Driveline Relocation and Vacuum-Assisted Closure for Ventricular Assist Device Driveline Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Protocol
2.2. Prophylactic Antibiotic Regimen and Driveline Protocol
2.3. Standard Driveline Dressing Protocol
2.4. Case Definitions
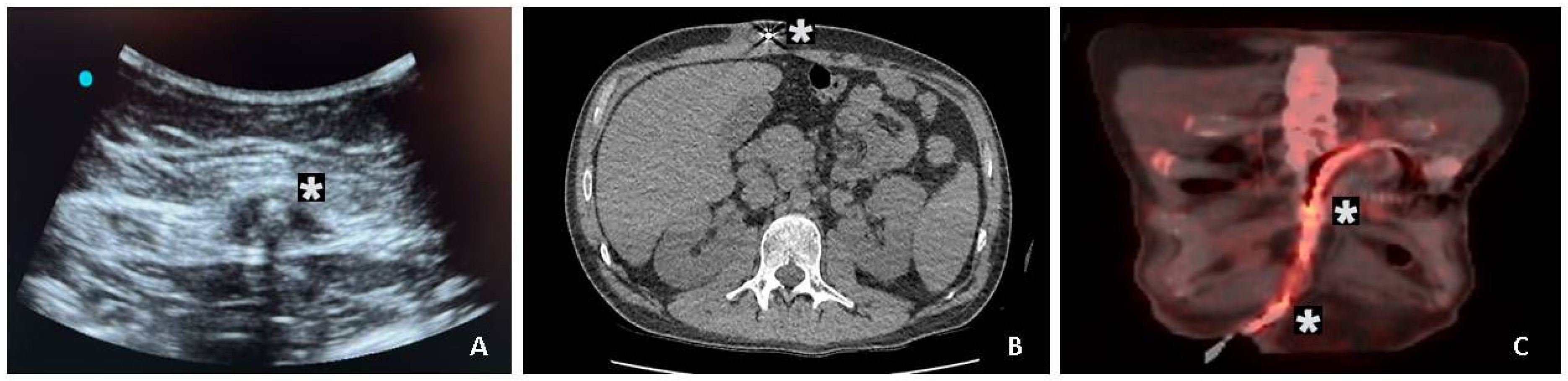
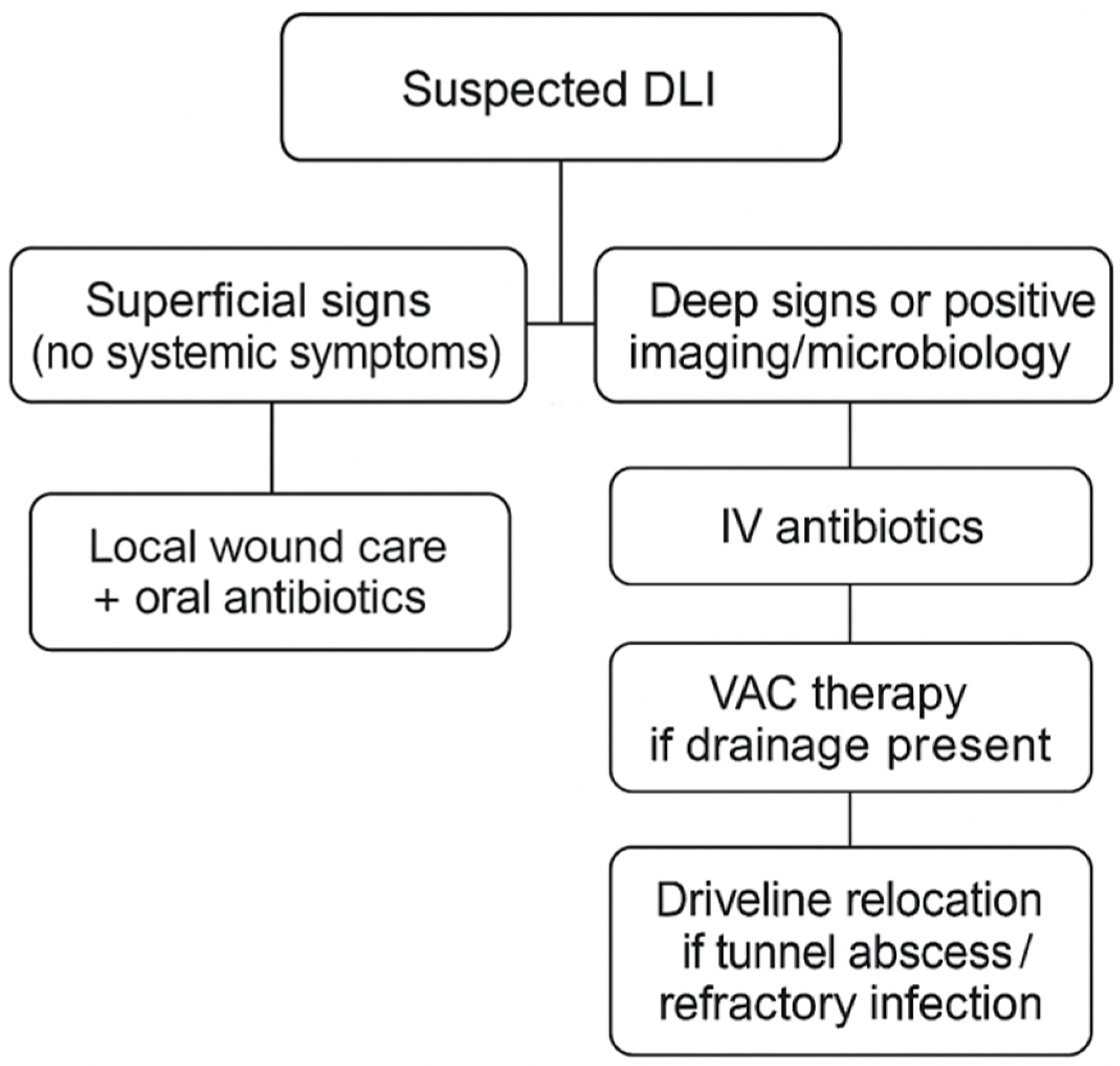
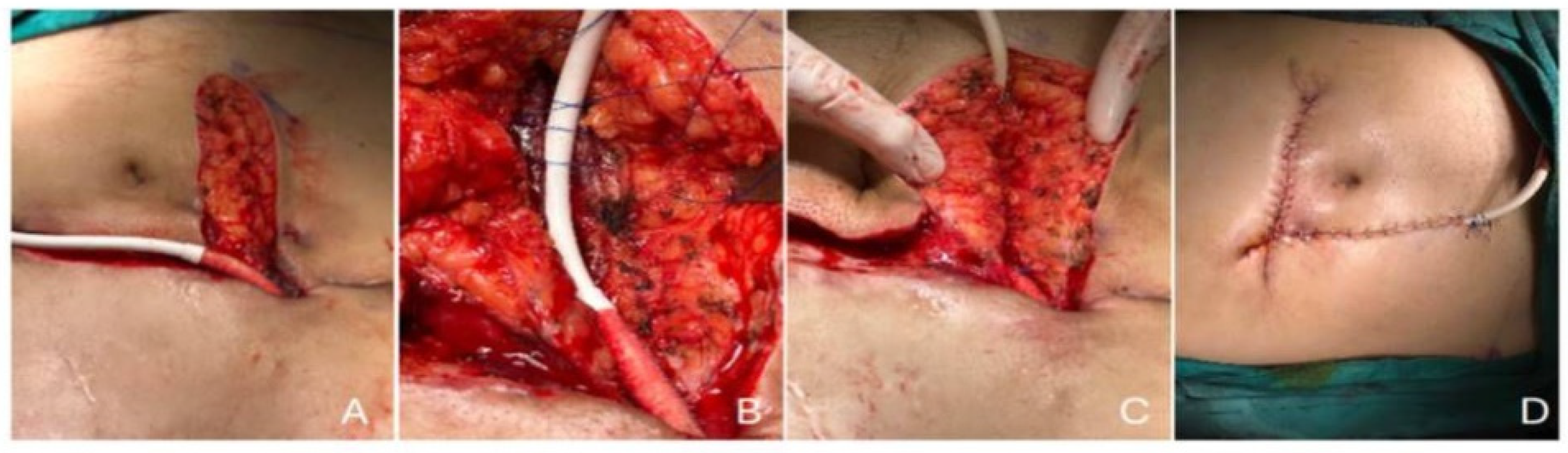
2.5. Imaging and Surgical Management
2.6. Setting
2.7. Statistical Analysis
3. Results
3.1. Culture Results
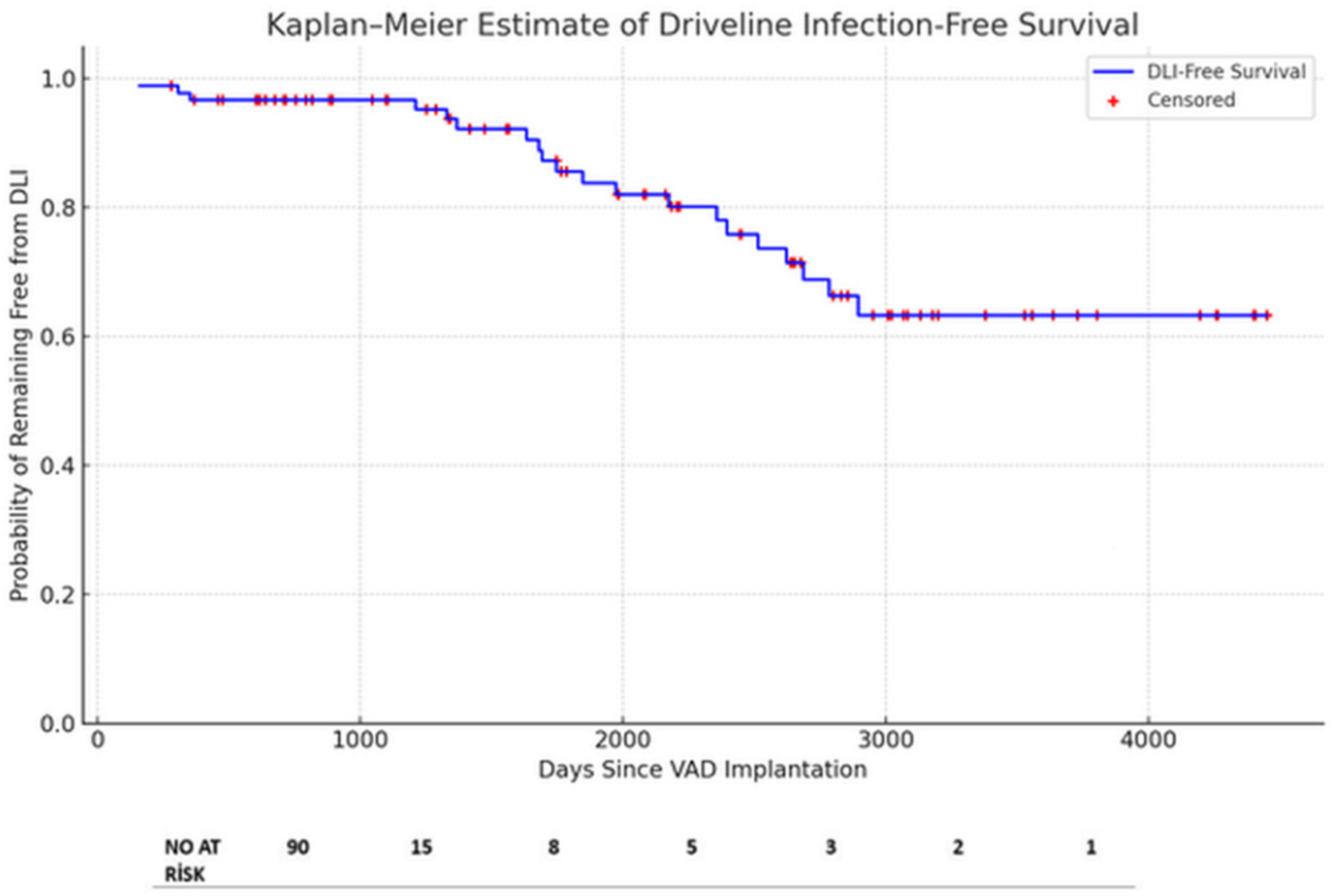
3.2. Outcomes and Infection Management
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdvHF | Advanced heart failure |
| BiVAD | Biventricular assist device |
| BMI | Body mass index |
| CoNS | Coagulase-negative staphylococci |
| DL | Driveline |
| DLES | Driveline exit site |
| DLIs | Driveline infections |
| DMCS | Durable mechanical circulatory support |
| HM2 | HeartMate 2 |
| HM3 | HeartMate 3 |
| HVAD | HeartWare |
| ISHLT | International Society for Heart and Lung Transplantation |
| LV | Left ventricle |
| LVAD | Left ventricular assist device |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| PAP | Pulmonary artery pressure |
| VAC | Vacuum-assisted closure |
| VAD | Ventricular assist device |
References
- Peled, Y.; Ducharme, A.; Kittleson, M.; Bansal, N.; Stehlik, J.; Amdani, S.; Saeed, D.; Cheng, R.; Clarke, B.; Dobbels, F.; et al. International Society for Heart and Lung Transplantation Guidelines for the Evaluation and Care of Cardiac Transplant Candidates-2024. J. Heart Lung Transplant. 2024, 43, 1529–1628.e54. [Google Scholar]
- Aslam, S.; Cowger, J.; Shah, P.; Stosor, V.; Copeland, H.; Reed, A.; Morales, D.; Giblin, G.; Mathew, J.; Morrissey, O.; et al. The International Society for Heart and Lung Transplantation (ISHLT): 2024 infection definitions for durable and acute mechanical circulatory support devices. J. Heart Lung Transplant. 2024, 43, 1039–1050. [Google Scholar] [CrossRef]
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Farber, G.; Hannan, M.M.; Kukucka, M.; de Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardiothorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Bernhardt, A.M.; Schloglhofer, T.; Lauenroth, V.; Mueller, F.; Mueller, M.; Schoede, A.; Klopsch, C. Prevention and early treatment of driveline infections in ventricular assist device patients—The DESTINE staging proposal and the first standard of care protocol. J. Crit. Care 2020, 56, 106–112. [Google Scholar] [CrossRef]
- Leuck, A.M. Left ventricular assist device driveline infections: Recent advances and future goals. J. Thorac. Dis. 2015, 7, 2151–2157. [Google Scholar] [CrossRef]
- Patel, C.B.; Blue, L.; Cagliostro, B.; Bailey, S.H.; Entwistle, J.W.; John, R.; Thohan, V.; Cleveland, J.C., Jr.; Goldstein, D.J.; Uriel, N.; et al. Left ventricular assist systems and infection-related outcomes: A comprehensive analysis of the MOMENTUM 3 trial. J. Heart Lung Transplant. 2020, 39, 774–781. [Google Scholar] [CrossRef]
- Krzelj, K.; Petricevic, M.; Gasparovic, H.; Biocina, B.; McGiffin, D. Ventricular Assist Device Driveline Infections: A Systematic Review. Thorac. Cardiovasc. Surg. 2022, 70, 493–504. [Google Scholar] [CrossRef]
- Hannan, M.M.; Husain, S.; Mattner, F.; Danziger-Isakov, L.; Drew, R.J.; Corey, G.R.; Schueler, S.; Holman, W.L.; Lawler, L.P.; Gordon, S.M.; et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J. Heart Lung Transplant. 2011, 30, 375–384. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Naftel, D.; Holman, W.; Bellumkonda, L.; Pamboukian, S.V.; Pagani, F.D.; Kirklin, J. Continuous-flow devices and percutaneous site infections: Clinical outcomes. J. Heart Lung Transplant. 2012, 31, 1151–1157. [Google Scholar] [CrossRef]
- Simpson, M.T.; Ning, Y.; Kurlansky, P.; Colombo, P.C.; Yuzefpolskaya, M.; Uriel, N.; Naka, Y.; Takeda, K. Outcomes of treatment for deep left ventricular assist device infection. J. Thorac. Cardiovasc. Surg. 2024, 167, 1824–1832.2. [Google Scholar] [CrossRef]
- Gordon, R.J.; Weinberg, A.D.; Pagani, F.D.; Slaughter, M.S.; Pappas, P.S.; Naka, Y.; Goldstein, D.J.; Dembitsky, W.P.; Giacalone, J.C.; Ferrante, J.; et al. Prospective, multicenter study of ventricular assist device infections. Circulation 2013, 127, 691–702. [Google Scholar] [CrossRef]
- John, R.; Aaronson, K.D.; Pae, W.E.; Acker, M.A.; Hathaway, D.R.; Najarian, K.B.; Slaughter, M.S.; HeartWare Bridge to Transplant, A.T.I. Drive-line infections and sepsis in patients receiving the HVAD system as a left ventricular assist device. J. Heart Lung Transplant. 2014, 33, 1066–1073. [Google Scholar] [CrossRef]
- Contreras, F.J.; Pinsker, B.L.; Katz, J.N.; Russell, S.D.; Schroder, J.; Bryner, B.; Gunn, A.H.; Amin, K.; Milano, C. Value of nutritional indices in predicting survival free from pump replacement and driveline infections in centrifugal left ventricular assist devices. JTCVS Open 2024, 19, 175–182. [Google Scholar] [CrossRef]
- Dettbarn, E.; Prenga, M.; Stein, J.; Muller, M.; Hoermandinger, C.; Schoenrath, F.; Falk, V.; Potapov, E.; Mulzer, J.; Knierim, J. Driveline infections in left ventricular assist devices-Incidence, epidemiology, and staging proposal. Artif. Organs 2024, 48, 83–90. [Google Scholar] [CrossRef]
- Pagani, F.D. Driveline Infections Associated With Durable Left Ventricular Assist Device Support: An Ounce of Prevention is Worth a Pound of Cure. ASAIO J. 2022, 68, 1459–1460. [Google Scholar] [CrossRef]
- Cagliostro, B.; Levin, A.P.; Fried, J.; Stewart, S.; Parkis, G.; Mody, K.P.; Garan, A.R.; Topkara, V.; Takayama, H.; Naka, Y.; et al. Continuous-flow left ventricular assist devices and usefulness of a standardized strategy to reduce drive-line infections. J. Heart Lung Transplant. 2016, 35, 108–114. [Google Scholar] [CrossRef]
- Yarboro, L.T.; Bergin, J.D.; Kennedy, J.L.; Ballew, C.C.; Benton, E.M.; Ailawadi, G.; Kern, J.A. Technique for minimizing and treating driveline infections. Ann. Cardiothorac. Surg. 2014, 3, 557–562. [Google Scholar] [CrossRef]
- Kusne, S.; Mooney, M.; Danziger-Isakov, L.; Kaan, A.; Lund, L.H.; Lyster, H.; Wieselthaler, G.; Aslam, S.; Cagliostro, B.; Chen, J.; et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J. Heart Lung Transplant. 2017, 36, 1137–1153. [Google Scholar] [CrossRef]
- Bauer, T.M.; Choi, J.H.; Luc, J.G.Y.; Weber, M.P.; Moncho Escriva, E.; Patel, S.; Maynes, E.J.; Boyle, A.J.; Samuels, L.E.; Entwistle, J.W.; et al. Device exchange versus nonexchange modalities in left ventricular assist device-specific infections: A systematic review and meta-analysis. Artif. Organs 2019, 43, 448–457. [Google Scholar] [CrossRef]
- Wadiwala, I.; Garg, P.; Alamouti-Fard, E.; Landolfo, K.; Sareyyupoglu, B.; Ahmed, M.E.; Jacob, S.; Pham, S. Absorbable antibiotic beads for treatment of LVAD driveline infections. Artif. Organs 2024, 48, 559–566. [Google Scholar] [CrossRef]
- Juraszek, A.; Smolski, M.; Kolsut, P.; Szymanski, J.; Litwinski, P.; Kusmierski, K.; Zakrzewska-Koperska, J.; Sterlinski, M.; Dziodzio, T.; Kusmierczyk, M. Prevalence and management of driveline infections in mechanical circulatory support—A single center analysis. J. Cardiothorac. Surg. 2021, 16, 216. [Google Scholar] [CrossRef]
- Ząbek, A.; Boczar, K.; Holcman, K.; Kostkiewicz, M.; Ulman, M.; Rydlewska, A.; Golińska-Grzybała, K.; Dębski, M.; Lelakowski, J.; Wierzbicki, K. Initial experience with transvenous lead extraction in patients with left ventricular assist devices. Kardiol. Pol. 2025, 83, 339–341. [Google Scholar] [CrossRef]
- Seretny, J.; Pidborochynski, T.; Buchholz, H.; Freed, D.H.; MacArthur, R.; Dubyk, N.; Cunliffe, L.; Zelaya, O.; Conway, J. Decreasing driveline infections in patients supported on ventricular assist devices: A care pathway approach. BMJ Open Qual. 2022, 11, e001815. [Google Scholar] [CrossRef]
- Trachtenberg, B.H.; Cordero-Reyes, A.; Elias, B.; Loebe, M. A review of infections in patients with left ventricular assist devices: Prevention, diagnosis and management. Methodist. Debakey Cardiovasc. J. 2015, 11, 28–32. [Google Scholar] [CrossRef]
- Jakus, N.; Brugts, J.J.; Claggett, B.; Timmermans, P.; Pouleur, A.C.; Rubiś, P.; Van Craenenbroeck, E.M.; Gaizauskas, E.; Barge-Caballero, E.; Paolillo, S.; et al. Improved survival of left ventricular assist device carriers in Europe according to implantation eras: Results from the PCHF-VAD registry. Eur. J. Heart Fail. 2022, 24, 1305–1315. [Google Scholar] [CrossRef]
- Vadalà, G.; Madaudo, C.; Fontana, A.; Sucato, V.; Bicelli, G.; Maniscalco, L.; Parlati, A.L.M.; Panarello, G.; Sciacca, S.; Pilato, M.; et al. Gastrointestinal Bleeding During Long-Term Left Ventricular Assist Device Support: External Validation of UTAH Bleeding Risk Score. J. Cardiovasc. Dev. Dis. 2025, 12, 105. [Google Scholar] [CrossRef]
- Spano, G.; Buffle, E.; Walti, L.N.; Mihalj, M.; Cameron, D.R.; Martinelli, M.; Furholz, M.; Que, Y.A.; Hayward, C.; Reineke, D.; et al. Ten-year retrospective cohort analysis of ventricular assist device infections. Artif. Organs 2023, 47, 898–905. [Google Scholar] [CrossRef]
- Puschel, A.; Skusa, R.; Bollensdorf, A.; Gross, J. Local Treatment of Driveline Infection with Bacteriophages. Antibiotics 2022, 11, 1310. [Google Scholar] [CrossRef]
- Topkara, V.K.; Kondareddy, S.; Malik, F.; Wang, I.W.; Mann, D.L.; Ewald, G.A.; Moazami, N. Infectious complications in patients with left ventricular assist device: Etiology and outcomes in the continuous-flow era. Ann. Thorac. Surg. 2010, 90, 1270–1277. [Google Scholar] [CrossRef]
- Raymond, A.L.; Kfoury, A.G.; Bishop, C.J.; Davis, E.S.; Goebel, K.M.; Stoker, S.; Selzman, C.H.; Clayson, S.E.; Smith, H.; Cowley, C.G.; et al. Obesity and left ventricular assist device driveline exit site infection. ASAIO J. 2010, 56, 57–60. [Google Scholar] [CrossRef]
- Koval, C.E.; Stosor, V.; on behalf of the AST ID Community of Practice. Ventricular assist device-related infections and solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13552. [Google Scholar] [CrossRef] [PubMed]
- Toba, F.A.; Akashi, H.; Arrecubieta, C.; Lowy, F.D. Role of biofilm in Staphylococcus aureus and Staphylococcus epidermidis ventricular assist device driveline infections. J. Thorac. Cardiovasc. Surg. 2011, 141, 1259–1264. [Google Scholar] [CrossRef]
- Holcman, K.; Ząbek, A.; Boczar, K.; Rubiś, P.; Ćmiel, B.; Szot, W.; Stępień, A.; Graczyk, K.; Podolec, P.; Kostkiewicz, M. The [99mTc]Tc-HMPAO-labelled white blood cell SPECT/CT as a novel criterion for infective endocarditis diagnosis. Int. J. Cardiol. 2024, 417, 132545. [Google Scholar] [CrossRef] [PubMed]
- Traykov, V.; Bongiorni, M.G.; Boriani, G.; Burri, H.; Costa, R.; Dagres, N.; Deharo, J.C.; Epstein, L.M.; Erba, P.A.; Snygg-Martin, U.; et al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: Results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace 2019, 21, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.M.; Pamirsad, M.A.; Brand, C.; Reichart, D.; Tienken, M.; Barten, M.J.; Schaefer, A.; Grahn, H.; Rybczynski, M.; Deuse, T.; et al. The value of fluorine-18 deoxyglucose positron emission tomography scans in patients with ventricular assist device specific infections dagger. Eur. J. Cardiothorac. Surg. 2017, 51, 1072–1077. [Google Scholar] [CrossRef]
- Cikes, M.; Jakus, N.; Claggett, B.; Brugts, J.J.; Timmermans, P.; Pouleur, A.C.; Rubis, P.; Van Craenenbroeck, E.M.; Gaizauskas, E.; Grundmann, S.; et al. Cardiac implantable electronic devices with a defibrillator component and all-cause mortality in left ventricular assist device carriers: Results from the PCHF-VAD registry. Eur. J. Heart Fail. 2019, 21, 1129–1141. [Google Scholar] [CrossRef]


| All Patients (n = 90) | DLIs Group (n = 20) | Non-DLIs Group (n = 70) | p-Values | |
|---|---|---|---|---|
| Sex—No. (%) Male | 74 (82.2%) | 17 (85%) | 57 (81.4%) | 0.502 |
| Age—yr Mean Range | 43.6 ± 17.7 10–70 | 31.5 ± 15.9 12–67 | 47.1 ± 16.7 10–70 | 0.01 |
| Age group—No. (%) Pediatric Adult | 17 (18.9%) 73 (81.1%) | 8 (40.0%) 12 (60%) | 9 (12.9%) 61 (87.1%) | 0.21 |
| BMI Mean Range | 25.5 ± 5.6 13.0–40.2 | 24.9 ± 5.7 14.4–32.5 | 25.6 ± 5.6 13.0–40.2 | 0.77 |
| Hypertension—No. (%) | 21 (23.3%) | 5 (25%) | 16 (22.9%) | 0.47 |
| Diabetes—No. (%) | 18 (20%) | 4 (20%) | 14 (20%) | 0.58 |
| Chronic Kidney Failure—No. (%) | 6 (6.7%) | 0 (0%) | 6 (8.6%) | 0.21 |
| Hyperlipidemia—No. (%) | 42 (46.7%) | 2 (10%) | 40 (57.1%) | 0.04 |
| COPD—No. (%) | 5 (6.7%) | 1 (5%) | 4 (5.7%) | 0.37 |
| Previous Stroke—No. (%) | 3 (3.3%) | 0 (0%) | 3 (4.3%) | 0.99 |
| History of Peripheral Vascular Disease—No. (%) | 4 (4.4%) | 1 (5%) | 3 (4.3%) | 0.97 |
| Smoking History | 35 (38.9%) | 6 (30%) | 29 (41.4%) | 0.39 |
| Previous Implantable Defibrillator—No. (%) | 17 (18.9%) | 4 (20%) | 13 (18.6%) | 0.36 |
| Hemoglobin (g/dL), mean ± SD | 10.2 ± 0.7 | 10.4 ± 0.2 | 9.9 ± 0.8 | 0.78 |
| Platelet Count (×109/L)—mean ± SD | 112.7 ± 17.8 | 115.8 ± 21.2 | 121.0 ± 19.4 | 0.99 |
| Albumin Level (g/L)—mean ± SD | 32.2 ± 4.7 | 28.8 ± 2.4 | 33.6 ± 5.3 | 0.03 |
| Multi-Organ Failure—No. (%) | 12 (13.33%) | 2 (10%) | 10 (14.3%) | 0.46 |
| VAD Duration Time (days) Mean Range | 561.7 ± 833.2 1–4112 | 1277.85 ± 621.6 149–2779 | 356.3 ± 773.0 1- 4112 | 0.001 |
| Heart Failure Etiology—No. (%) DCMP ICMP Other | 42 (46.7%) 44 (48.9%) 4 (4.4%) | 15 (75%) 4 (20%) 1 (5%) | 27 (38.6%) 40 (57.1%) 3 (4.3%) | 0.02 |
| LVEF% Mean Range | 18.62 ± 6.30 10–35 | 22.11 ± 8.2 10–35 | 17.6 ± 5.21 10–31 | 0.22 |
| Type of Device—No. (%) HeartWare HVAD HeartMate 2 HeartMate 3 BiVAD | 39 (43.3%) 10 (11.1%) 38 (42.2%) 3 (3.3%) | 11 (55%) 1 (5%) 8 (40%) 0 (0%) | 28 (40%) 9 (12.9%) 30 (42.9%) 3 (4.3%) | 0.34 |
| CPB Times—minutes Mean Range | 202.4 ± 71.9 111–433 | 175 ± 50.8 144–283 | 209.5 101–433 | 0.10 |
| Intention of VAD—No. (%) Bridge to transplant Bridge to destination | 77 (85.6%) 13 (14.4%) | 19 (95%) 1 (5%) | 58 (85.9%) 12 (11.1%) | 0.16 |
| Number of Days from VAD Implantation to First DLI | All Patients (n = 20) |
|---|---|
| Median Interquartile Range | 513 404 |
| DL Infection Symptoms—No. (%) Drainage from the DLES Fever Erythema Pain in the DL | 20 (100%) 5 (25%) 7 (35%) 4 (20%) |
| Increased Acute-Phase Reactants—No. (%) | 20 (100%) |
| Signs of Infection by Ultrasonography—No. (%) | 12 (60%) |
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Age <52 y | 11.78 (1.6–88.6) | 0.02 | 9.77 (1.3–74.5) | 0.03 |
| Hyperlipidemia Yes/No | 0.14 (0.03–0.059) | 0.01 | ||
| Albumin Level (g/dL) <30.35 g/dL | 12.79 (1.70–95.73) | 0.01 | 10.55 (1.40–79.35) | 0.02 |
| Etiology DCMP vs. ICMP Others vs. ICMP | 3.19 (1.04–9.69) 3.47 (0.376–32.02) | 0.04 0.27 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saricaoglu, M.C.; Kandemir, M.; Saricaoglu, E.M.; Karacuha, A.F.; Kadiroglu, E.; Abdullahi, M.F.; Inan, M.B.; Azap, A.; Akar, A.R. Driveline Relocation and Vacuum-Assisted Closure for Ventricular Assist Device Driveline Infections. J. Cardiovasc. Dev. Dis. 2025, 12, 211. https://doi.org/10.3390/jcdd12060211
Saricaoglu MC, Kandemir M, Saricaoglu EM, Karacuha AF, Kadiroglu E, Abdullahi MF, Inan MB, Azap A, Akar AR. Driveline Relocation and Vacuum-Assisted Closure for Ventricular Assist Device Driveline Infections. Journal of Cardiovascular Development and Disease. 2025; 12(6):211. https://doi.org/10.3390/jcdd12060211
Chicago/Turabian StyleSaricaoglu, Mehmet Cahit, Melisa Kandemir, Elif M. Saricaoglu, Ali Fuat Karacuha, Ezel Kadiroglu, Mustafa Farah Abdullahi, Mustafa Bahadir Inan, Alpay Azap, and Ahmet Ruchan Akar. 2025. "Driveline Relocation and Vacuum-Assisted Closure for Ventricular Assist Device Driveline Infections" Journal of Cardiovascular Development and Disease 12, no. 6: 211. https://doi.org/10.3390/jcdd12060211
APA StyleSaricaoglu, M. C., Kandemir, M., Saricaoglu, E. M., Karacuha, A. F., Kadiroglu, E., Abdullahi, M. F., Inan, M. B., Azap, A., & Akar, A. R. (2025). Driveline Relocation and Vacuum-Assisted Closure for Ventricular Assist Device Driveline Infections. Journal of Cardiovascular Development and Disease, 12(6), 211. https://doi.org/10.3390/jcdd12060211







