, A.A.; Marchetti, C.; Bogani, G.; Ceccacci, I.; Mueller, M.D.; Benedetti Panici, P. PI3K/AKT/mTOR Pathway in Ovarian Cancer Treatment: Are We on the Right Track? Geburtshilfe Frauenheilkd 2017, 77, 1095–1103. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef]
- Kaplan, B.; Qazi, Y.; Wellen, J.R. Strategies for the management of adverse events associated with mTOR inhibitors. Transpl. Rev. 2014, 28, 126–133. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grünwald, V.; Thompson, J.A.; Figlin, R.A.; Hollaender, N.; et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet 2008, 372, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Deedwania, P. Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: Benefits of vasodilating β-blockers. J. Clin. Hypertens. 2011, 13, 52–59. [Google Scholar] [CrossRef]
- Attard, G.; Reid, A.H.; Auchus, R.J.; Hughes, B.A.; Cassidy, A.M.; Thompson, E.; Babu Oommen, N.; Folkerd, E.; Dowsett, M.; Arlt, W.; et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J. Clin. Endocrinol. Metab. 2012, 97, 507–516. [Google Scholar] [CrossRef]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivaschenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Martinez-Martin, F.J.; Kuzior, A.; Hernandez-Lazaro, A.; de Leon-Durango, R.J.; Rios-Gomez, C.; Santana-Ojeda, B.; Perez-Rivero, J.M.; Fernandez-Trujillo-Comenge, P.M.; Gonzalez-Diaz, P.; Arnas-Leon, C.; et al. Incidence of hypertension in young transgender people after a 5-year follow-up: Association with gender-affirming hormonal therapy. Hypertens. Res. 2023, 46, 219–225. [Google Scholar] [CrossRef]
- Tonia, T.; Mettler, A.; Robert, N.; Schwarzer, G.; Seidenfeld, J.; Weingart, O.; Hyde, C.; Engert, A.; Bohlius, J. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst. Rev. 2012, 12, Cd003407. [Google Scholar] [CrossRef]
- Pufall, M.A. Glucocorticoids and Cancer. Adv. Exp. Med. Biol. 2015, 872, 315–333. [Google Scholar] [PubMed]
- Vaziri, N.D. Mechanism of erythropoietin-induced hypertension. Am. J. Kidney Dis. 1999, 33, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Zhou, X.J.; Naqvi, F.; Smith, J.; Oveisi, F.; Wang, Z.Q.; Purdy, R.E. Role of nitric oxide resistance in erythropoietin-induced hypertension in rats with chronic renal failure. Am. J. Physiol. 1996, 271, E113–E122. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Geller, D.S. Glucocorticoid-induced hypertension. Pediatr. Nephrol. 2012, 27, 1059–1066. [Google Scholar] [CrossRef]
- Baid, S.; Nieman, L.K. Glucocorticoid excess and hypertension. Curr. Hypertens. Rep. 2004, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.G.; Nguyen, T.V.; Day, R.O. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann. Intern. Med. 1994, 121, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.B.; Silva, E.N.; Ribeiro, M.L.; Martins Wde, A. Hypertension in patients with cancer. Arq. Bras. Cardiol. 2015, 104, 246–252. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Walsh, S.B.; McCormick, J.A.; Zietse, R.; Unwin, R.J.; Ellison, D.H. Pathogenesis of calcineurin inhibitor-induced hypertension. J. Nephrol. 2012, 25, 269–275. [Google Scholar] [CrossRef]
- Divac, N.; Naumović, R.; Stojanović, R.; Prostran, M. The Role of Immunosuppressive Medications in the Pathogenesis of Hypertension and Efficacy and Safety of Antihypertensive Agents in Kidney Transplant Recipients. Curr. Med. Chem. 2016, 23, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.M. Influence of the new immunosuppressive combinations on arterial hypertension after renal transplantation. Kidney Int. Suppl. 2002, 82, S81–S87. [Google Scholar] [CrossRef]
- Bursztyn, M.; Zelig, O.; Or, R.; Nagler, A. Isradipine for the prevention of cyclosporine-induced hypertension in allogeneic bone marrow transplant recipients: A randomized, double-blind study. Transplantation 1997, 63, 1034–1036. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhao, Y. Incidence and risk of hypertension with ramucirumab in cancer patients: A meta-analysis of published studies. Clin. Drug Investig. 2015, 35, 221–228. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, X.; Xu, T.; Xu, X.; Liu, Z. Bevacizumab significantly increases the risks of hypertension and proteinuria in cancer patients: A systematic review and comprehensive meta-analysis. Oncotarget 2017, 8, 51492–51506. [Google Scholar] [CrossRef]
- Ge, P.; Han, C.; Reyila, A.; Liu, D.; Hong, W.; Liu, J.; Zhang, J.; Han, X.; Li, X.; Huang, M.; et al. Risk of antiangiogenic adverse events in metastatic colorectal cancer patients receiving aflibercept in combination with chemotherapy: A meta-analysis. Medicine 2023, 102, e34793. [Google Scholar] [CrossRef]
- Qi, W.X.; Lin, F.; Sun, Y.J.; Tang, L.N.; He, A.N.; Yao, Y.; Shen, Z. Incidence and risk of hypertension with pazopanib in patients with cancer: A meta-analysis. Cancer Chemother. Pharmacol. 2013, 71, 431–439. [Google Scholar] [CrossRef] [PubMed]
- PARAPLATIN (Carboplatin) [Prescribing Information]: Food and Drug Administration. 2022. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f88e0483-09be-4f60-be8f-964c02e3ece0 (accessed on 1 June 2025).
- TORISEL (Temsirolimus) [Prescribing Information]: Food and Drug Administration. 2025. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95b7dc92-2180-42f1-8699-3c28f609e674 (accessed on 1 June 2025).
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Furman, R.R.; Byrd, J.C.; Owen, R.G.; O’Brien, S.M.; Brown, J.R.; Hillmen, P.; Stephens, D.M.; Chernyukhin, N.; Lezhava, T.; Hamdy, A.M.; et al. Pooled analysis of safety data from clinical trials evaluating acalabrutinib monotherapy in mature B-cell malignancies. Leukemia 2021, 35, 3201–3211. [Google Scholar] [CrossRef] [PubMed]
- DANZITEN (Nilotinib) [Prescribing Information]: Food and Drug Administration. 2024. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d288d165-3505-49bb-9b2e-124490d65f49 (accessed on 1 June 2025).
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [PubMed]
- Kallioinen, N.; Hill, A.; Horswill, M.S.; Ward, H.E.; Watson, M.O. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: A systematic review. J. Hypertens. 2017, 35, 421–441. [Google Scholar] [CrossRef]
- Johnson, K.C.; Whelton, P.K.; Cushman, W.C.; Cutler, J.A.; Evans, G.W.; Snyder, J.K.; Ambrosius, W.T.; Beddhu, S.; Cheung, A.K.; Fine, L.J.; et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018, 71, 848–857. [Google Scholar] [CrossRef]
- Kronish, I.M.; Kent, S.; Moise, N.; Shimbo, D.; Safford, M.M.; Kynerd, R.E.; O’Beirne, R.; Sullivan, A.; Muntner, P. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J. Am. Soc. Hypertens. 2017, 11, 573–580. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.J.; Mant, J.; Haque, M.S.; Bray, E.P.; Bryan, S.; Greenfield, S.M.; Jones, M.I.; Jowett, S.; Little, P.; Penaloza, C.; et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: The TASMIN-SR randomized clinical trial. JAMA 2014, 312, 799–808. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G. Ital. Cardiol. 2018, 19, 3s–73s. [Google Scholar]
- Rao, V.U.; Reeves, D.J.; Chugh, A.R.; O’Quinn, R.; Fradley, M.G.; Raghavendra, M.; Dent, S.; Barac, A.; Lenihan, D. Clinical Approach to Cardiovascular Toxicity of Oral Antineoplastic Agents: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2693–2716. [Google Scholar] [CrossRef]
- Maitland, M.L.; Bakris, G.L.; Black, H.R.; Chen, H.X.; Durand, J.B.; Elliott, W.J.; Ivy, S.P.; Leier, C.V.; Lindfield, J.; Liu, G.; et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J. Natl. Cancer Inst. 2010, 102, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Sahni, G. Onco-Hypertension: Changing Paradigm of Treating Hypertension in Patients With Cancer. J. Clin. Oncol. 2023, 41, 958–963. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, S. Risks and management of hypertension in cancer patients undergoing targeted therapy: A review. Clin. Hypertens. 2022, 28, 14. [Google Scholar] [CrossRef]
- van Dorst, D.C.H.; Dobbin, S.J.H.; Neves, K.B.; Herrmann, J.; Herrmann, S.M.; Versmissen, J.; Mathijssen, R.H.J.; Jan Danser, A.H.; Lang, N.N. Hypertension and Prohypertensive Antineoplastic Therapies in Cancer Patients. Circ. Res. 2021, 128, 1040–1061. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, T.; Arín, R.M.; Casis, E.; Torres-Jacome, J.; Sanchez-Chapula, J.A.; Casis, O. Mechanisms responsible for the altered cardiac repolarization dispersion in experimental hypothyroidism. Acta Physiol. 2012, 204, 502–512. [Google Scholar] [CrossRef]
- Beavers, C.J.; Rodgers, J.E.; Bagnola, A.J.; Beckie, T.M.; Campia, U.; Di Palo, K.E.; Okwuosa, T.M.; Przespolewski, E.R.; Dent, S.; American Heart Association Clinical Pharmacology Committee; et al. Cardio-Oncology Drug Interactions: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e811–e838. [Google Scholar] [CrossRef] [PubMed]
- Nazer, B.; Humphreys, B.D.; Moslehi, J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: Focus on hypertension. Circulation 2011, 124, 1687–1691. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Geraylow, K.R.; Khazaei, M.; Nassiri, M.; Hassanian, S.M.; Ferns, G.A.; Avan, A. Angiotensin-converting Enzyme Inhibitors and Angiotensin Receptor Blockers as Potential Therapeutic Options for Pancreatic Cancer. Curr. Cancer Drug Targets 2022, 22, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, T.; Gavras, I. Renin-Angiotensin Inhibition in Combating Malignancy: A Review. Anticancer. Res. 2019, 39, 4597–4602. [Google Scholar] [CrossRef]
- Waliany, S.; Sainani, K.L.; Park, L.S.; Zhang, C.A.; Srinivas, S.; Witteles, R.M. Increase in Blood Pressure Associated With Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor. JACC Cardio Oncol. 2019, 1, 24–36. [Google Scholar] [CrossRef]
- Philip, L.J.; Findlay, S.G.; Gill, J.H. Baseline blood pressure and development of cardiotoxicity in patients treated with anthracyclines: A systematic review. Int. J. Cardiol. Cardiovasc. Risk Prev. 2022, 15, 200153. [Google Scholar] [CrossRef] [PubMed]
- Perini, M.V.; Dmello, R.S.; Nero, T.L.; Chand, A.L. Evaluating the benefits of renin-angiotensin system inhibitors as cancer treatments. Pharmacol. Ther. 2020, 211, 107527. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Izzedine, H.; Albiges, L.; Escudier, B. Hypertension and Angiotensin System Inhibitors in Patients with Metastatic Renal Cell Carcinoma. Oncol. Rev. 2016, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Curwen, J.O.; Musgrove, H.L.; Kendrew, J.; Richmond, G.H.; Ogilvie, D.J.; Wedge, S.R. Inhibition of vascular endothelial growth factor-a signaling induces hypertension: Examining the effect of cediranib (recentin; AZD2171) treatment on blood pressure in rat and the use of concomitant antihypertensive therapy. Clin. Cancer Res. 2008, 14, 3124–3131. [Google Scholar] [CrossRef]
- Araújo, W.F.; Naves, M.A.; Ravanini, J.N.; Schor, N.; Teixeira, V.P. Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol. Oncol. 2015, 33, 389.e1–389.e7. [Google Scholar] [CrossRef]
- McKay, R.R.; Rodriguez, G.E.; Lin, X.; Kaymakcalan, M.D.; Hamnvik, O.P.R.; Sabbisetti, V.S.; Bhatt, R.S.; Simantov, R.; Choueiri, T.K. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 2015, 21, 2471–2479. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. ACE (Angiotensin-Converting Enzyme) Inhibitors/Angiotensin Receptor Blockers Are Associated With Lower Colorectal Cancer Risk: A Territory-Wide Study with Propensity Score Analysis. Hypertension 2020, 76, 968–975. [Google Scholar] [CrossRef]
- Mir, O.; Coriat, R.; Ropert, S.; Cabanes, L.; Blanchet, B.; Camps, S.; Billemont, B.; Knebelmann, B.; Goldwasser, F. Treatment of bevacizumab-induced hypertension by amlodipine. Investig. New Drugs 2012, 30, 702–707. [Google Scholar] [CrossRef]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef]
- Alshnbari, A.S.; Millar, S.A.; O’Sullivan, S.E.; Idris, I. Effect of sodium-glucose cotransporter-2 inhibitors on endothelial function: A systematic review of preclinical studies. Diabetes Ther. 2020, 11, 1947–1963. [Google Scholar] [CrossRef]
- Ren, C.; Sun, K.; Zhang, Y.; Hu, Y.; Hu, B.; Zhao, J.; He, Z.; Ding, R.; Wang, W.; Liang, C. Sodium-Glucose CoTransporter-2 Inhibitor Empagliflozin Ameliorates Sunitinib-Induced Cardiac Dysfunction via Regulation of AMPK-mTOR Signaling Pathway-Mediated Autophagy. Front. Pharmacol. 2021, 12, 664181. [Google Scholar] [CrossRef] [PubMed]
| Medication Class | Mechanism of Action | Example Medications | Relative Incidence of All-Grade HTN † | Impaired NO Homeostasis | Vascular Endothelial Dysfunction | Capillary Rarefaction | Impaired Natriuresis | Angiotensin-Mediated Vasoconstriction | Direct Vascular Toxicity | Abnormal Vascular Remodeling | Oxidative Stress | Impaired VEGF Signaling | Renal Toxicity | Sympathetic Dysfunction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VSPI | Disruption of VEGF-mediated angiogenesis | Ramucirumab [149] (anti-VEGF-R2 Ab ) | ↑ | + | + | + | + | + | ||||||
| Bevacizumab [150] (anti-VEGF-A Ab) | ↑ | |||||||||||||
| Aflibercept [151] (anti-VEGF-Trap ligand Ab) | ↑↑ | |||||||||||||
| Pazopanib [152] (tyrosine kinase inhibitor) | ↑↑ | |||||||||||||
| Proteosome inhibitors [6,73,75,76,77] | Inhibition of ubiquitin-proteosome cascade | Carfilzomib | ↑↑ | + | + | + | + | |||||||
| Bortezomib | ↑ | |||||||||||||
| Alkylating agents [6,75,112,113,114,115] | Impaired genome replication and transcription via DNA cross-linking | Busulfan | ↑↑ | + | + | + | + | + | + | + | ||||
| Ifosfamide | ↑ | |||||||||||||
| Platinum-containing compounds [6,110,114,119,120,121,122,153] | Impaired genome replication and transcription via DNA cross-linking | Cisplatin | ↑↑↑ | + | + | + | + | + | ||||||
| Carboplatin | ↑ | |||||||||||||
| mTOR inhibitors [6,129,130,131,154] | Disruption of cellular metabolism, growth, and proliferation via serine-threonine kinase inhibition | Everolimus | ↑ | + | + | + | ||||||||
| Temsirolimus | ↑ | |||||||||||||
| BRAF/MEK [92,93,94,95,96,155] | MAPK pathway inhibition | Encorafenib/binimetinib | ↑ | + | ||||||||||
| Vemurafenib/cobimetinib | ↑ | |||||||||||||
| BTK inhibitors [88,89,90,156] | Impeded B cell receptor signaling | Acalabrutinib | ↑ | + | + | + | + | |||||||
| Ibrutinib | ↑↑↑ | |||||||||||||
| BCR-ABL TK inhibitors ‡ [123,124,125,126,157] | Inhibition of BCR-ABL TK-mediated effects upon cellular division and apoptosis | Ponatinib | ↑↑ | + | + | |||||||||
| Nilotinib | ↑ |
| Medication Class | Mechanism of Action | Example Medications | Mechanism of HTN | |
|---|---|---|---|---|
| Adjuvant Therapies | Corticosteroids | Predominantly glucocorticoid activity | Prednisone, dexamethasone | Predominantly mineralocorticoid-induced water and Na retention |
| Calcineurin inhibitors | Impaired T cell activation via inhibition of transcription factors | Cyclosporine, tacrolimus | RAAS activation, sympathetic dysfunction, increased proximal tubule Na resorption (NCC-mediated), endothelial dysfunction (ET1 mediated), oxidative stress, impaired NO homeostasis | |
| Erythropoiesis stimulating agents | Increased erythrocyte production | Epoetin alfa, epoetin beta | Increased blood viscosity, arterial remodeling, impaired NO homeostasis | |
| NSAIDs | Decreased prostaglandin synthesis via inhibition of COX enzymes | Ketorolac, ibuprofen | Impaired natriuresis | |
| Radiation Therapy | Cellular apoptosis | N/A | Carotid baroreceptor dysfunction, radiation nephropathy, radiation-induced renal artery stenosis | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arriola-Montenegro, J.; Roth, J.; Gonzalez Suarez, M.L. Navigating the Complexities of Cancer Treatment-Induced Hypertension. J. Cardiovasc. Dev. Dis. 2025, 12, 235. https://doi.org/10.3390/jcdd12060235
Arriola-Montenegro J, Roth J, Gonzalez Suarez ML. Navigating the Complexities of Cancer Treatment-Induced Hypertension. Journal of Cardiovascular Development and Disease. 2025; 12(6):235. https://doi.org/10.3390/jcdd12060235
Chicago/Turabian StyleArriola-Montenegro, Jose, John Roth, and Maria L. Gonzalez Suarez. 2025. "Navigating the Complexities of Cancer Treatment-Induced Hypertension" Journal of Cardiovascular Development and Disease 12, no. 6: 235. https://doi.org/10.3390/jcdd12060235
APA StyleArriola-Montenegro, J., Roth, J., & Gonzalez Suarez, M. L. (2025). Navigating the Complexities of Cancer Treatment-Induced Hypertension. Journal of Cardiovascular Development and Disease, 12(6), 235. https://doi.org/10.3390/jcdd12060235





