IL-1R8 Downregulation and Concomitant TLR7 and TLR9 Upregulation Are Related to the Pathogenesis of Canine Diffuse Large B-Cell Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. RNA Extraction and Reverse Transcription
2.2. Real-Time qRT-PCR
2.3. Immunohistochemistry
2.4. Statistical Analysis
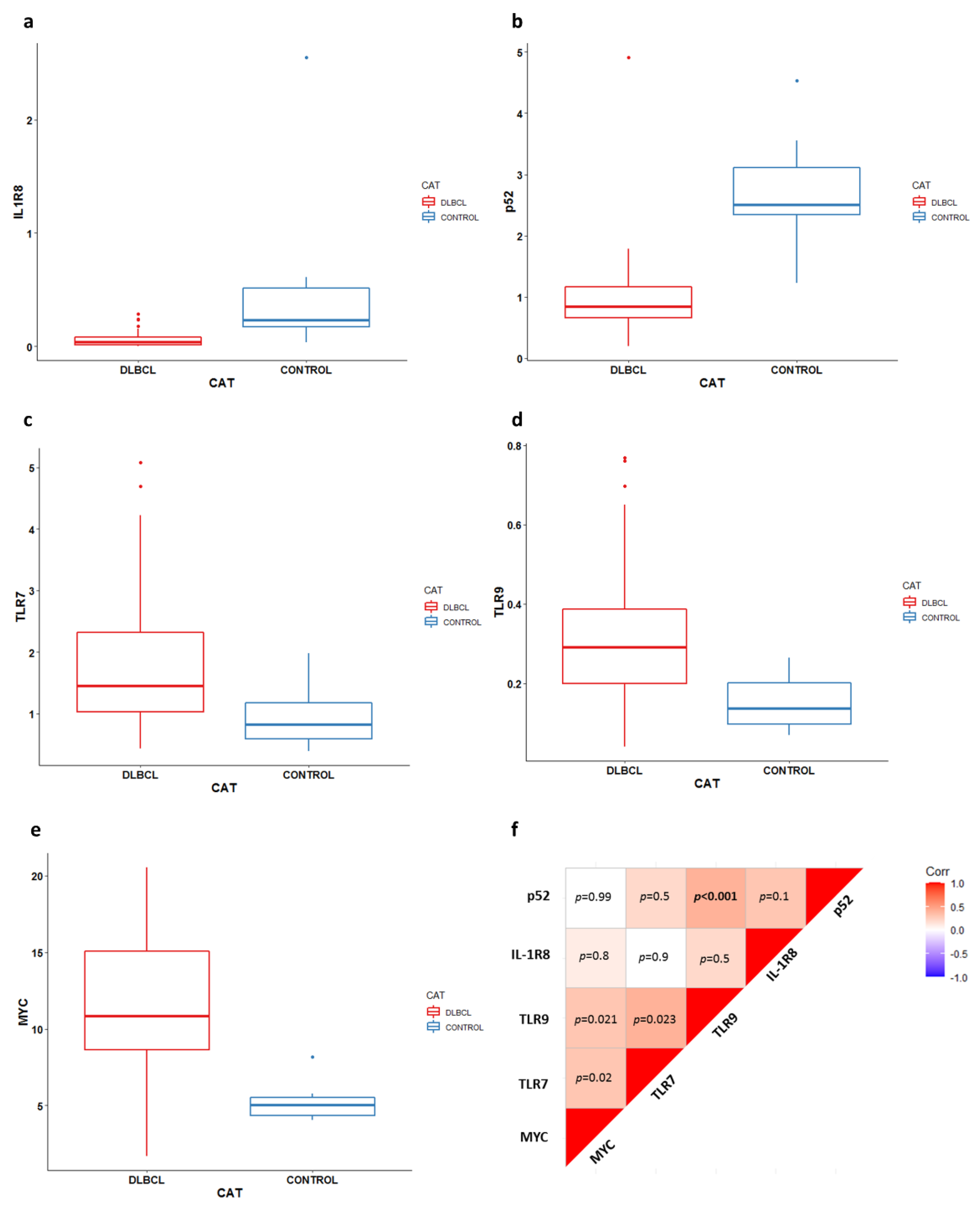
3. Results
3.1. Study Population
3.2. Gene Expression Analysis
3.3. Immunohistochemistry
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Miniscalco, B.; Morello, E.; Comazzi, S.; Gelain, M.E.; Aresu, L.; Riondato, F. Flow cytometric evaluation of ki67 for the determination of malignancy grade in canine lymphoma. Vet. Comp. Oncol. 2015, 13, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Martini, V.; Stefanello, D.; Moretti, P.; Ferrari, R.; Comazzi, S.; Laganga, P.; Riondato, F.; Aresu, L. Peripheral blood lymphocyte/monocyte ratio as a useful prognostic factor in dogs with diffuse large B-cell lymphoma receiving chemoimmunotherapy. Vet. J. 2015, 206, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Aresu, L. Canine Lymphoma, More Than a Morphological Diagnosis: What We Have Learned about Diffuse Large B-Cell Lymphoma. Front. Vet. Sci. 2016, 3, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraresso, S.; Aricò, A.; Sanavia, T.; Da Ros, S.; Milan, M.; Cascione, L.; Comazzi, S.; Martini, V.; Giantin, M.; Di Camillo, B.; et al. DNA methylation profiling reveals common signatures of tumorigenesis and defines epigenetic prognostic subtypes of canine Diffuse Large B-cell Lymphoma. Sci. Rep. 2017, 7, 11591. [Google Scholar] [CrossRef] [PubMed]
- Aresu, L.; Marconato, L.; Martini, V.; Fanelli, A.; Licenziato, L.; Foiani, G.; Melchiotti, E.; Nicoletti, A.; Vascellari, M. Prognostic Value of PD-L1, PD-1 and CD8A in Canine Diffuse Large B-Cell Lymphoma Detected by RNAscope. Vet. Sci. 2021, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, T.J.; Georgel, P.T. Genetic and epigenetic determinants of diffuse large B-cell lymphoma. Blood Cancer J. 2020, 10, 123. [Google Scholar] [CrossRef]
- Mudaliar, M.A.; Haggart, R.D.; Miele, G.; Sellar, G.; Tan, K.A.; Goodlad, J.R.; Milne, E.; Vail, D.M.; Kurzman, I.; Crowther, D.; et al. Comparative gene expression profiling identifies common molecular signatures of NF-κB activation in canine and human diffuse large B cell lymphoma (DLBCL). PLoS ONE 2013, 8, e72591. [Google Scholar] [CrossRef]
- Richards, K.L.; Motsinger-Reif, A.A.; Chen, H.W.; Fedoriw, Y.; Fan, C.; Nielsen, D.M.; Small, G.W.; Thomas, R.; Smith, C.; Dave, S.S.; et al. Gene profiling of canine B-cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer Res. 2013, 73, 5029–5039. [Google Scholar] [CrossRef] [Green Version]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [Green Version]
- Riva, F.; Polentarutti, N.; Tribbioli, G.; Mantovani, A.; Garlanda, C.; Turin, L. The expression pattern of TIR8 is conserved among vertebrates. Vet. Immunol. Immunopathol. 2009, 131, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.; Qin, J.; Zhao, Z.; Qian, Y.; Naramura, M.; Tian, L.; Towne, J.; Sims, J.E.; Stark, G.R.; Li, X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 2003, 4, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Riva, F.; Polentarutti, N.; Buracchi, C.; Sironi, M.; De Bortoli, M.; Muzio, M.; Bergottini, R.; Scanziani, E.; Vecchi, A.; et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc. Natl. Acad. Sci. USA 2004, 101, 3522–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lech, M.; Kulkarni, O.P.; Pfeiffer, S.; Savarese, E.; Krug, A.; Garlanda, C.; Mantovani, A.; Anders, H.J. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J. Exp. Med. 2008, 205, 1879–1888. [Google Scholar] [CrossRef]
- Bulek, K.; Swaidani, S.; Qin, J.; Lu, Y.; Gulen, M.F.; Herjan, T.; Min, B.; Kastelein, R.A.; Aronica, M.; Kosz-Vnenchak, M.; et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J. Immunol. 2009, 182, 2601–2609. [Google Scholar] [CrossRef] [Green Version]
- Bertilaccio, M.T.; Simonetti, G.; Dagklis, A.; Rocchi, M.; Rodriguez, T.V.; Apollonio, B.; Mantovani, A.; Ponzoni, M.; Ghia, P.; Garlanda, C.; et al. Lack of TIR8/SIGIRR triggers progression of chronic lymphocytic leukemia in mouse models. Blood 2011, 118, 660–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aresu, L.; Ferraresso, S.; Marconato, L.; Cascione, L.; Napoli, S.; Gaudio, E.; Kwee, I.; Tarantelli, C.; Testa, A.; Maniaci, C.; et al. New molecular and therapeutic insights into canine diffuse large B-cell lymphoma elucidates the role of the dog as a model for human disease. Haematologica 2019, 104, e256–e259. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; San Myint, M.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, L.N. TNM Classification of Tumours in Domestic Animals; World Health Organization: Washington, DC, USA, 1980. [Google Scholar]
- Aresu, L.; Buracco, P.; De Maria, R.; Iussich, S.; Martano, M.; Morello, E.; Bettini, G.; Comazzi, S.; Riondato, F.; Marconato, L. The Italian-Canine Cancer Biobank: Our 10-year challenge. Hematol. Oncol. 2019, 37, 314–315. [Google Scholar] [CrossRef]
- Marconato, L.; Frayssinet, P.; Rouquet, N.; Comazzi, S.; Leone, V.F.; Laganga, P.; Rossi, F.; Vignoli, M.; Pezzoli, L.; Aresu, L. Randomized, placebo-controlled, double-blinded chemoimmunotherapy clinical trial in a pet dog model of diffuse large B-cell lymphoma. Clin. Cancer Res. 2014, 20, 668–677. [Google Scholar] [CrossRef] [Green Version]
- Riva, F.; Rahman, M.M.; Turin, L.; Ceciliani, F.; Russo, S.; Tribbioli, G.; Lecchi, C. TIR8 receptor expression in bovine tissues. Vet. Immunol. Immunopathol. 2010, 136, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Michels, G.M.; Khanna, C.; Selting, K.A.; London, C.A.; Group, V.C.O. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)—A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2010, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Avery, A.C. The Genetic and Molecular Basis for Canine Models of Human Leukemia and Lymphoma. Front. Oncol. 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Bushell, K.R.; Kim, Y.; Chan, F.C.; Ben-Neriah, S.; Jenks, A.; Alcaide, M.; Fornika, D.; Grande, B.M.; Arthur, S.; Gascoyne, R.D.; et al. Genetic inactivation of TRAF3 in canine and human B-cell lymphoma. Blood 2015, 125, 999–1005. [Google Scholar] [CrossRef]
- Riva, F.; Ponzoni, M.; Supino, D.; Bertilaccio, M.T.S.; Polentarutti, N.; Massara, M.; Pasqualini, F.; Carriero, R.; Innocenzi, A.; Anselmo, A.; et al. IL1R8 Deficiency Drives Autoimmunity-Associated Lymphoma Development. Cancer Immunol. Res. 2019, 7, 874–885. [Google Scholar] [CrossRef] [Green Version]
- Elvers, I.; Turner-Maier, J.; Swofford, R.; Koltookian, M.; Johnson, J.; Stewart, C.; Zhang, C.Z.; Schumacher, S.E.; Beroukhim, R.; Rosenberg, M.; et al. Exome sequencing of lymphomas from three dog breeds reveals somatic mutation patterns reflecting genetic background. Genome Res. 2015, 25, 1634–1645. [Google Scholar] [CrossRef] [Green Version]
- Giannuzzi, D.; Marconato, L.; Cascione, L.; Comazzi, S.; Elgendy, R.; Pegolo, S.; Cecchinato, A.; Bertoni, F.; Aresu, L.; Ferraresso, S. Mutational landscape of canine B-cell lymphoma profiled at single nucleotide resolution by RNA-seq. PLoS ONE 2019, 14, e0215154. [Google Scholar] [CrossRef] [Green Version]
- Molgora, M.; Barajon, I.; Mantovani, A.; Garlanda, C. Regulatory Role of IL-1R8 in Immunity and Disease. Front. Immunol. 2016, 7, 149. [Google Scholar] [CrossRef] [Green Version]
- Lech, M.; Skuginna, V.; Kulkarni, O.P.; Gong, J.; Wei, T.; Stark, R.W.; Garlanda, C.; Mantovani, A.; Anders, H.J. Lack of SIGIRR/TIR8 aggravates hydrocarbon oil-induced lupus nephritis. J. Pathol. 2010, 220, 596–607. [Google Scholar] [CrossRef]
- Aricò, A.; Ferraresso, S.; Bresolin, S.; Marconato, L.; Comazzi, S.; Te Kronnie, G.; Aresu, L. Array-based comparative genomic hybridization analysis reveals chromosomal copy number aberrations associated with clinical outcome in canine diffuse large B-cell lymphoma. PLoS ONE 2014, 9, e111817. [Google Scholar] [CrossRef]
- Zhao, J.; Zepp, J.; Bulek, K.; Li, X. SIGIRR, a negative regulator of colon tumorigenesis. Drug Discov. Today Dis. Mech. 2011, 8, e63–e69. [Google Scholar] [CrossRef] [Green Version]
- Assumpção, A.L.F.V.; Jark, P.C.; Hong, C.C.; Lu, Z.; Ruetten, H.M.; Heaton, C.M.; Pinkerton, M.E.; Pan, X. STAT3 Expression and Activity are Up-Regulated in Diffuse Large B Cell Lymphoma of Dogs. J. Vet. Intern. Med. 2018, 32, 361–369. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, F.; Filipe, J.; Fanelli, A.; Marconato, L.; Inglesi, A.; Scanziani, E.; Soldati, S.; Licenziato, L.; Comazzi, S.; Minoli, L.; et al. IL-1R8 Downregulation and Concomitant TLR7 and TLR9 Upregulation Are Related to the Pathogenesis of Canine Diffuse Large B-Cell Lymphoma. Vet. Sci. 2022, 9, 209. https://doi.org/10.3390/vetsci9050209
Riva F, Filipe J, Fanelli A, Marconato L, Inglesi A, Scanziani E, Soldati S, Licenziato L, Comazzi S, Minoli L, et al. IL-1R8 Downregulation and Concomitant TLR7 and TLR9 Upregulation Are Related to the Pathogenesis of Canine Diffuse Large B-Cell Lymphoma. Veterinary Sciences. 2022; 9(5):209. https://doi.org/10.3390/vetsci9050209
Chicago/Turabian StyleRiva, Federica, Joel Filipe, Antonella Fanelli, Laura Marconato, Alessia Inglesi, Eugenio Scanziani, Sabina Soldati, Luca Licenziato, Stefano Comazzi, Lucia Minoli, and et al. 2022. "IL-1R8 Downregulation and Concomitant TLR7 and TLR9 Upregulation Are Related to the Pathogenesis of Canine Diffuse Large B-Cell Lymphoma" Veterinary Sciences 9, no. 5: 209. https://doi.org/10.3390/vetsci9050209
APA StyleRiva, F., Filipe, J., Fanelli, A., Marconato, L., Inglesi, A., Scanziani, E., Soldati, S., Licenziato, L., Comazzi, S., Minoli, L., & Aresu, L. (2022). IL-1R8 Downregulation and Concomitant TLR7 and TLR9 Upregulation Are Related to the Pathogenesis of Canine Diffuse Large B-Cell Lymphoma. Veterinary Sciences, 9(5), 209. https://doi.org/10.3390/vetsci9050209






