Mutations in Exons 8 and 11 of c-kit Gene in Canine Subcutaneous Mast Cell Tumors and Their Association with Cell Proliferation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Signalment
2.3. Histologic Grading
2.4. KIT Expression Patterns
2.5. Ki67 Index, AgNOR Count, and Combined AgNOR x Ki67 Score
2.6. Screening for Mutations in Exons 8 and 11 of c-kit
2.7. Statistical Analyses
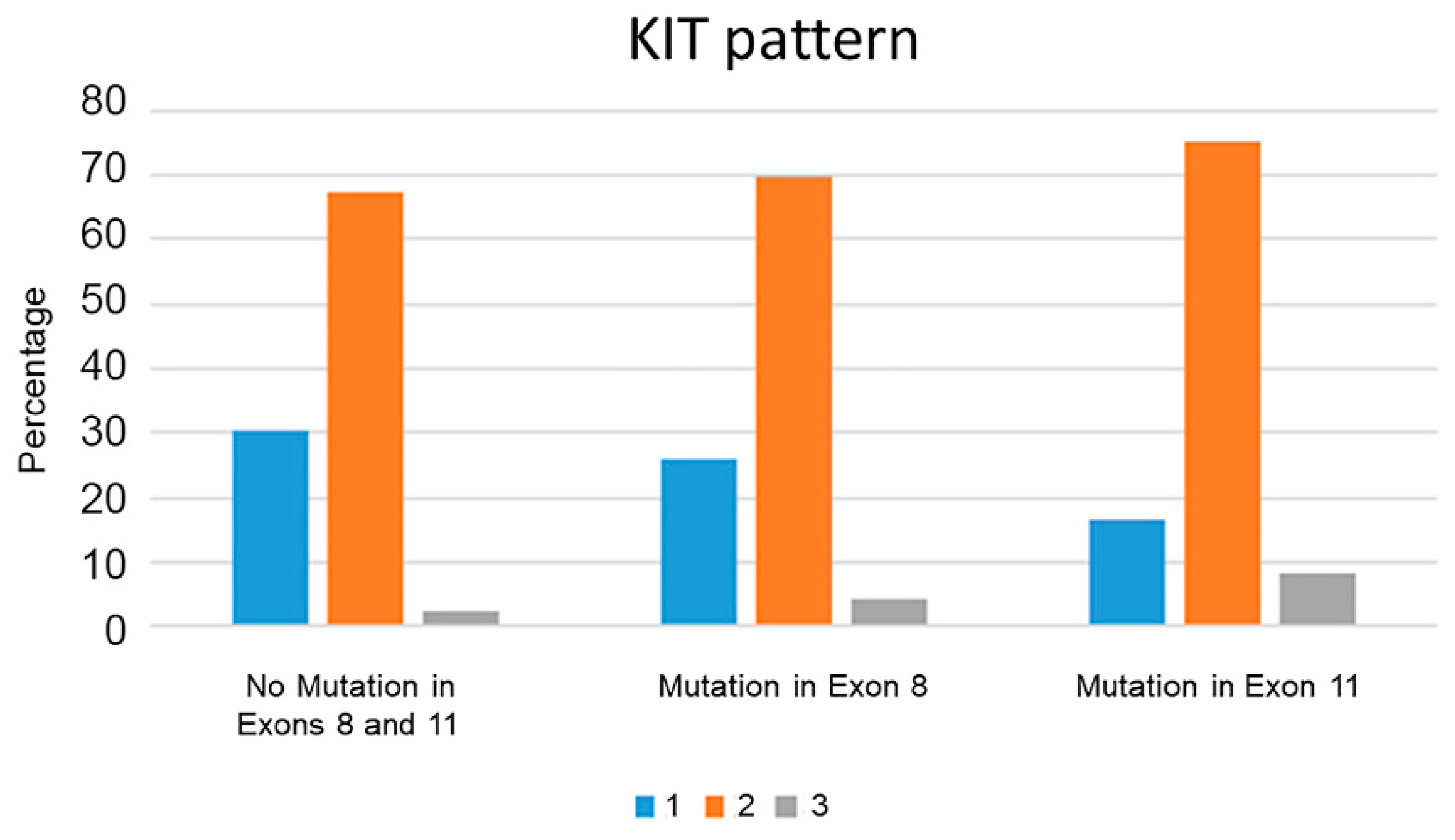
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiupel, M.; Camus, M. Diagnosis and Prognosis of Canine Cutaneous Mast Cell Tumors. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Tamlin, V.S.; Bottema, C.D.K.; Peaston, A.E. Comparative aspects of mast cell neoplasia in animals and the role of KIT in prognosis and treatment. Vet. Med. Sci. 2019, 6, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Leibman, N.; Monette, S.; Craft, D.M.; Bergman, P.J. Prognostic Indicators and Clinical Outcome in Dogs with Subcutaneous Mast Cell Tumors Treated with Surgery Alone: 43 Cases. J. Am. Anim. Hosp. Assoc. 2020, 56, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Horta, R.S.; LaValle, G.E.; Monteiro, L.N.; Souza, M.C.C.; Cassali, G.D.; Araújo, R.B. Assessment of Canine Mast Cell Tumor Mortality Risk Based on Clinical, Histologic, Immunohistochemical, and Molecular Features. Vet. Pathol. 2018, 55, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.; Mrkonjich, L.; Walker, K.; Rohrbach, B. Canine Subcutaneous Mast Cell Tumour: Diagnosis and Prognosis. J. Comp. Pathol. 2007, 136, 231–239. [Google Scholar] [CrossRef]
- Thompson, J.J.; Pearl, D.L.; Yager, J.A.; Best, S.J.; Coomber, B.L.; Foster, R.A. Canine Subcutaneous Mast Cell Tumor: Characterization and Prognostic indices. Vet. Pathol. 2010, 48, 156–168. [Google Scholar] [CrossRef]
- Kiupel, M.; Webster, J.D.; Bailey, K.L.; Best, S.; DeLay, J.; Detrisac, C.J.; Fitzgerald, S.D.; Gamble, D.; Ginn, P.E.; Goldschmidt, M.H.; et al. Proposal of a 2-Tier Histologic Grading System for Canine Cutaneous Mast Cell Tumors to More Accurately Predict Biological Behavior. Vet. Pathol. 2010, 48, 147–155. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Ehler, W.J.; MacEwen, E.G. Canine Cutaneous Mast Cell Tumor: Morphologic Grading and Survival Time in 83 Dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fujino, Y.; Watanabe, M.; Takahashi, M.; Nakagawa, T.; Takeuchi, A.; Bonkobara, M.; Kobayashi, T.; Ohno, K.; Uchida, K.; et al. Validation of the prognostic value of histopathological grading or c-kit mutation in canine cutaneous mast cell tumours: A retrospective cohort study. Vet. J. 2013, 196, 492–498. [Google Scholar] [CrossRef]
- Thompson, J.J.; Yager, J.A.; Best, S.J.; Pearl, D.L.; Coomber, B.L.; Torres, R.N.; Kiupel, M.; Foster, R.A. Canine Subcutaneous Mast Cell Tumors: Cellular Proliferation and KIT Expression as Prognostic Indices. Vet. Pathol. 2010, 48, 169–181. [Google Scholar] [CrossRef]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Miller, R.A.; Kaneene, J.B.; Kiupel, M. Cellular Proliferation in Canine Cutaneous Mast Cell Tumors: Associations with c-KIT and Its Role in Prognostication. Vet. Pathol. 2007, 44, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Gil da Costa, R.M. C-kit as a prognostic and therapeutic marker in canine cutaneous mast cell tumours: From laboratory to clinic. Vet. J. 2015, 205, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Letard, S.; Yang, Y.; Hanssens, K.; Palmérini, F.; Leventhal, P.S.; Guéry, S.; Moussy, A.; Kinet, J.-P.; Hermine, O.; Dubreuil, P. Gain-of-Function Mutations in the Extracellular Domain of KIT Are Common in Canine Mast Cell Tumors. Mol. Cancer Res. 2008, 6, 1137–1145. [Google Scholar] [CrossRef]
- London, C.A.; Galli, S.J.; Yuuki, T.; Hu, Z.-Q.; Helfand, S.C.; Geissler, E.N. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp. Hematol. 1999, 27, 689–697. [Google Scholar] [CrossRef]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Kaneene, J.B.; Miller, R.; Resau, J.H.; Kiupel, M. The Role of c-KIT in Tumorigenesis: Evaluation in Canine Cutaneous Mast Cell Tumors. Neoplasia 2006, 8, 104–111. [Google Scholar] [CrossRef]
- Hadzijusufovic, E.; Peter, B.; Gleixner, K.V.; Schuch, K.; Pickl, W.F.; Thaiwong, T.; Yuzbasiyan-Gurkan, V.; Mirkina, I.; Willmann, M.; Valent, P. H1-receptor antagonists terfenadine and loratadine inhibit spontaneous growth of neoplastic mast cells. Exp. Hematol. 2010, 38, 896–907. [Google Scholar] [CrossRef]
- Isotani, M.; Ishida, N.; Tominaga, M.; Tamura, K.; Yagihara, H.; Ochi, S.; Kato, R.; Kobayashi, T.; Fujita, M.; Fujino, Y.; et al. Effect of Tyrosine Kinase Inhibition by Imatinib Mesylate on Mast Cell Tumors in Dogs. J. Vet. Intern. Med. 2008, 22, 985–988. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G.; et al. Multi-center, Placebo-controlled, Double-blind, Randomized Study of Oral Toceranib Phosphate (SU11654), a Receptor Tyrosine Kinase Inhibitor, for the Treatment of Dogs with Recurrent (Either Local or Distant) Mast Cell Tumor Following Surgical Excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Chien, M.B.; Kass, P.H.; Moore, P.F.; London, C.A. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c- kit in mast cell tumors of dogs. Am. J. Vet. Res. 2002, 63, 1718–1723. [Google Scholar] [CrossRef]
- Cameron, L.R.J.; Grahn, R.A.; Chien, M.B.; Lyons, L.A.; London, C.A. Detection of c-kit Mutations in Canine Mast Cell Tumors using Fluorescent Polyacrylamide Gel Electrophoresis. J. Vet. Diagn. Investig. 2004, 16, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Tamlin, V.; Kessell, A.; Mccoy, R.; Dobson, E.; Smith, T.; Hebart, M.; Brown, L.; Mitrovic, D.; Peaston, A. Prevalence of exon 11 internal tandem duplications in the C-KIT proto-oncogene in Australian canine mast cell tumours. Aust. Vet. J. 2017, 95, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Vozdova, M.; Kubickova, S.; Fictum, P.; Cernohorska, H.; Fröhlich, J.; Rubes, J. Mutation and methylation status of KIT and TP53 in canine cutaneous and subcutaneous mast cell tumours. Vet. Comp. Oncol. 2019, 18, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Vozdova, M.; Kubickova, S.; Fictum, P.; Fröhlich, J.; Jelinek, F.; Rubes, J. Prevalence and prognostic value of c-kit and TP53 mutations in canine mast cell tumours. Vet. J. 2019, 247, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Vozdova, M.; Kubickova, S.; Pal, K.; Fröhlich, J.; Fictum, P.; Rubes, J. Recurrent gene mutations detected in canine mast cell tumours by next generation sequencing. Vet. Comp. Oncol. 2020, 18, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Zemke, D.; Yamini, B.; Yuzbasiyan-Gurkan, V. Mutations in the juxtamembrane domain of c-KIT are associated with higher grade mast cell tumors in dogs. Vet. Pathol. 2002, 39, 529–535. [Google Scholar] [CrossRef]
- Mochizuki, H.; Thomas, R.; Moroff, S.; Breen, M. Genomic profiling of canine mast cell tumors identifies DNA copy number aberrations associated with KIT mutations and high histological grade. Chromosom. Res. 2017, 25, 129–143. [Google Scholar] [CrossRef]
- Brocks, B.A.W.; Bertram, C.A.; Bartel, A.; Kirpensteijn, J.; Collins-Webb, A.; Catlin, C.; Thaiwong, T.; Kiupel, M. Internal Tandem Duplication of Exon 8 of c-kit Is Associated With Longer Total Survival in Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2020, 58, 315–324. [Google Scholar] [CrossRef]
- Martins, A.; Faria, F.; Mesquita, J.; Rtner, F.; Amorim, I. Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Vet. J. 2021, 11, 619. [Google Scholar] [CrossRef]
- Pierini, A.; Lubas, G.; Gori, E.; Binanti, D.; Millanta, F.; Marchetti, V. Epidemiology of Breed-Related Mast Cell Tumour Occurrence and Prognostic Significance of Clinical Features in a Defined Population of Dogs in West-Central Italy. Vet. Sci. 2019, 6, 53. [Google Scholar] [CrossRef]
- Thompson, J.J.; Morrison, J.A.; Pearl, D.L.; Boston, S.E.; Wood, G.; Foster, R.A.; Coomber, B.L. Receptor Tyrosine Kinase Expression Profiles in Canine Cutaneous and Subcutaneous Mast Cell Tumors. Vet. Pathol. 2015, 53, 545–558. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Marconato, L.; Sabattini, S.; Kiupel, M. Mutations in Exons 8 and 11 of c-kit Gene in Canine Subcutaneous Mast Cell Tumors and Their Association with Cell Proliferation. Vet. Sci. 2022, 9, 493. https://doi.org/10.3390/vetsci9090493
Chen P, Marconato L, Sabattini S, Kiupel M. Mutations in Exons 8 and 11 of c-kit Gene in Canine Subcutaneous Mast Cell Tumors and Their Association with Cell Proliferation. Veterinary Sciences. 2022; 9(9):493. https://doi.org/10.3390/vetsci9090493
Chicago/Turabian StyleChen, Polly, Laura Marconato, Silvia Sabattini, and Matti Kiupel. 2022. "Mutations in Exons 8 and 11 of c-kit Gene in Canine Subcutaneous Mast Cell Tumors and Their Association with Cell Proliferation" Veterinary Sciences 9, no. 9: 493. https://doi.org/10.3390/vetsci9090493
APA StyleChen, P., Marconato, L., Sabattini, S., & Kiupel, M. (2022). Mutations in Exons 8 and 11 of c-kit Gene in Canine Subcutaneous Mast Cell Tumors and Their Association with Cell Proliferation. Veterinary Sciences, 9(9), 493. https://doi.org/10.3390/vetsci9090493





