Lifestyle and Pharmacological Interventions to Prevent Anthracycline-Related Cardiotoxicity in Cancer Patients
Abstract
1. Introduction
2. Epidemiology of Anthracycline-Induced Cardiotoxicity
2.1. Incidence and Prevalence in Adult and Pediatric Populations
2.2. Anthracycline Use Across Different Malignancies
2.3. Cumulative Dose and Cardiotoxicity Thresholds
2.4. Identification of High-Risk Populations
2.5. Long-Term Outcomes and Healthcare Burden
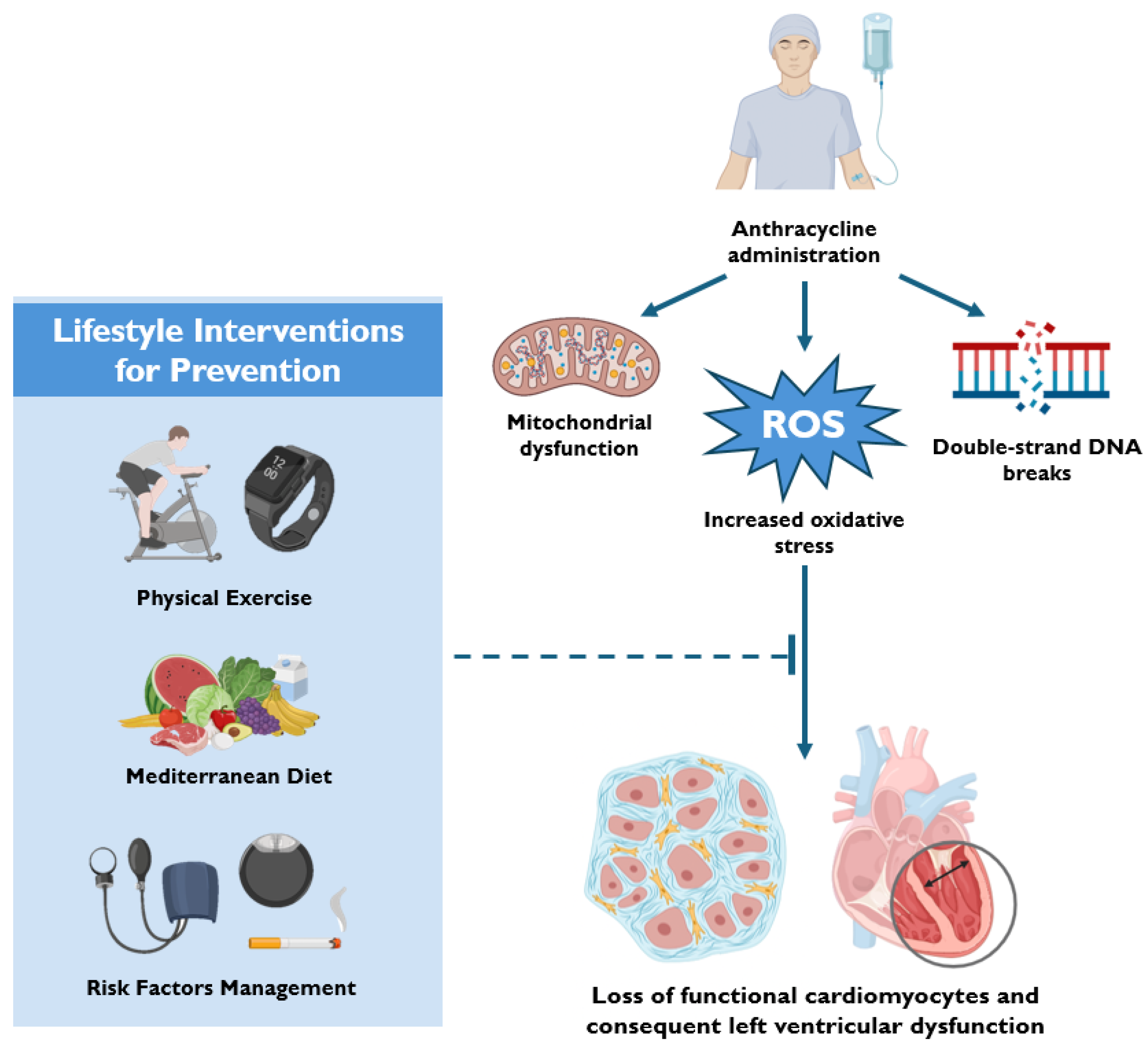
3. Mechanisms of Anthracycline-Induced Cardiotoxicity
3.1. Oxidative Stress and Mitochondrial Dysfunction
3.2. Disruption of Calcium Homeostasis
3.3. Activation of Cell Death Pathways
3.4. DNA Damage and Epigenetic Modifications
3.5. Synergistic Effects with Other Cancer Therapies
4. Lifestyle Interventions for Prevention
5. Pharmacological Strategies for Prevention
6. Novel Therapeutic Pharmacological Interventions
6.1. SGLT2 Inhibitors
6.2. GLP-1 Receptor Agonists
6.3. Vericiguat
6.4. Sacubitril/Valsartan
7. Role of Multidisciplinary Cardio-Oncology Teams: Integrating Prevention and Management
8. Personalized Strategies Using Risk Stratification Tools
9. Long-Term Follow-Up Protocols
9.1. Biomarkers, Imaging, and Lifestyle Reassessment in Survivors
9.2. Integration of Cardio-Protective Strategies into Clinical Workflows
10. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lotrionte, M.; Biondi-Zoccai, G.; Abbate, A.; Lanzetta, G.; D’Ascenzo, F.; Malavasi, V.; Peruzzi, M.; Frati, G.; Palazzoni, G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am. J. Cardiol. 2013, 112, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J.-Cardiovasc. Imaging 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Camilli, M.; Cipolla, C.M.; Dent, S.; Minotti, G.; Cardinale, D.M. Anthracycline Cardiotoxicity in Adult Cancer Patients: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncolgy 2024, 6, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, I.; Chianca, M.; Cipolla, C.M.; Cardinale, D.M. Anthracycline-induced cardiomyopathy: Risk prediction, prevention and treatment. Nat. Rev. Cardiol. 2025. [Google Scholar] [CrossRef]
- Fabiani, I.; Chianca, M.; Aimo, A.; Emdin, M.; Dent, S.; Fedele, A.; Cipolla, C.M.; Cardinale, D.M. Use of new and emerging cancer drugs: What the cardiologist needs to know. Eur. Heart J. 2024, 45, 1971–1987. [Google Scholar] [CrossRef]
- Balough, E.; Ariza, A.; Asnani, A.; Hoeger, C.W. Cardiotoxicity of Anthracyclines. Cardiol. Clin. 2025, 43, 111–127. [Google Scholar] [CrossRef]
- Iervolino, A.; Spadafora, L.; Spadaccio, C.; Iervolino, V.; Biondi Zoccai, G.; Andreotti, F. Myocardial Cell Preservation from Potential Cardiotoxic Drugs: The Role of Nanotechnologies. Pharmaceutics 2022, 15, 87. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Martinoni, A.; LabTech, A.T.; Civelli, M.; Lamantia, G.; Cinieri, S.; Martinelli, G.; Cipolla, C.M.; Fiorentini, C. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J. Am. Coll. Cardiol. 2000, 36, 517–522. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Bansal, N.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Chemotherapy-induced cardiotoxicity in children. Expert Opin. Drug Metab. Toxicol. 2017, 13, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Tini, G.; Cuomo, A.; Battistoni, A.; Sarocchi, M.; Mercurio, V.; Ameri, P.; Volpe, M.; Porto, I.; Tocchetti, C.G.; Spallarossa, P. Baseline cardio-oncologic risk assessment in breast cancer women and occurrence of cardiovascular events: The HFA/ICOS risk tool in real-world practice. Int. J. Cardiol. 2022, 349, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Ferdinandy, P.; Salvatorelli, E.; Menna, P.; Minotti, G. Anthracyclines, Diastolic Dysfunction and the road to Heart Failure in Cancer survivors: An untold story. Prog. Cardiovasc. Dis. 2024, 86, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Madaudo, C.; Alagna, G.; Santoro, M.; Rossetto, L.; Siragusa, S.; Novo, G. The new HFA/ICOS risk assessment tool to identify patients with chronic myeloid leukaemia at high risk of cardiotoxicity. ESC Heart Fail. 2022, 9, 1914–1919. [Google Scholar] [CrossRef]
- Giallauria, F.; Vitelli, A.; Maresca, L.; De Magistris, M.S.; Chiodini, P.; Mattiello, A.; Gentile, M.; Mancini, M.; Grieco, A.; Russo, A.; et al. Exercise training improves cardiopulmonary and endothelial function in women with breast cancer: Findings from the Diana-5 dietary intervention study. Intern. Emerg. Med. 2016, 11, 183–189. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef]
- Semeraro, G.C.; Lamantia, G.; Cipolla, C.M.; Cardinale, D. How to identify anthracycline-induced cardiotoxicity early and reduce its clinical impact in everyday practice. Kardiol. Pol. 2021, 79, 114–122. [Google Scholar] [CrossRef]
- Upshaw, J.N.; Parson, S.K.; Buchsbaum, R.J.; Schlam, I.; Ruddy, K.J.; Durani, U.; Epperla, N.; Leong, D.P. Dexrazoxane to Prevent Cardiotoxicity in Adults Treated with Anthracyclines: JACC: CardioOncology Controversies in Cardio-Oncology. JACC CardioOncology 2024, 6, 322–324. [Google Scholar] [CrossRef]
- Avagimyan, A.; Pogosova, N.; Kakturskiy, L.; Sheibani, M.; Challa, A.; Kogan, E.; Fogacci, F.; Mikhaleva, L.; Vandysheva, R.; Yakubovskaya, M.; et al. Doxorubicin-related cardiotoxicity: Review of fundamental pathways of cardiovascular system injury. Cardiovasc. Pathol. 2024, 73, 107683. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Huang, Y. Anthracycline-induced cardiotoxicity: An overview from cellular structural perspective. Biomed. Pharmacother. 2024, 179, 117312. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, P.; Huang, Y. Anthracycline-induced cardiotoxicity: Mechanisms, monitoring, and prevention. Front. Cardiovasc. Med. 2023, 10, 1242596. [Google Scholar] [CrossRef] [PubMed]
- Menna, P.; Recalcati, S.; Cairo, G.; Minotti, G. An introduction to the metabolic determinants of anthracycline cardiotoxicity. Cardiovasc. Toxicol. 2007, 7, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Sousa, R.P.; Cadete, V.J.; Lopaschuk, G.D.; Palmeira, C.M.; Bjork, J.A.; Wallace, K.B. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology 2010, 270, 92–98. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef]
- Hanna, A.D.; Lam, A.; Tham, S.; Dulhunty, A.F.; Beard, N.A. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol. Pharmacol. 2014, 86, 438–449. [Google Scholar] [CrossRef]
- Li, H.; Xia, B.; Chen, W.; Zhang, Y.; Gao, X.; Chinnathambi, A.; Alharbi, S.A.; Zhao, Y. Nimbolide prevents myocardial damage by regulating cardiac biomarkers, antioxidant level, and apoptosis signaling against doxorubicin-induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2020, 34, e22543. [Google Scholar] [CrossRef]
- Renu, K.; Abilash, V.G.; Tirupathi Pichiah, P.B.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Gharanei, M.; Hussain, A.; Janneh, O.; Maddock, H.L. Doxorubicin induced myocardial injury is exacerbated following ischaemic stress via opening of the mitochondrial permeability transition pore. Toxicol. Appl. Pharmacol. 2013, 268, 149–156. [Google Scholar] [CrossRef]
- An, J.; Li, P.; Li, J.; Dietz, R.; Donath, S. ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J. Mol. Med. 2009, 87, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Tewey, K.M.; Rowe, T.C.; Yang, L.; Halligan, B.D.; Liu, L.F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 1984, 226, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Schirone, L.; Vecchio, D.; Valenti, V.; Forte, M.; Relucenti, M.; Angelini, A.; Zaglia, T.; Schiavon, S.; D’ambrosio, L.; Sarto, G.; et al. MST1 mediates doxorubicin-induced cardiomyopathy by SIRT3 downregulation. Cell. Mol. Life. Sci. 2023, 80, 245. [Google Scholar] [CrossRef] [PubMed]
- Schirone, L.; D’Ambrosio, L.; Forte, M.; Genovese, R.; Schiavon, S.; Spinosa, G.; Iacovone, G.; Valenti, V.; Frati, G.; Sciarretta, S. Mitochondria and Doxorubicin-Induced Cardiomyopathy: A Complex Interplay. Cells 2022, 11, 2000. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Nakano, K.; Vousden, K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Fan, X.; He, Y.; Wu, G.; Chen, H.; Cheng, X.; Zhan, Y.; An, C.; Chen, T.; Wang, X. Sirt3 activates autophagy to prevent DOX-induced senescence by inactivating PI3K/AKT/mTOR pathway in A549 cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2023, 1870, 119411. [Google Scholar] [CrossRef]
- Li, D.L.; Wang, Z.V.; Ding, G.; Tan, W.; Luo, X.; Criollo, A.; Xie, M.; Jiang, N.; May, H.; Kyrychenko, V.; et al. Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation 2016, 133, 1668–1687. [Google Scholar] [CrossRef]
- Tavakoli Dargani, Z.; Singla, D.K. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H460–H471. [Google Scholar] [CrossRef]
- Sauter, K.A.; Wood, L.J.; Wong, J.; Iordanov, M.; Magun, B.E. Doxorubicin and daunorubicin induce processing and release of interleukin-1beta through activation of the NLRP3 inflammasome. Cancer Biol. Ther. 2011, 11, 1008–1016. [Google Scholar] [CrossRef]
- Meng, L.; Lin, H.; Zhang, J.; Lin, N.; Sun, Z.; Gao, F.; Luo, H.; Ni, T.; Luo, W.; Chi, J.; et al. Doxorubicin induces cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional stabilization of NLR family pyrin domain containing 3. J. Mol. Cell. Cardiol. 2019, 136, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhong, T.; Ma, Y.; Wan, X.; Qin, A.; Yao, B.; Zou, H.; Song, Y.; Yin, D. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 2020, 242, 117186. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kang, Y.M.; Liu, W.; Zang, W.J.; Bao, C.Y.; Qin, D.N. Inhibition of cyclooxygenase-2 reduces hypothalamic excitation in rats with adriamycin-induced heart failure. PLoS ONE 2012, 7, e48771. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Cervantes, M.; Froufe, H.J.C.; Egas, C.; Cunha-Oliveira, T.; Sassone-Corsi, P.; Oliveira, P.J. Doxorubicin persistently rewires cardiac circadian homeostasis in mice. Arch. Toxicol. 2020, 94, 257–271. [Google Scholar] [CrossRef]
- Forte, M.; D’Ambrosio, L.; Schiattarella, G.G.; Salerno, N.; Perrone, M.A.; Loffredo, F.S.; Bertero, E.; Pilichou, K.; Manno, G.; Valenti, V.; et al. Mitophagy modulation for the treatment of cardiovascular diseases. Eur. J. Clin. Investig. 2024, 54, e14199. [Google Scholar] [CrossRef]
- Song, R.; Yang, Y.; Lei, H.; Wang, G.; Huang, Y.; Xue, W.; Wang, Y.; Yao, L.; Zhu, Y. HDAC6 inhibition protects cardiomyocytes against doxorubicin-induced acute damage by improving alpha-tubulin acetylation. J. Mol. Cell. Cardiol. 2018, 124, 58–69. [Google Scholar] [CrossRef]
- Guglin, M.; Krischer, J.; Tamura, R.; Fink, A.; Bello-Matricaria, L.; McCaskill-Stevens, W.; Munster, P.N. Randomized Trial of Lisinopril Versus Carvedilol to Prevent Trastuzumab Cardiotoxicity in Patients with Breast Cancer. J. Am. Coll. Cardiol. 2019, 73, 2859–2868. [Google Scholar] [CrossRef]
- Vitale, R.; Marzocco, S.; Popolo, A. Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account. Int. J. Mol. Sci. 2024, 25, 7477. (In English) [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Kim, Y.; Restrepo-Cordoba, M.A.; Lunde, I.G.; Wakimoto, H.; Smith, A.M.; Toepfer, C.N.; Getz, K.; Gorham, J.; Patel, P.; et al. Genetic Variants Associated with Cancer Therapy-Induced Cardiomyopathy. Circulation 2019, 140, 31–41. [Google Scholar] [CrossRef]
- Hahn, V.S.; Zhang, K.W.; Sun, L.; Narayan, V.; Lenihan, D.J.; Ky, B. Heart Failure with Targeted Cancer Therapies: Mechanisms and Cardioprotection. Circ. Res. 2021, 128, 1576–1593. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Lenihan, D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2017, 13, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Passantino, A.; Dalla Vecchia, L.A.; Corra, U.; Scalvini, S.; Pistono, M.; Bussotti, M.; Gambarin, F.I.; Scrutinio, D.; La Rovere, M.T. The Future of Exercise-Based Cardiac Rehabilitation for Patients with Heart Failure. Front. Cardiovasc. Med. 2021, 8, 709898. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Gathright, E.C.; Khadanga, S.; Ozemek, C.; Thomas, R.J. Core Components of Cardiac Rehabilitation Programs: 2024 Update: A Scientific Statement From the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef] [PubMed]
- Tranchita, E.; Murri, A.; Grazioli, E.; Cerulli, C.; Emerenziani, G.P.; Ceci, R.; Caporossi, D.; Dimauro, I.; Parisi, A. The Beneficial Role of Physical Exercise on Anthracyclines Induced Cardiotoxicity in Breast Cancer Patients. Cancers 2022, 14, 2288. [Google Scholar] [CrossRef]
- Naaktgeboren, W.R.; Binyam, D.; Stuiver, M.M.; Aaronson, N.K.; Teske, A.J.; van Harten, W.H.; Groen, W.G.; May, A.M. Efficacy of Physical Exercise to Offset Anthracycline-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis of Clinical and Preclinical Studies. J. Am. Heart. Assoc. 2021, 10, e021580. [Google Scholar] [CrossRef]
- Ghignatti, P.; Nogueira, L.J.; Lehnen, A.M.; Leguisamo, N.M. Cardioprotective effects of exercise training on doxorubicin-induced cardiomyopathy: A systematic review with meta-analysis of preclinical studies. Sci. Rep. 2021, 11, 6330. [Google Scholar] [CrossRef]
- Pahlavani, H.A. Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front. Cell Dev. Biol. 2022, 10, 950927. [Google Scholar] [CrossRef]
- Schuttler, D.; Clauss, S.; Weckbach, L.T.; Brunner, S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells 2019, 8, 1128. [Google Scholar] [CrossRef]
- Jiang, J.; Ni, L.; Zhang, X.; Chatterjee, E.; Lehmann, H.I.; Li, G.; Xiao, J. Keeping the Heart Healthy: The Role of Exercise in Cardiac Repair and Regeneration. Antioxid. Redox Signal. 2023, 39, 1088–1107. [Google Scholar] [CrossRef]
- Nijholt, K.T.; Sanchez-Aguilera, P.I.; Voorrips, S.N.; de Boer, R.A.; Westenbrink, B.D. Exercise: A molecular tool to boost muscle growth and mitochondrial performance in heart failure? Eur. J. Heart Fail. 2022, 24, 287–298. [Google Scholar] [CrossRef]
- Howden, E.J.; Bigaran, A.; Beaudry, R.; Fraser, S.; Selig, S.; Foulkes, S.; Antill, Y.; Nightingale, S.; Loi, S.; Haykowsky, M.J.; et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur. J. Prev. Cardiol. 2019, 26, 305–315. [Google Scholar] [CrossRef]
- Dimeo, F.; Pagonas, N.; Seibert, F.; Arndt, R.; Zidek, W.; Westhoff, T.H. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension 2012, 60, 653–658. [Google Scholar] [CrossRef]
- Lu, L.; Mei, D.F.; Gu, A.G.; Wang, S.; Lentzner, B.; Gutstein, D.E.; Zwas, D.; Homma, S.; Yi, G.-H.; Wang, J. Exercise training normalizes altered calcium-handling proteins during development of heart failure. J. Appl. Physiol. 2002, 92, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Linke, A. Impact of exercise training on cardiovascular disease and risk. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Gilchrist, S.C.; Barac, A.; Ades, P.A.; Alfano, C.M.; Franklin, B.A.; Jones, L.W.; La Gerche, A.; Ligibel, J.A.; Lopez, G.; Madan, K.; et al. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e997–e1012. [Google Scholar] [CrossRef]
- Herranz-Gomez, A.; Cuenca-Martinez, F.; Suso-Marti, L.; Varangot-Reille, C.; Calatayud, J.; Blanco-Díaz, M.; Casaña, J. Effectiveness of HIIT in patients with cancer or cancer survivors: An umbrella and mapping review with meta-meta-analysis. Scand. J. Med. Sci. Sports 2022, 32, 1522–1549. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef]
- Kang, D.W.; Wilson, R.L.; Christopher, C.N.; Normann, A.J.; Barnes, O.; Lesansee, J.D.; Choi, G.; Dieli-Conwright, C.M. Exercise Cardio-Oncology: Exercise as a Potential Therapeutic Modality in the Management of Anthracycline-Induced Cardiotoxicity. Front. Cardiovasc. Med. 2021, 8, 805735. [Google Scholar] [CrossRef]
- Frazelle, M.L.; Friend, P.J. Optimizing the Teachable Moment for Health Promotion for Cancer Survivors and Their Families. J. Adv. Pract. Oncol. 2016, 7, 422–433. (In English) [Google Scholar] [PubMed]
- Shephard, R.J. Maximal oxygen intake and independence in old age. Br. J. Sports Med. 2009, 43, 342–346. [Google Scholar] [CrossRef]
- Stephenson, E.; McLaughlin, M.; Bray, J.W.; Saxton, J.M.; Vince, R.V. Nutrition Modulation of Cardiotoxicity in Breast Cancer: A Scoping Review. Nutrients 2024, 16, 3777. [Google Scholar] [CrossRef]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Llanos-Gonzalez, E.; Alcain, F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants 2021, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaur, H.; Devi, P.; Mohan, V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol. Ther. 2009, 124, 259–268. [Google Scholar] [CrossRef]
- Weant, K.A.; Smith, K.M. The role of coenzyme Q10 in heart failure. Ann. Pharmacother. 2005, 39, 1522–1526. [Google Scholar] [CrossRef]
- Nonn, L.; Peng, L.; Feldman, D.; Peehl, D.M. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: Implications for prostate cancer prevention by vitamin D. Cancer Res. 2006, 66, 4516–4524. [Google Scholar] [CrossRef]
- Zittermann, A.; Schleithoff, S.S.; Koerfer, R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br. J. Nutr. 2005, 94, 483–492. [Google Scholar] [CrossRef]
- Lee, K.J.; Wright, G.; Bryant, H.; Wiggins, L.A.; Zotto, V.L.D.; Schuler, M.; Malozzi, C.; Cohen, M.V.; Gassman, N.R. Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7439. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.U.; Clear, K.Y.J.; Cornelius, Z.; Bawaneh, A.; Feliz-Mosquea, Y.R.; Wilson, A.S.; Ruggiero, A.D.; Cruz-Diaz, N.; Shi, L.; Kerr, B.A.; et al. Diet impacts triple-negative breast cancer growth, metastatic potential, chemotherapy responsiveness, and doxorubicin-mediated cardiac dysfunction. Physiol. Rep. 2022, 10, e15192. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Rev. Esp. Cardiol. 2022, 75, 429. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Rivera-Theurel, F.; Scott, J.M.; Nadler, M.B.; Foulkes, S.; Leong, D.; Nilsen, T.; Porter, C.; Haykowsky, M.; Abdel-Qadir, H.; et al. Cardio-oncology rehabilitation and exercise: Evidence, priorities, and research standards from the ICOS-CORE working group. Eur. Heart J. 2025, ehaf100. [Google Scholar] [CrossRef] [PubMed]
- Nakamae, H.; Tsumura, K.; Terada, Y.; Nakane, T.; Nakamae, M.; Ohta, K.; Yamane, T.; Hino, M. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 2005, 104, 2492–2498, Erratum in Cancer 2015, 121, 3048. https://doi.org/10.1002/cncr.29458. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M.; et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef]
- Kaya, M.G.; Ozkan, M.; Gunebakmaz, O.; Akkaya, H.; Kaya, E.G.; Akpek, M.; Kalay, N.; Dikilitas, M.; Yarlioglues, M.; Karaca, H.; et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A randomized control study. Int. J. Cardiol. 2013, 167, 2306–2310. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R., Jr.; Cruz, F.d.D.; Brandão, S.M.G.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Filho, R.K.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef]
- Bosch, X.; Rovira, M.; Sitges, M.; Domènech, A.; Ortiz-Pérez, J.T.; de Caralt, T.M.; Morales-Ruiz, M.; Perea, R.J.; Monzó, M.; Esteve, J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J. Am. Coll. Cardiol. 2013, 61, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Heck, S.L.; Mecinaj, A.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.E.; Fagerland, M.W.; Gravdehaug, B.; Røsjø, H.; Steine, K.; Geisler, J.; et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): Extended Follow-Up of a 2x2 Factorial, Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Candesartan and Metoprolol. Circulation 2021, 143, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.K.; Villa, D.; Tsang, T.S.M.; Starovoytov, A.; Gelmon, K.; Virani, S.A. Effect of Eplerenone on Diastolic Function in Women Receiving Anthracycline-Based Chemotherapy for Breast Cancer. JACC CardioOncology 2019, 1, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; D’Agostino, R., Jr.; Crotts, T.; Craver, K.; Hackney, M.H.; Jordan, J.H.; Ky, B.; Wagner, L.I.; Herrington, D.M.; Yeboah, J.; et al. Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid. 2022, 1, EVIDoa2200097. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Houbois, C.; Marwick, T.H.; Kei, T.; Saha, S.; Runeckles, K.; Huang, F.; Shalmon, T.; Thorpe, K.E.; Pezo, R.C.; et al. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 515–525. [Google Scholar] [CrossRef]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 2023, 330, 528–536. [Google Scholar] [CrossRef]
- Sobczuk, P.; Czerwinska, M.; Kleibert, M.; Cudnoch-Jedrzejewska, A. Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system-from molecular mechanisms to therapeutic applications. Heart Fail. Rev. 2022, 27, 295–319. [Google Scholar] [CrossRef]
- Bozcali, E.; Dedeoglu, D.B.; Karpuz, V.; Suzer, O.; Karpuz, H. Cardioprotective effects of zofenopril, enalapril and valsartan against ischaemia/reperfusion injury as well as doxorubicin cardiotoxicity. Acta Cardiol. 2012, 67, 87–96. [Google Scholar] [CrossRef]
- Dessi, M.; Madeddu, C.; Piras, A.; Cadeddu, C.; Antoni, G.; Mercuro, G.; Mantovani, G. Long-term, up to 18 months, protective effects of the angiotensin II receptor blocker telmisartan on Epirubin-induced inflammation and oxidative stress assessed by serial strain rate. Springerplus 2013, 2, 198. [Google Scholar] [CrossRef]
- Lother, A.; Bergemann, S.; Kowalski, J.; Huck, M.; Gilsbach, R.; Bode, C.; Hein, L. Inhibition of the cardiac myocyte mineralocorticoid receptor ameliorates doxorubicin-induced cardiotoxicity. Cardiovasc. Res. 2018, 114, 282–290. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Tawara, S.; Fukumoto, Y.; Seto, M.; Yano, K.; Shimokawa, H. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ. J. 2009, 73, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Maack, C.; Kartes, T.; Kilter, H.; Schafers, H.-J.; Nickenig, G.; Bohm, M.; Laufs, U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 2003, 108, 1567–1574. [Google Scholar] [CrossRef]
- Huelsenbeck, J.; Henninger, C.; Schad, A.; Lackner, K.J.; Kaina, B.; Fritz, G. Inhibition of Rac1 signaling by lovastatin protects against anthracycline-induced cardiac toxicity. Cell Death Dis. 2011, 2, e190. [Google Scholar] [CrossRef]
- Bhalraam, U.; Veerni, R.B.; Paddock, S.; Meng, J.; Piepoli, M.; López-Fernández, T.; Tsampasian, V.; Vassiliou, V.S. Impact of sodium-glucose cotransporter-2 inhibitors on heart failure outcomes in cancer patients and survivors: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2025, zwaf026. [Google Scholar] [CrossRef] [PubMed]
- Gongora, C.A.; Drobni, Z.D.; Silva, T.Q.A.C.; Zafar, A.; Gong, J.; Zlotoff, D.A.; Gilman, H.K.; Hartmann, S.E.; Sama, S.; Nikolaidou, S.; et al. Sodium-Glucose Co-Transporter-2 Inhibitors and Cardiac Outcomes Among Patients Treated with Anthracyclines. JACC Heart Fail. 2022, 10, 559–567. [Google Scholar] [CrossRef]
- Dabour, M.S.; George, M.Y.; Daniel, M.R.; Blaes, A.H.; Zordoky, B.N. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology 2024, 6, 159–182. [Google Scholar] [CrossRef]
- Sabatino, J.; De Rosa, S.; Tamme, L.; Iaconetti, C.; Sorrentino, S.; Polimeni, A.; Mignogna, C.; Amorosi, A.; Spaccarotella, C.; Yasuda, M.; et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020, 19, 66. [Google Scholar] [CrossRef]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- Daniele, A.J.; Gregorietti, V.; Costa, D.; Lopez-Fernandez, T. Use of EMPAgliflozin in the prevention of CARDiotoxicity: The EMPACARD—PILOT trial. Cardio-Oncology 2024, 10, 58. [Google Scholar] [CrossRef]
- Li, X.; Luo, W.; Tang, Y.; Wu, J.; Zhang, J.; Chen, S.; Zhou, L.; Tao, Y.; Tang, Y.; Wang, F.; et al. Semaglutide attenuates doxorubicin-induced cardiotoxicity by ameliorating BNIP3-Mediated mitochondrial dysfunction. Redox. Biol. 2024, 72, 103129. [Google Scholar] [CrossRef] [PubMed]
- HamaSalih, R.M. Effects of Semaglutide in Doxorubicin-Induced Cardiac Toxicity in Wistar Albino Rats. Cancer Manag. Res. 2024, 16, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, H.; Xu, T.; Mei, X.; Wang, X.; Yang, Q.; Luo, Z.; Zeng, Q.; Xu, D.; Ren, H. Vericiguat attenuates doxorubicin-induced cardiotoxicity through the PRKG1/PINK1/STING axis. Transl. Res. 2024, 273, 90–103. (In English) [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Berretta, M.; Bisceglia, I.; Giacobbe, I.; Iovine, M.; Giordano, V.; Arianna, R.; Barbato, M.; Izzo, F.; Maurea, C.; et al. The sGCa Vericiguat Exhibit Cardioprotective and Anti-Sarcopenic Effects through NLRP-3 Pathways: Potential Benefits for Anthracycline-Treated Cancer Patients. Cancers 2024, 16, 1487. [Google Scholar] [CrossRef]
- Tajstra, M.; Dyrbus, M.; Rutkowski, T.; Składowski, K.; Sosnowska-Pasiarska, B.; Góźdź, S.; Radecka, B.; Staszewski, M.; Majsnerowska, A.; Myrda, K.; et al. Sacubitril/valsartan for cardioprotection in breast cancer (MAINSTREAM): Design and rationale of the randomized trial. ESC Heart Fail. 2023, 10, 3174–3183. [Google Scholar] [CrossRef]
- Hu, F.; Yan, S.; Lin, L.; Qiu, X.; Lin, X.; Wang, W. Sacubitril/valsartan attenuated myocardial inflammation, fibrosis, apoptosis and promoted autophagy in doxorubicin-induced cardiotoxicity mice via regulating the AMPKalpha-mTORC1 signaling pathway. Mol. Cell. Biochem. 2025, 480, 1891–1908. [Google Scholar] [CrossRef]
- Rivero-Santana, B.; Saldana-Garcia, J.; Caro-Codon, J.; Zamora, P.; Moliner, P.; Monzonis, A.M.; Zatarain, E.; Álvarez-Ortega, C.; Gómez-Prieto, P.; Pernas, S.; et al. Anthracycline-induced cardiovascular toxicity: Validation of the Heart Failure Association and International Cardio-Oncology Society risk score. Eur. Heart J. 2024, 46, 273–284. [Google Scholar] [CrossRef]
- Butel-Simoes, L.E.; Ngo, D.T.M.; Sverdlov, A.L. Navigating cardiotoxicity risk in cancer therapy: The importance of the HFA-ICOS score. Eur. Heart J. 2025, 46, 285–287. [Google Scholar] [CrossRef]
- Ky, B.; French, B.; Khan, A.M.; Plappert, T.; Wang, A.; Chirinos, J.A.; Fang, J.C.; Sweitzer, N.K.; Borlaug, B.A.; Kass, D.A.; et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J. Am. Coll. Cardiol. 2013, 62, 1165–1172. [Google Scholar] [CrossRef]
- Saunderson, C.E.D.; Plein, S.; Manisty, C.H. Role of cardiovascular magnetic resonance imaging in cardio-oncology. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 383–396. [Google Scholar] [CrossRef]
- Baldassarre, L.A.; Ganatra, S.; Lopez-Mattei, J.; Yang, E.H.; Zaha, V.G.; Wong, T.C.; Ayoub, C.; DeCara, J.M.; Dent, S.; Deswal, A.; et al. Advances in Multimodality Imaging in Cardio-Oncology: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef]
- Todorova, V.K.; Hsu, P.C.; Wei, J.Y.; Lopez-Candales, A.; Chen, J.Z.; Su, L.J.; Makhoul, I. Biomarkers of inflammation, hypercoagulability and endothelial injury predict early asymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. Am. J. Cancer Res. 2020, 10, 2933–2945. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33042627 (accessed on 1 January 2025). [PubMed]
- Lee, W.E.; Genetzakis, E.; Barsha, G.; Vescovi, J.; Mifsud, C.; Vernon, S.T.; Nguyen, T.V.; Gray, M.P.; Grieve, S.M.; Figtree, G.A. Expression of Myeloperoxidase in Patient-Derived Endothelial Colony-Forming Cells-Associations with Coronary Artery Disease and Mitochondrial Function. Biomolecules 2024, 14, 1308. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Q.; Tan, Y.; Qiao, J.; Liu, Q.; Yang, B.; Yang, S.; Cui, L. Early detection of anthracycline-induced cardiotoxicity. Clin. Chim. Acta 2025, 565, 120000. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | No. of Participants | Cancer Type (%) | Intervention | Follow-Up | Outcome |
|---|---|---|---|---|---|---|
| ACE-i/ARBs | ||||||
| Nakame et al. (2005) [86] | Randomized, placebo-controlled | 40 | Lymphoma | Valsartan 80 mg daily | 0.25 months | ventricular remodeling and arrhythmia incidence LVEF unchanged in the treatment arm |
| Cardinale et al. (2006) [87] | Randomized, placebo-controlled | 114 | AML, lymphoma, MM, breast | Enalapril from dose of 2.5 mg to 20 mg daily | 12 months | LVEF and EDV and ESV only in untreated patients |
| Beta-Blockers | ||||||
| Kaya et al. (2013) [88] | Double-blind, placebo-controlled | 45 | Breast | Nebivolol 5 mg daily | 6 months | Unchanged LVESD and LVEDD in the nebivolol group declines in LVEF in the intervention arm |
| CECCY trial Avila et al. (2018) [89] | Double-blind, placebo-controlled | 200 | Breast | Carvedilol with incremental dose | 6 months | No impact of carvedilol in LVEF reduction |
| Beta-Blockers + ACE-i/ARBs | ||||||
| OVERCOME trial Bosch X et al. (2013) [90] | Double-blind, placebo-controlled | 90 | ALL, AML, lymphoma, MM | Enalapril + carvedilol | 6 months | LVEF unchanged in the intervention group while significantly in controls |
| PRADA trial Heck et al. (2021) [91] | 2 × 2 factorial, randomized-placebo controlled trial | 120 | Breast | Metoprolol combined candesartan | 23 months | Candesartan + metoprolol > no change in LVEF, GLS, or LVESD candesartan alone > declines in GLS and EDV [91,92] |
| MRAs | ||||||
| ELEVATE trial Davis et al. (2019) [93] | Randomized placebo-controlled trial | 44 | Breast | Eplerenone 50 mg daily | 6 months | No significant differences in LV systolic or diastolic dysfunction were observed compared to the placebo group |
| Statins | ||||||
| PREVENT trial Hundley et al. (2022) [94] | Double-blind, placebo-controlled | 279 | Breast (85%), lymphoma (15%) | Atorvastatin 40 mg daily | 24 months | No difference in final LVEF, adjusted for baseline |
| SPARE-HF trial Thavendiranathan et al. (2023) [95] | Double-blind, placebo-controlled | 112 | Breast (65%), lymphoma (21%), sarcoma (6%), thymoma (5%), leukemia (3%) | Atorvastatin 40 mg daily | 2.5 months | No difference in final LVEF, adjusted for baseline |
| STOP-CA trial Neilan et al. (2023) [96] | Double-blind, placebo-controlled | 300 | Lymphoma (100%) | Atorvastatin 40 mg daily | 12 months | incidence of CTRCD in the statin arm |
| Patient-Specific Factors | |
|---|---|
| Pre-existing cardiovascular conditions | Hypertension, diabetes, hyperlipidemia, coronary artery disease, or previous heart failure. |
| Age | Older age increases susceptibility to cardiotoxicity due to general decline in cardiac function with aging. |
| Genetic predisposition | Specific genetic factors can increase sensitivity to chemotherapy-induced heart damage. |
| Gender | Gender-related differences may exist, with studies showing women may be at higher risk. |
| Lifestyle factors | Smoking, sedentary lifestyle, and poor dietary habits can increase cardiovascular risk. |
| Treatment-Specific Factors | |
| Type and cumulative dose of chemotherapy agents | Anthracyclines (like doxorubicin) and trastuzumab are cardiotoxic, especially at higher cumulative doses. |
| Radiotherapy | Chest irradiation increases the risk, particularly for left-sided breast cancer treatments. |
| Combination therapies | Some chemotherapy regimens, when used with other medications that affect cardiac function, may increase the risk. |
| Baseline Cardiac Function | |
| Left ventricular ejection fraction (LVEF) | Lower baseline LVEF can indicate higher risk of developing significant cardiotoxicity. |
| Cancer-Specific Factors | |
| Type of cancer | Certain cancers (e.g., breast cancer or hematologic malignancies) carry different risks for chemotherapy-induced cardiac damage. |
| Stage of cancer | The stage of cancer and its treatment protocol impact the risk of cardiotoxicity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadafora, L.; Di Muro, F.M.; Intonti, C.; Massa, L.; Monelli, M.; Pedretti, R.F.E.; Palazzo Adriano, E.; Guarini, P.; Cantiello, G.; Bernardi, M.; et al. Lifestyle and Pharmacological Interventions to Prevent Anthracycline-Related Cardiotoxicity in Cancer Patients. J. Cardiovasc. Dev. Dis. 2025, 12, 212. https://doi.org/10.3390/jcdd12060212
Spadafora L, Di Muro FM, Intonti C, Massa L, Monelli M, Pedretti RFE, Palazzo Adriano E, Guarini P, Cantiello G, Bernardi M, et al. Lifestyle and Pharmacological Interventions to Prevent Anthracycline-Related Cardiotoxicity in Cancer Patients. Journal of Cardiovascular Development and Disease. 2025; 12(6):212. https://doi.org/10.3390/jcdd12060212
Chicago/Turabian StyleSpadafora, Luigi, Francesca Maria Di Muro, Chiara Intonti, Ludovica Massa, Mauro Monelli, Roberto Franco Enrico Pedretti, Edvige Palazzo Adriano, Pasquale Guarini, Gaia Cantiello, Marco Bernardi, and et al. 2025. "Lifestyle and Pharmacological Interventions to Prevent Anthracycline-Related Cardiotoxicity in Cancer Patients" Journal of Cardiovascular Development and Disease 12, no. 6: 212. https://doi.org/10.3390/jcdd12060212
APA StyleSpadafora, L., Di Muro, F. M., Intonti, C., Massa, L., Monelli, M., Pedretti, R. F. E., Palazzo Adriano, E., Guarini, P., Cantiello, G., Bernardi, M., Russo, F., Cacciatore, S., Sabouret, P., Golino, M., Biondi Zoccai, G., Zimatore, F. R., & Dalla Vecchia, L. A. (2025). Lifestyle and Pharmacological Interventions to Prevent Anthracycline-Related Cardiotoxicity in Cancer Patients. Journal of Cardiovascular Development and Disease, 12(6), 212. https://doi.org/10.3390/jcdd12060212











