Sudden Cardiac Death Due to Ventricular Arrhythmia in Acute Coronary Occlusion: Potential Roles of the Sinoatrial Nodal Artery and Conus Artery
Abstract
1. Introduction
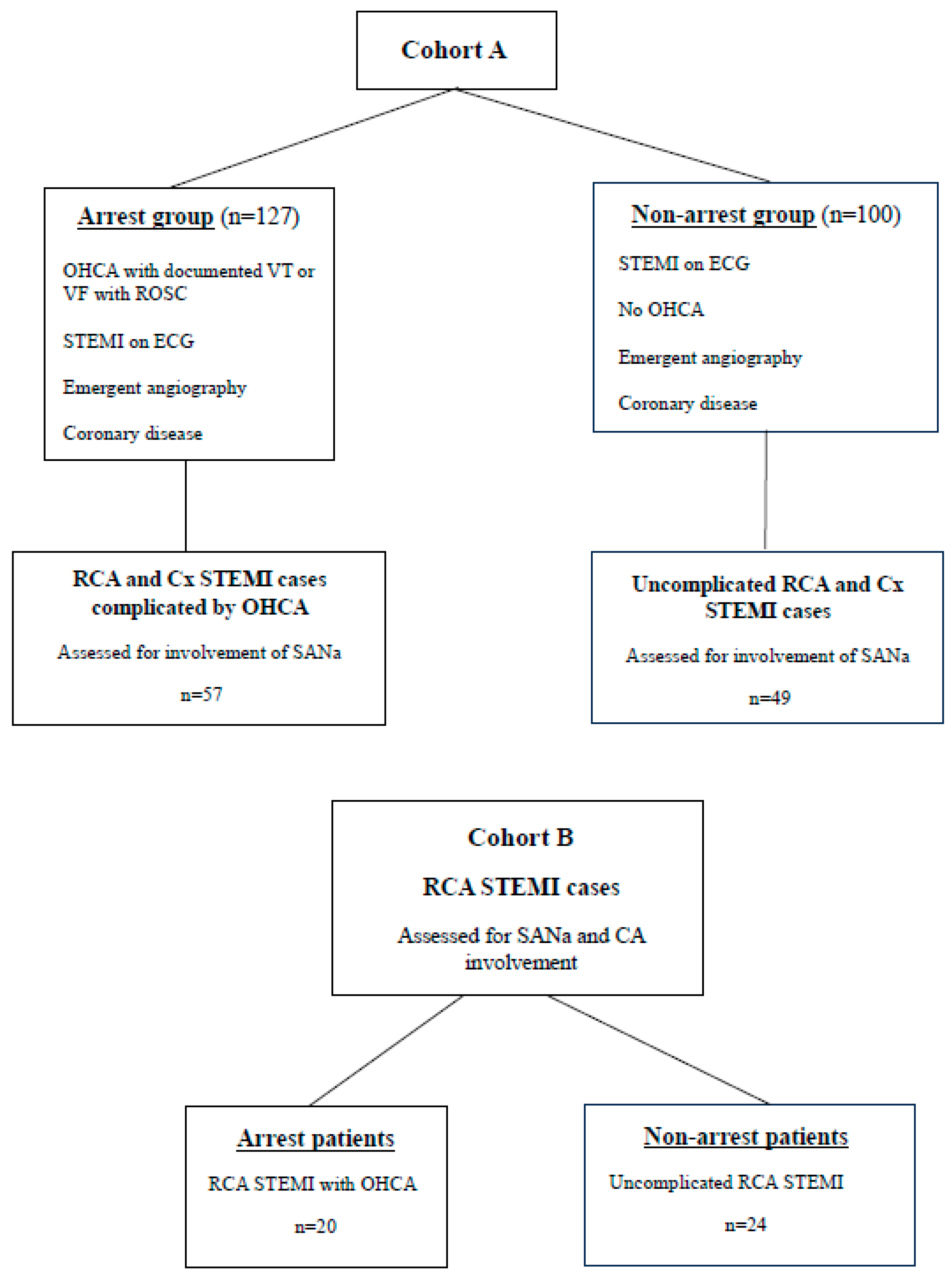
2. Methods
3. Results
3.1. Clinical Characteristics
3.2. Electrocardiogram Findings
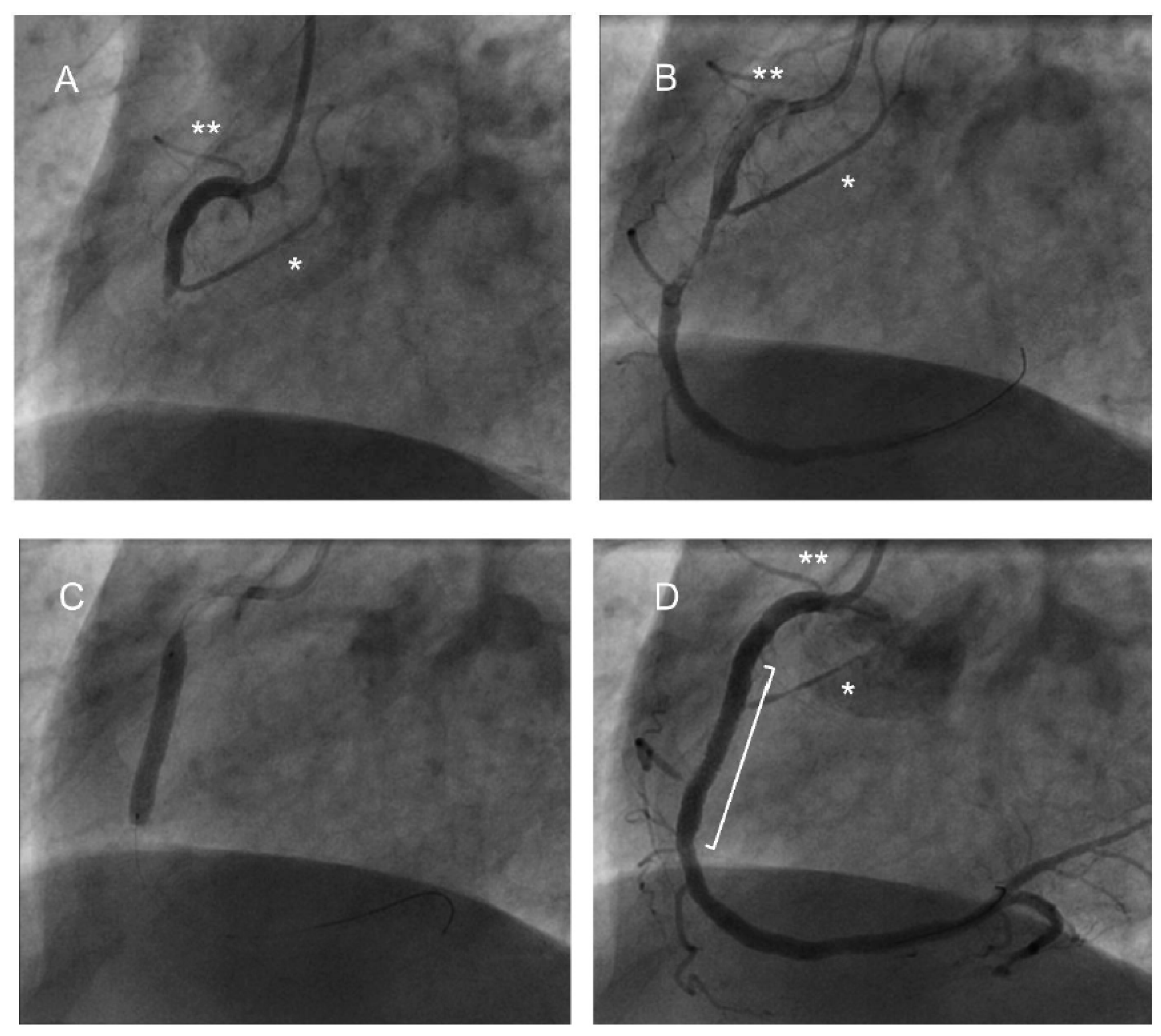
3.3. Coronary Angiogram Findings
3.4. Cardiac Intervention
3.5. In-Hospital Outcomes and Long Term Mortality Data
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar]
- Myerburg, R.J.; Castellanos, A. Cardiac arrest and sudden cardiac death. In Heart Disease: A Textbook of Cardiovascular Medicine; Braunwald, E., Ed.; WB Saunders: NY, USA, 1997; pp. 756–779. [Google Scholar]
- Deo, R.; Albert, C. Epidemiology and genetics of sudden cardiac death. Circulation 2012, 125, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Devitt, P.A.; Gaine, S.P.; Magdy, J.; Coughlan, J.J.; Szirt, R. Non-dominant right coronary artery occlusion. J. Am. Coll. Cardiol. Case Rep. 2022, 4, 156–160. [Google Scholar]
- Shabbir, M.A.; Jhand, A.; Velagapudi, P. A case report of non-dominant right coronary artery occlusion. Eur. Heart J. Case Rep. 2023, 7, ytad303. [Google Scholar] [CrossRef] [PubMed]
- Chadda, K.D.; Banka, V.; Helfant, R.H. Rate dependent ventricular ectopia following acute coronary occlusion: The concept of an optimal antiarrhythmic heart rate. Circulation 1974, 49, 654. [Google Scholar] [CrossRef]
- Umemura, M.; Ho, D.; Nozawa, N.; Balginnyam, E.; Iwatsubo, K.; Saito, T.; Endo, T.; Ishikawa, Y.; Umemura, S.; Kimura, K. Acute myocardial infarction with isolated conus branch occlusion. J. Electrocardiol. 2012, 45, 285–287. [Google Scholar] [CrossRef][Green Version]
- Calmac, L.; Horodinschi, R.N.; Stanciulescu, D.; Scafa-Udriste, A. Malignant Arrhythmia in Acute Conus Artery Occlusion. J. Clin. Stud. Rev. Rep. 2021, 3, 1–4. [Google Scholar] [CrossRef]
- Kuno, T.; Fujisawa, T.; Numasawa, Y.; Takahashi, T. The electrocardiogram change of conus branch occlusion during right coronary artery angioplasty. Case Rep. Int. Med. 2015, 2, 7–9. [Google Scholar] [CrossRef]
- Abdallah, M.S.; Wang, K.; Magnuson, E.A.; Osnabrugge, R.L.; Kappetein, A.P.; Morice, M.-C.; Mohr, F.A.; Serruys, P.W.; Cohen, D.J. Quality of Life After Surgery or DES in Patients with 3-Vessel or Left Main Disease. J. Am. Coll. Cardiol. 2017, 69, 2039–2050. [Google Scholar] [CrossRef]
- Parasca, C.A.; Head, S.J.; Milojevic, M.; Mack, M.J.; Serruys, P.W.; Morice, M.C.; Mohr, F.W.; Feldman, T.E.; Colombo, A.; Dawkins, K.D.; et al. Incidence, Characteristics, Predictors, and Outcomes of Repeat Revascularization After Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting: The SYNTAX Trial at 5 Years. JACC Cardiovasc. Interv. 2016, 9, 2493–2507. [Google Scholar] [CrossRef]
- Perera, D.; Stables, R.; Booth, J.; Thomas, M.; Redwood, S. The balloon pump-assisted coronary intervention study (BCIS-1): Rationale and design. Am. Heart J. 2009, 158, 910–916.e2. [Google Scholar] [CrossRef]
- Califf, R.M.; Phillips, H.R.I.I.I.; Hindman, M.C.; Mark, D.B.; Lee, K.L.; Behar, V.S.; Johnson, R.A.; Pryor, D.B.; Rosati, R.A.; Wagner, G.S. Prognostic value of a coronary artery jeopardy score. J. Am. Coll. Cardiol. 1985, 5, 1055–1063. [Google Scholar] [CrossRef]
- Kosugi, S.; Shinouchi, K.; Ueda, Y.; Abe, H.; Sogabe, T.; Ishida, K.; Mishima, T.; Ozaki, T.; Takayasu, K.; Iida, Y.; et al. Clinical and angiographic features of patients with out-of-hospital cardiac arrest and acute myocardial infarction. J. Am. Coll. Cardiol. 2020, 76, 1934–1943. [Google Scholar] [CrossRef]
- Jabbari, R.; Engstrøm, T.; Glinge, C.; Risgaard, B.; Jabbari, J.; Winkel, B.G.; Terkelsen, C.J.; Tilsted, H.; Jensen, L.O.; Hougaard, M.; et al. Incidence and risk factors of ventricular fibrillation before primary angioplasty in patients with first ST-elevation myocardial infarction: A nationwide study in Denmark. J. Am. Heart Assoc. 2015, 4, e001399. [Google Scholar] [CrossRef]
- Karam, N.; Bataille, S.; Marijon, E.; Tafflet, M.; Benamer, H.; Caussin, C.; Garot, P.; Juliard, J.M.; Pires, V.; Boche, T.; et al. for the e-MUST study investigators. Incidence, mortality and outcome predictors of sudden cardiac arrest complicating myocardial infarction prior to hospital admission. Circ. Cardiovasc. Interv. 2019, 12, e007081. [Google Scholar] [CrossRef]
- Zipes, D.P. The clinical significance of bradycardic rhythms in acute myocardial infarction. Am. J. Cardiol. 1969, 24, 814–825. [Google Scholar] [CrossRef]
- Serrano, C.V.; A Bortolotto, L.; César, L.A.M.; Solimene, M.C.; Mansur, A.P.; Nicolau, J.C.; Ramires, J.A.F. Sinus bradycardia as a predictor of right coronary artery occlusion in patients with inferior myocardial infarction. Int. J. Cardiol. 1999, 68, 75–82. [Google Scholar] [CrossRef]
- Dawes, G.S.; Comroe, J.H. Chemoreflexes from the heart and lungs. Physiol. Rev. 1954, 34, 167–201. [Google Scholar] [CrossRef]
- Thames, M.D.; Klopfenstein, H.S.; Abboud, F.M.; Mark, A.L.; Walker, J.L. Preferential distribution of inhibitory cardiac receptors with vagal afferents to the inferoposterior wall of the left ventricle activated during coronary occlusion in the dog. Circ. Res. 1978, 43, 512–519. [Google Scholar] [CrossRef]
- Arrowood, J.A.; Mohanty, P.K.; Hodgson, J.M.; Dibner-Dunlap, M.E.; Thames, M.D. Ventricular sensory endings mediate reflex bradycardia during coronary arteriography in humans. Circulation 1989, 80, 1293–1300. [Google Scholar] [CrossRef]
- Scherlag, B.J.; Hope, R.R.; Williams, D.O.; El-Sherif, N.; Lazzara, R. Mechanisms of ectopic rhythm formation due to myocardial ischemia. Effects of heart rate and ventricular premature beats in the conduction system of the heart. In Structure, Functional and Clinical Implications; Wellens, H.J.J., Lie, K.I., Janse, M.J., Eds.; Stenfert Kroese BV: Leiden, The Netherlands, 1976; pp. 633–649. [Google Scholar]
- Adgey, A.A.J.; Mulholland, H.C.; Geddes, J.S.; Keegan, D.A.J.; Pantridge, J.F. Incidence and management of early bradyarrhythmias complicating acute myocardial infarction. Lancet 1968, 2, 1097. [Google Scholar] [CrossRef]
- Han, J.; Millet, K.; Chizzonitti, B.; Moe, G.K. Temporal dispersion of recovery of excitability in atrium and ventricles as a function of heart rate. Am. Heart J. 1966, 71, 481. [Google Scholar] [CrossRef]
- Scherlag, B.J.; Kabell, G.; Harrison, L.; Lazzara, R. Mechanisms of bradycardia-induced ventricular arrhythmias in myocardial ischemia and infarction. Circulation 1982, 65, 7. [Google Scholar] [CrossRef]
- Nerantizis, C.E.; Toutouzas, P.; Avgoustakis, D. The importance of the sinus node artery in the blood supply of the atrial myocardium: An anatomical study of 360 cases. Acta Cardiol. 1983, 38, 35–47. [Google Scholar]
- Vikse, J.; Henry, B.M.; Roy, J.; Walocha, J.A.; Tomaszewski, K.A. Anatomical variations in the sinoatrial nodal artery: A meta-analysis and clinical considerations. PLOS ONE 2016, 11, e0148331. [Google Scholar]
- Pejkovi, B.; Krajn, I.; Anderhuber, F.; Kosuti, D. Anatomical aspects of the arterial blood supply to the sinoatrial and atrioventricular nodes of the human heart. J. Int. Med. Res. 2008, 36, 691–698. [Google Scholar] [CrossRef]
- Kini, S.; Bis, K.G.; Weaver, L. Normal and variant coronary arterial and venous anatomy on high-resolution CT angiography. AJR Am. J. Roentgenol. 2007, 188, 1665–1674. [Google Scholar] [CrossRef]
- Hernández, F.H.; Garrido-Lestache, E.B.; Arribas, P.; Gutierrez, J. Recurrent ventricular fibrillation and ST segment elevation in the right precordial leads due to acute occlusion of the conus branch. Rev. Esp. Cardiol. 2011, 64, 1226–1227. [Google Scholar] [CrossRef]
- Eichhofer, J.; Curzen, N. Images in cardiovascular medicine: Unexpected profound transient anterior ST elevation after occlusion of the conus branch of the right coronary artery during angioplasty. Circulation 2005, 111, e113-4. [Google Scholar] [CrossRef]
- Celik, T.; Yuksel, U.C.; Kursaklioglu, H.; Iyisoy, A.; Kose, S.; Isik, E. Precordial ST-segment elevation in acute occlusion of the proximal right coronary artery. J. Electrocardiolgy 2006, 39, 301–304. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.; Lin, Y.; Chen, S.; Chen, Y. Electrical and structural insights into right ventricular outflow tract arrhythmogenesis. Int. J. Mol. Sci. 2023, 22, 11795. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.A.; Tyler, J.M.; Henry, T.D.; Garberich, R.; Sharkey, S.W.; Schmidt, C.W.; Henry, J.T.; Eckman, P.; Megaly, M.; Brilakis, E.S.; et al. Clinical characteristics and outcomes of STEMI patients with cardiogenic shock and cardiac arrest. J. Am. Coll. Cardiol. Intv. 2020, 13, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, B.; Giugliano, R.P.; Cannon, C.P.; Roe, M.T.; Sharma, A.; Page, C.; White, J.A.; Lokhnygina, Y.; Braunwald, E.; Blazing, M.A. Cardiovascular events and long-term risk of sudden death among stabilized patients after acute coronary syndrome: Insights from IMPROVE-IT. J. Am. Heart Assoc. 2022, 11, e022733. [Google Scholar] [CrossRef] [PubMed]


| Variables | Arrest Patients | Non-Arrest Patients | p-Value |
|---|---|---|---|
| Age | 62 (±13) | 62 (±14) | NS |
| Gender, male | 76% | 71% | NS |
| Hypertension | 27% | 34% | NS |
| Diabetes mellitus | 13% | 15% | NS |
| Prior documented CAD | 19% | 15% | NS |
| Electrocardiogram: | |||
| Heart rate (bpm) | 80 (±20) | 77 (±15) | NS |
| Atrial arrhythmia | 14% | 9% | NS |
| Complete heart block | 4% | 2% | NS |
| STE > 1 mm V1-V2 (Cohort A: RCA culprits only) | 62% | 23% | <0.005 |
| STE > 1 mm V1-V2 (Cohort B) | 85% | 21% | <0.005 |
| Angiography: | |||
| Single vessel disease | 57% | 60% | NS |
| Culprit vessel | LMS 0% LAD 44% RCA 34% Cx 16% SVG/3VD 6% | LMS 1% LAD 38% RCA 49% Cx 10% SVG 2% | NS |
| Proximal lesion | 55% | 41% | <0.05 |
| SYNTAX score | 14 (±10) | 13 (±8) | NS |
| Modified Jeopardy score | 6 (3) | 6 (2) | NS |
| SANa involvement of RCA/Cx | 77% | 33% | <0.005 |
| CA involvement of RCA (Cohort B) | 74% | 21% | <0.005 |
| Index event intervention: | |||
| No intervention | 4% | 5% | NS |
| Percutaneous coronary intervention | 92% | 93% | NS |
| Coronary artery bypass graft | 3% | 0% | NS |
| Device | 1% | 2% | NS |
| Long-term outcomes: | |||
| Deceased | n = 28 | n = 14 | NS |
| Death ≤ 30 days | 43% | 7% | <0.05 |
| RCA Infarcts Complicated by Cardiac Arrest (n = 20) | Uncomplicated RCA Infarcts (n = 24) | |
|---|---|---|
| STE > 1 mm V1-V2 | 85% | 21% |
| SANa or CA involvement | 74% | 21% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhar-Amato, J.; Roy, A.; Lambert, B.; Kassou, S.; Hoole, S.P.; Agarwal, S. Sudden Cardiac Death Due to Ventricular Arrhythmia in Acute Coronary Occlusion: Potential Roles of the Sinoatrial Nodal Artery and Conus Artery. J. Cardiovasc. Dev. Dis. 2025, 12, 210. https://doi.org/10.3390/jcdd12060210
Bhar-Amato J, Roy A, Lambert B, Kassou S, Hoole SP, Agarwal S. Sudden Cardiac Death Due to Ventricular Arrhythmia in Acute Coronary Occlusion: Potential Roles of the Sinoatrial Nodal Artery and Conus Artery. Journal of Cardiovascular Development and Disease. 2025; 12(6):210. https://doi.org/10.3390/jcdd12060210
Chicago/Turabian StyleBhar-Amato, Justine, Aurojit Roy, Benjamin Lambert, Sofia Kassou, Stephen P. Hoole, and Sharad Agarwal. 2025. "Sudden Cardiac Death Due to Ventricular Arrhythmia in Acute Coronary Occlusion: Potential Roles of the Sinoatrial Nodal Artery and Conus Artery" Journal of Cardiovascular Development and Disease 12, no. 6: 210. https://doi.org/10.3390/jcdd12060210
APA StyleBhar-Amato, J., Roy, A., Lambert, B., Kassou, S., Hoole, S. P., & Agarwal, S. (2025). Sudden Cardiac Death Due to Ventricular Arrhythmia in Acute Coronary Occlusion: Potential Roles of the Sinoatrial Nodal Artery and Conus Artery. Journal of Cardiovascular Development and Disease, 12(6), 210. https://doi.org/10.3390/jcdd12060210






