Pathological Findings in Gastrointestinal Neoplasms and Polyps in 860 Cats and a Pilot Study on miRNA Analyses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Histopathology
2.2. Immunohistochemistry
2.3. miRNA Analysis
2.4. Statistics
3. Results
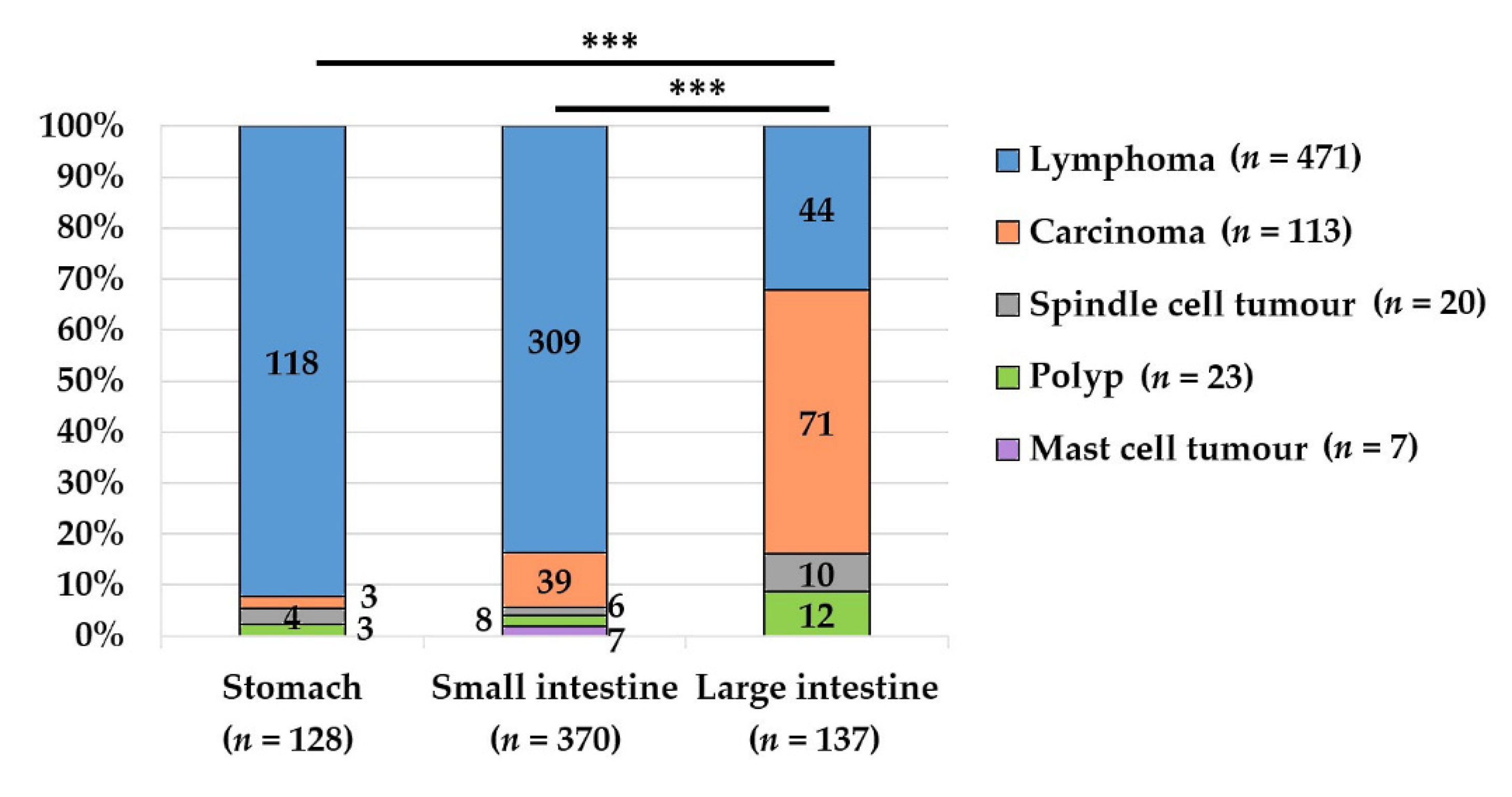
3.1. Total Cat Population with Gastrointestinal Non-Inflammatory Masses (n = 860)
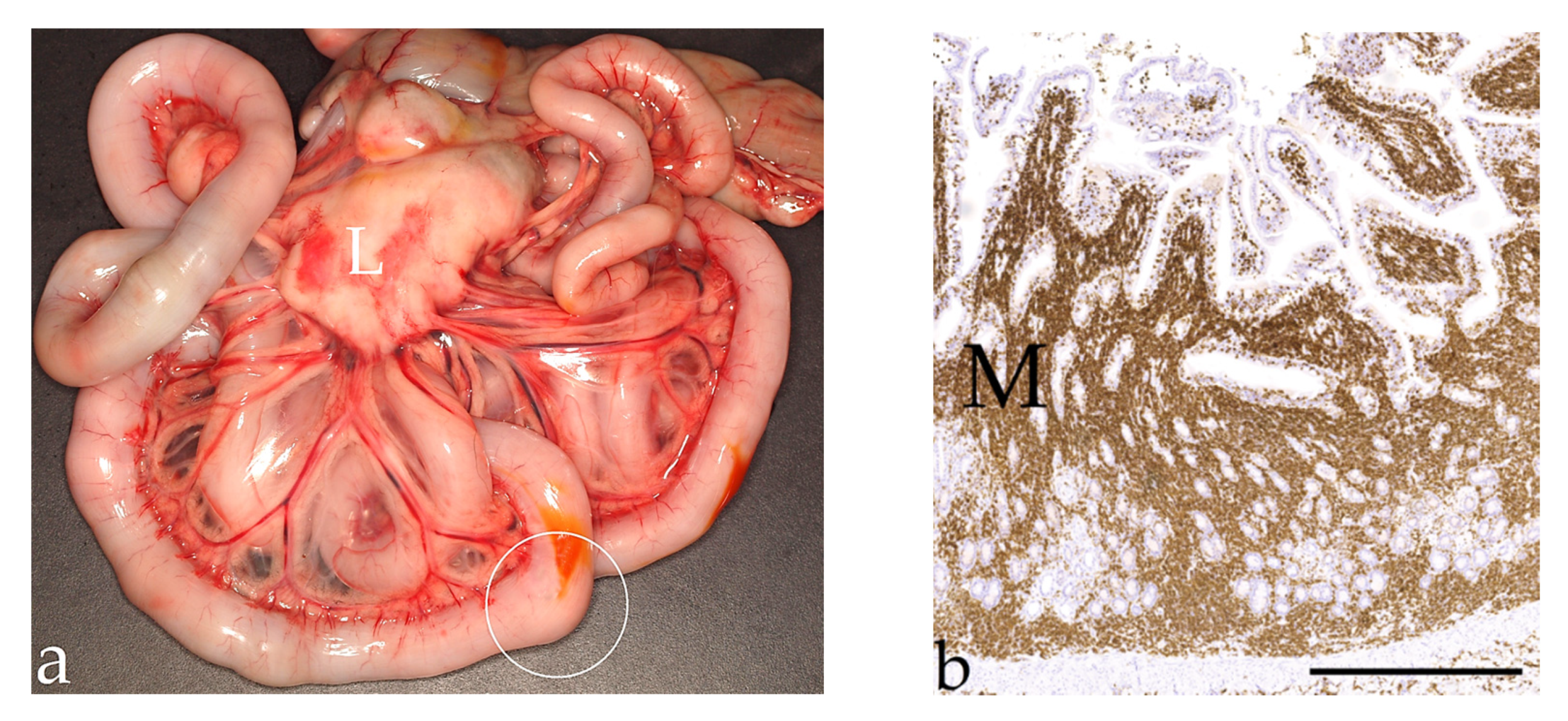
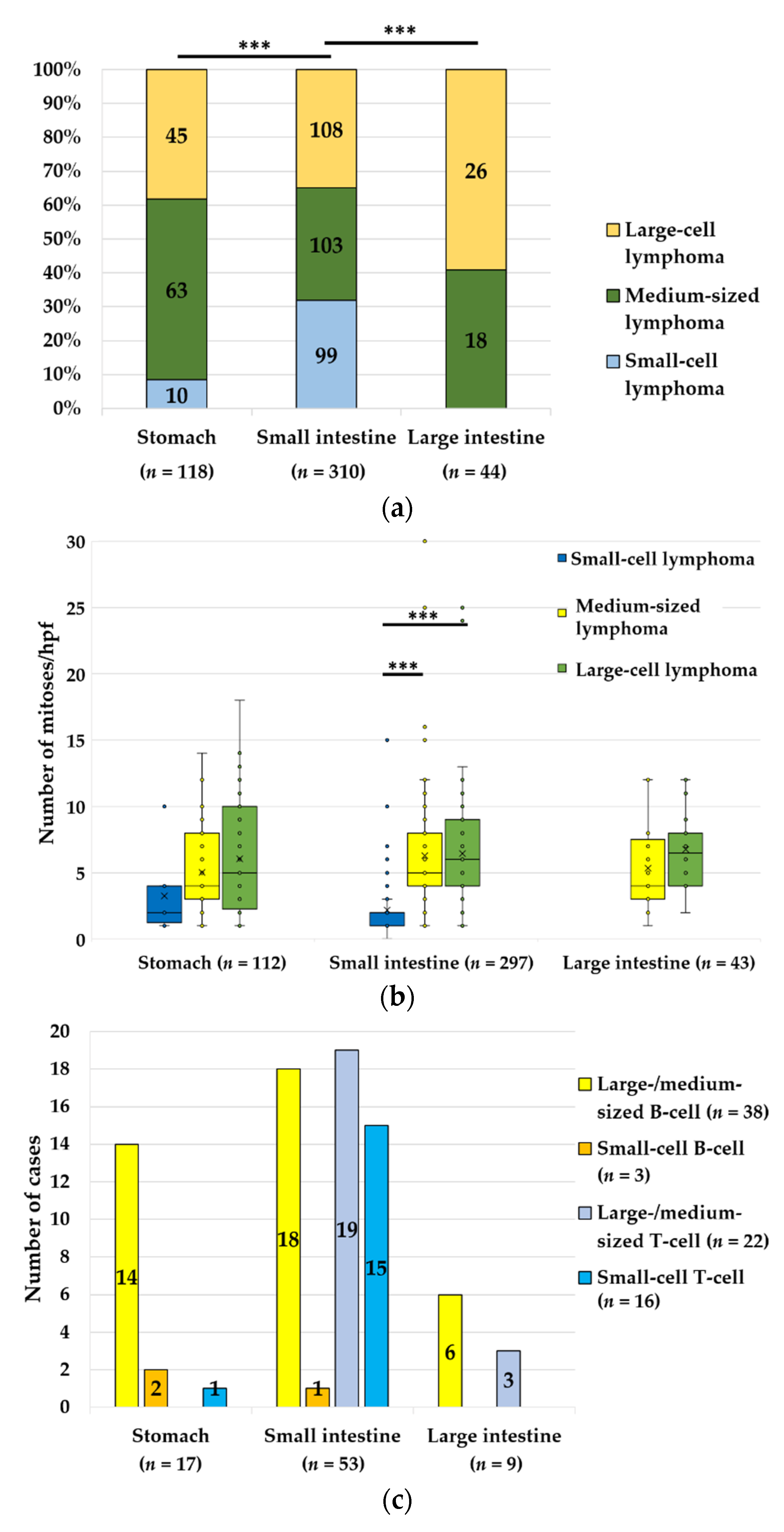
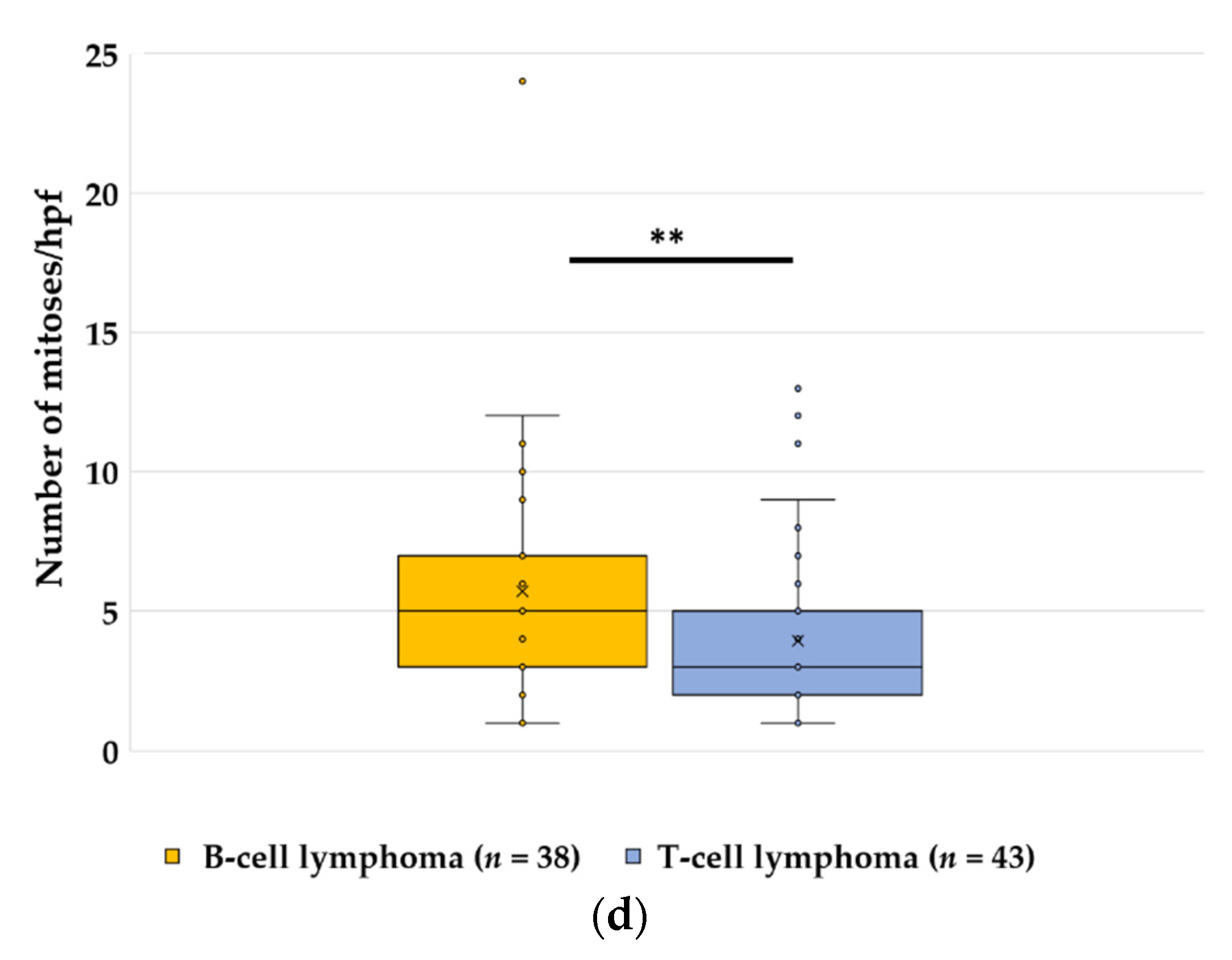
3.2. Lymphoma, n = 679
3.3. Carcinoma, n = 122
3.4. Spindle Cell Tumours, n = 29
3.5. Polyps, n = 23
3.6. Mast Cell Tumours, n = 7
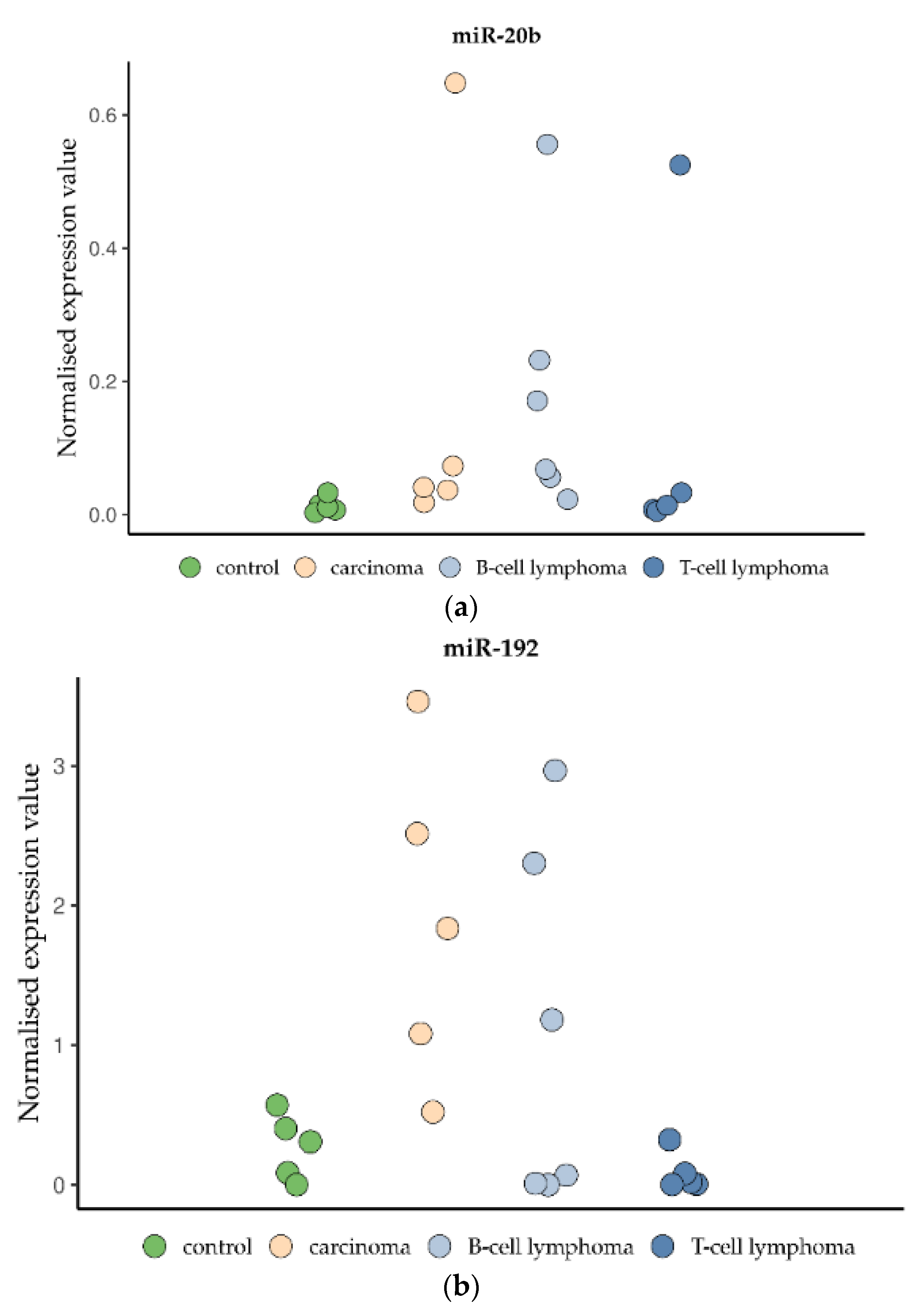
3.7. miRNA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Willard, M.D. Alimentary neoplasia in geriatric dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Gaschen, L. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Shamir, S.K.; Singh, A.; Mayhew, P.D.; Runge, J.J.; Case, J.B.; Steffey, M.A.; Balsa, I.M.; Culp, W.T.N.; Giuffrida, M.A.; Kilkenny, J.J.; et al. Evaluation of minimally invasive small intestinal exploration and targeted abdominal organ biopsy with use of a wound retraction device in dogs: 27 cases (2010–2017). J. Am. Vet. Med. Assoc. 2019, 255, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, U.; Bertazzolo, W.; Bottero, E.; de Lorenzi, D.; Marconato, L.; Masserdotti, C.; Zatelli, A.; Zini, E. Diagnostic value of cytologic examination of gastrointestinal tract tumors in dogs and cats: 83 cases (2001–2004). J. Am. Vet. Med. Assoc. 2006, 229, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, S.; Harder, J.; Nolte, I.; Marsilio, S.; Hewicker-Trautwein, M. Chronic inflammatory and non-inflammatory diseases of the gastrointestinal tract in cats: Diagnostic advantages of full-thickness intestinal and extraintestinal biopsies. J. Feline Med. Surg. 2010, 12, 97–103. [Google Scholar] [CrossRef]
- Daure, E.; Jania, R.; Jennings, S.; d’Anjou, M.-A.; Penninck, D. Ultrasonographic and clinicopathological features of pyloroduodenal adenomatous polyps in cats. J. Feline Med. Surg. 2017, 19, 141–145. [Google Scholar] [CrossRef]
- Rissetto, K.; Villamil, J.A.; Selting, K.A.; Tyler, J.; Henry, C.J. Recent trends in feline intestinal neoplasia: An epidemiologic study of 1,129 cases in the veterinary medical database from 1964 to 2004. J. Am. Anim. Hosp. Assoc. 2011, 47, 28–36. [Google Scholar] [CrossRef]
- Schleis, S.E. Cancer screening tests for small animals. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 871–881. [Google Scholar] [CrossRef]
- Wiley, C.; Wise, C.F.; Breen, M. Novel Noninvasive Diagnostics. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 781–791. [Google Scholar] [CrossRef]
- Wang, L.; Sharif, H.; Saellström, S.; Rönnberg, H.; Eriksson, S. Feline thymidine kinase 1: Molecular characterization and evaluation of its serum form as a diagnostic biomarker. BMC Vet. Res. 2021, 17, 316. [Google Scholar] [CrossRef]
- Chibuk, J.; Flory, A.; Kruglyak, K.M.; Leibman, N.; Nahama, A.; Dharajiya, N.; van den Boom, D.; Jensen, T.J.; Friedman, J.S.; Shen, M.R.; et al. Horizons in Veterinary Precision Oncology: Fundamentals of Cancer Genomics and Applications of Liquid Biopsy for the Detection, Characterization, and Management of Cancer in Dogs. Front. Vet. Sci. 2021, 8, 664718. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.H. Circulating microRNAs as biomarkers in cancer diagnosis. Life Sci. 2020, 248, 117473. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Diomaiuto, E.; Principe, V.; de Luca, A.; Laperuta, F.; Alterisio, C.; Di Loria, A. Exosomes in Dogs and Cats: An Innovative Approach to Neoplastic and Non-Neoplastic Diseases. Pharmaceuticals 2021, 14, 766. [Google Scholar] [CrossRef]
- Howard, J.; Wyse, C.; Argyle, D.; Quinn, C.; Kelly, P.; McCann, A. Exosomes as Biomarkers of Human and Feline Mammary Tumours; A Comparative Medicine Approach to Unravelling the Aggressiveness of TNBC. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188431. [Google Scholar] [CrossRef]
- Laganà, A.; Dirksen, W.P.; Supsavhad, W.; Yilmaz, A.S.; Ozer, H.G.; Feller, J.D.; Vala, K.A.; Croce, C.M.; Rosol, T.J. Discovery and characterization of the feline miRNAome. Sci. Rep. 2017, 7, 9263. [Google Scholar] [CrossRef]
- Joos, D.; Leipig-Rudolph, M.; Weber, K. Tumour-specific microRNA expression pattern in canine intestinal T-cell-lymphomas. Vet. Comp. Oncol. 2020, 18, 502–508. [Google Scholar] [CrossRef]
- Gieger, T. Alimentary lymphoma in cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 419–432. [Google Scholar] [CrossRef]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of Histological Grading Systems in Veterinary Medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Meuten, D.J. (Ed.) Tumors in Domestic Animals, 5th ed.; Wiley/Blackwell: Ames, IA, USA, 2017; ISBN 9780813821795. [Google Scholar]
- Munday, J.S.; Löhr, C.V.; Kiupel, M. Tumors of the Alimentary Tract. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons Inc: Ames, IA, USA, 2017; pp. 499–601. ISBN 9781119181200. [Google Scholar]
- Wolfesberger, B.; Fuchs-Baumgartinger, A.; Greß, V.; Hammer, S.E.; Gradner, G.; Knödl, K.; Tichy, A.; Rütgen, B.C.; Beham-Schmid, C. World Health Organisation Classification of Lymphoid Tumours in Veterinary and Human Medicine: A Comparative Evaluation of Gastrointestinal Lymphomas in 61 Cats. J. Comp. Pathol. 2018, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Finotello, R.; Vasconi, M.E.; Sabattini, S.; Agnoli, C.; Giacoboni, C.; Annoni, M.; Dentini, A.; Bettini, G.; Guazzi, P.; Stefanello, D.; et al. Feline large granular lymphocyte lymphoma: An Italian Society of Veterinary Oncology (SIONCOV) retrospective study. Vet. Comp. Oncol. 2018, 16, 159–166. [Google Scholar] [CrossRef]
- Kiupel, M.; Smedley, R.C.; Pfent, C.; Xie, Y.; Xue, Y.; Wise, A.G.; DeVaul, J.M.; Maes, R.K. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet. Pathol. 2011, 48, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Gabor, L.J.; Canfield, P.J.; Malik, R. Immunophenotypic and histological characterisation of 109 cases of feline lymphosarcoma. Aust. Vet. J. 1999, 77, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Vernau, W.; Moore, P.F. Clonality Testing in Veterinary Medicine: A Review with Diagnostic Guidelines. Vet. Pathol. 2016, 53, 711–725. [Google Scholar] [CrossRef]
- Burkhard, M.J.; Bienzle, D. Making sense of lymphoma diagnostics in small animal patients. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1331–1347. [Google Scholar] [CrossRef]
- Groll, T.; Schopf, F.; Denk, D.; Mogler, C.; Schwittlick, U.; Aupperle-Lellbach, H.; Sarker, S.R.J.; Pfarr, N.; Weichert, W.; Matiasek, K.; et al. Bridging the Species Gap: Morphological and Molecular Comparison of Feline and Human Intestinal Carcinomas. Cancers 2021, 13, 5941. [Google Scholar] [CrossRef]
- Sabattini, S.; Giantin, M.; Barbanera, A.; Zorro Shahidian, L.; Dacasto, M.; Zancanella, V.; Prata, D.; Trivigno, E.; Bettini, G. Feline intestinal mast cell tumours: Clinicopathological characterisation and KIT mutation analysis. J. Feline Med. Surg. 2016, 18, 280–289. [Google Scholar] [CrossRef]
- Halsey, C.H.C.; Powers, B.E.; Kamstock, D.A. Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008). Vet. Comp. Oncol. 2010, 8, 72–79. [Google Scholar] [CrossRef]
- Barrett, L.E.; Skorupski, K.; Brown, D.C.; Weinstein, N.; Clifford, C.; Szivek, A.; Haney, S.; Kraiza, S.; Krick, E.L. Outcome following treatment of feline gastrointestinal mast cell tumours. Vet. Comp. Oncol. 2018, 16, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Laurenson, M.P.; Skorupski, K.A.; Moore, P.F.; Zwingenberger, A.L. Ultrasonography of intestinal mast cell tumors in the cat. Vet. Radiol. Ultrasound 2011, 52, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.; Cannon, M.J.; Lucke, V.M.; Day, M.J. Intestinal haemangiosarcoma in the cat: Clinical and pathological features of four cases. J. Small Anim. Pract. 2000, 41, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.N.; Drobatz, K.J.; Glassman, M.M.; Baez, J.L.; Aronson, L.R. Feline visceral hemangiosarcoma. J. Vet. Intern. Med. 2008, 22, 148–152. [Google Scholar] [CrossRef]
- Barrand, K.R.; Scudamore, C.L. Intestinal leiomyosarcoma in a cat. J. Small Anim. Pract. 1999, 40, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.; Brooks Brownlie, H.; Ogden, D.; Atencia, S. A case of gastric leiomyosarcoma in a domestic shorthair cat. JFMS Open Rep. 2018, 4, 2055116918818912. [Google Scholar] [CrossRef]
- Henker, L.C.; Dal Pont, T.P.; Dos Santos, I.R.; Bandinelli, M.B.; Zardo, I.L.; Rodrigues, R.; Pavarini, S.P. Duodenal leiomyosarcoma in a cat: Cytologic, pathologic, and immunohistochemical findings. Vet. Clin. Pathol. 2022. [Google Scholar] [CrossRef]
- Head, K.W.; Cullen, J.M.; Dubielzig, R.R. Histological Classification of Tumors of the Alimentary System of Domestic Animals; Armed Forces Institute of Pathology: Silver Spring, MD, USA, 2003; ISBN 9781881041863. [Google Scholar]
- MacDonald, J.M.; Mullen, H.S.; Moroff, S.D. Adenomatous polyps of the duodenum in cats: 18 cases (1985–1990). J. Am. Vet. Med. Assoc. 1993, 202, 647–651. [Google Scholar]
- Ayala, I.; Cabot, A.; Garcia-Martinez, J.D.; Escobar, M.T.; Alberca, F. Endoscopic Endocautery Polypectomy for the Treatment of Duodenal and Gastric Polyps in a Cat. Top. Companion Anim. Med. 2021, 44, 100537. [Google Scholar] [CrossRef]
- Kamstock, D.A.; Ehrhart, E.J.; Getzy, D.M.; Bacon, N.J.; Rassnick, K.M.; Moroff, S.D.; Liu, S.M.; Straw, R.C.; McKnight, C.A.; Amorim, R.L.; et al. Recommended guidelines for submission, trimming, margin evaluation, and reporting of tumor biopsy specimens in veterinary surgical pathology. Vet. Pathol. 2011, 48, 19–31. [Google Scholar] [CrossRef]
- Mulisch, M.; Welsch, U. (Eds.) Romeis-Mikroskopische Technik; 19. Aufl. 2015; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783642551901. [Google Scholar]
- Valli, V.E.; Jacobs, R.; Parodi, A.L.; Vernau, W.; Moore, P.F. Histological Classification of Hematopoietic Tumors of Domestic Animals; Armed Forces Institute of Pathology: Silver Spring, MD, USA, 2002; ISBN 9781881041757. [Google Scholar]
- Valli, V.E.; Jacobs, R.M.; Norris, A.; Couto, C.G.; Morrison, W.B.; McCaw, D.; Cotter, S.; Ogilvie, G.; Moore, A. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J. Vet. Diagn. Investig. 2000, 12, 295–306. [Google Scholar] [CrossRef]
- Weishaar, K.M.; Thamm, D.H.; Worley, D.R.; Kamstock, D.A. Correlation of nodal mast cells with clinical outcome in dogs with mast cell tumour and a proposed classification system for the evaluation of node metastasis. J. Comp. Pathol. 2014, 151, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.A.; Gallina, A.M.; Russell, T.S. Nonhematopoietic gastrointestinal neoplasia in cats: A retrospective study of 44 cases. Vet. Pathol. 1981, 18, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Slawienski, M.J.; Mauldin, G.E.; Mauldin, G.N.; Patnaik, A.K. Malignant colonic neoplasia in cats: 46 cases (1990–1996). J. Am. Vet. Med. Assoc. 1997, 211, 878–881. [Google Scholar] [PubMed]
- Morrice, M.; Polton, G.; Beck, S. Evaluation of the histopathological extent of neoplastic infiltration in intestinal tumours in cats. Vet. Med. Sci. 2019, 5, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Pohlman, L.M.; Higginbotham, M.L.; Welles, E.G.; Johnson, C.M. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet. Pathol. 2009, 46, 259–268. [Google Scholar] [CrossRef]
- Barrs, V.R.; Beatty, J.A. Feline alimentary lymphoma: 1. Classification, risk factors, clinical signs and non-invasive diagnostics. J. Feline Med. Surg. 2012, 14, 182–190. [Google Scholar] [CrossRef]
- Gustafson, T.L.; Villamil, A.; Taylor, B.E.; Flory, A. A retrospective study of feline gastric lymphoma in 16 chemotherapy-treated cats. J. Am. Anim. Hosp. Assoc. 2014, 50, 46–52. [Google Scholar] [CrossRef]
- Freiche, V.; Fages, J.; Paulin, M.V.; Bruneau, J.; Couronné, L.; German, A.J.; Penninck, D.; Hermine, O. Clinical, laboratory and ultrasonographic findings differentiating low-grade intestinal T-cell lymphoma from lymphoplasmacytic enteritis in cats. J. Vet. Intern. Med. 2021, 35, 2685–2696. [Google Scholar] [CrossRef]
- Freiche, V.; Cordonnier, N.; Paulin, M.V.; Huet, H.; Turba, M.E.; Macintyre, E.; Malamut, G.; Cerf-Bensussan, N.; Molina, T.J.; Hermine, O.; et al. Feline low-grade intestinal T cell lymphoma: A unique natural model of human indolent T cell lymphoproliferative disorder of the gastrointestinal tract. Lab. Investig. 2021, 101, 794–804. [Google Scholar] [CrossRef]
- Freiche, V.; Paulin, M.V.; Cordonnier, N.; Huet, H.; Turba, M.-E.; Macintyre, E.; Molina, T.-J.; Hermine, O.; Couronné, L.; Bruneau, J. Histopathologic, phenotypic, and molecular criteria to discriminate low-grade intestinal T-cell lymphoma in cats from lymphoplasmacytic enteritis. J. Vet. Intern. Med. 2021, 35, 2673–2684. [Google Scholar] [CrossRef]
- Moore, P.F.; Rodriguez-Bertos, A.; Kass, P.H. Feline gastrointestinal lymphoma: Mucosal architecture, immunophenotype, and molecular clonality. Vet. Pathol. 2012, 49, 658–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesari, A.; Bettini, G.; Vezzali, E. Feline intestinal T-cell lymphoma: Assessment of morphologic and kinetic features in 30 cases. J. Vet. Diagn. Investig. 2009, 21, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Swennes, A.G.; Parry, N.M.A.; Feng, Y.; Sawyer, E.; Lohr, B.R.; Twedt, D.C.; Fox, J.G. Enterohepatic Helicobacter spp. in cats with non-haematopoietic intestinal carcinoma: A survey of 55 cases. J. Med. Microbiol. 2016, 65, 814–820. [Google Scholar] [CrossRef] [PubMed]
- August, J.R. Gastrointestinal Disorders of the Cat. Vet. Clin. N. Am. Small Anim. Pract. 1983, 13, 585–597. [Google Scholar] [CrossRef]
- Gualtieri, M.; Monzeglio, M.G.; Scanziani, E. Gastric neoplasia. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 415–440. [Google Scholar]
- Orr, C.M.; Gruffydd-Jones, T.J.; Kelly, D.F. Ileal polyps in Siamese cats. J. Small Anim. Pract. 1980, 21, 669–674. [Google Scholar] [CrossRef]
- Uneyama, M.; Chambers, J.K.; Nakashima, K.; Uchida, K. Feline pyloric and duodenal adenoma: A histological and immunohistochemical study. Vet. Pathol. 2021, 58, 1025–1032. [Google Scholar] [CrossRef]
- Mallett, C.L.; Northrup, N.C.; Saba, C.F.; Rodriguez, C.O.; Rassnick, K.M.; Gieger, T.L.; Childress, M.O.; Howerth, E.W. Immunohistochemical characterization of feline mast cell tumors. Vet. Pathol. 2013, 50, 106–109. [Google Scholar] [CrossRef]
- Mancuso, G.; Bovio, E.; Rena, O.; Rrapaj, E.; Mercalli, F.; Veggiani, C.; Paganotti, A.; Andorno, S.; Boldorini, R. Prognostic impact of a 3-MicroRNA signature in cytological samples of small cell lung cancer. Cancer Cytopathol. 2016, 124, 621–629. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; da Silva, A.M.; Calin, G.; Pantel, K. Data Normalization Strategies for MicroRNA Quantification. Clin. Chem. 2015, 61, 1333–1342. [Google Scholar] [CrossRef]
- Landais, S.; Landry, S.; Legault, P.; Rassart, E. Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res. 2007, 67, 5699–5707. [Google Scholar] [CrossRef] [PubMed]
- Khuu, C.; Utheim, T.P.; Sehic, A. The Three Paralogous MicroRNA Clusters in Development and Disease, miR-17-92, miR-106a-363, and miR-106b-25. Scientifica 2016, 2016, 1379643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khella, H.W.Z.; Bakhet, M.; Allo, G.; Jewett, M.A.S.; Girgis, A.H.; Latif, A.; Girgis, H.; von Both, I.; Bjarnason, G.A.; Yousef, G.M. miR-192, miR-194 and miR-215: A convergent microRNA network suppressing tumor progression in renal cell carcinoma. Carcinogenesis 2013, 34, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ge, X.; Zhang, Y.; Xia, H.; Yuan, D.; Tang, Q.; Chen, L.; Pang, X.; Leng, W.; Bi, F. Plasma miR-122 and miR-192 as potential novel biomarkers for the early detection of distant metastasis of gastric cancer. Oncol. Rep. 2014, 31, 1863–1870. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Lyu, C.; Buchner, A.; Pohla, H. Diagnostic and Prognostic Role of miR-192 in Different Cancers: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2021, 2021, 8851035. [Google Scholar] [CrossRef]
- Elshafie, N.O.; Nascimento, N.C.d.; Lichti, N.I.; Kasinski, A.L.; Childress, M.O.; Santos, A.P.D. MicroRNA Biomarkers in Canine Diffuse Large B-Cell Lymphoma. Vet. Pathol. 2021, 58, 34–41. [Google Scholar] [CrossRef]
- Garnica, T.K.; Lesbon, J.C.C.; Ávila, A.C.F.C.M.; Rochetti, A.L.; Matiz, O.R.S.; Ribeiro, R.C.S.; Zoppa, A.; Nishiya, A.T.; Costa, M.T.; de Nardi, A.B.; et al. Liquid biopsy based on small extracellular vesicles predicts chemotherapy response of canine multicentric lymphomas. Sci. Rep. 2020, 10, 20371. [Google Scholar] [CrossRef]
- Craig, K.K.L.; Wood, G.A.; Keller, S.M.; Mutsaers, A.J.; Wood, R.D. MicroRNA profiling in canine multicentric lymphoma. PLoS ONE 2019, 14, e0226357. [Google Scholar] [CrossRef] [Green Version]



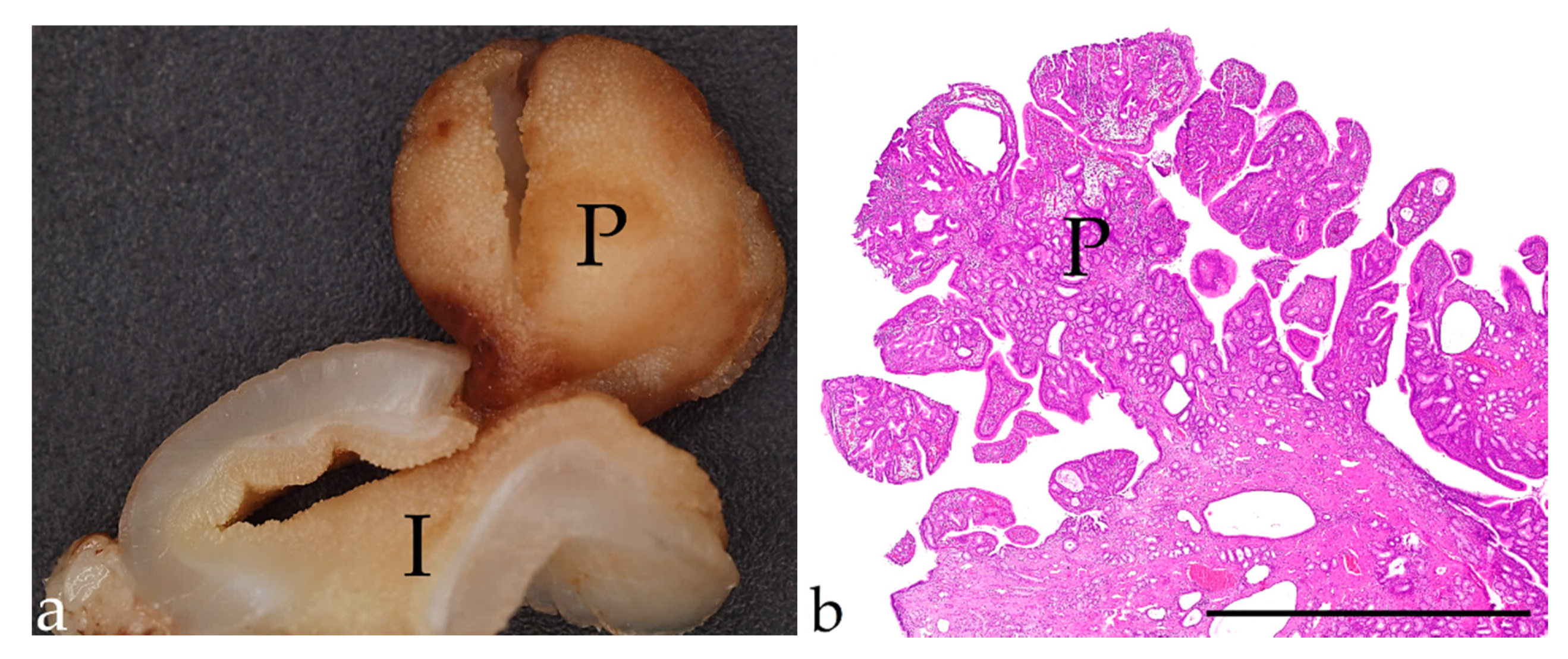
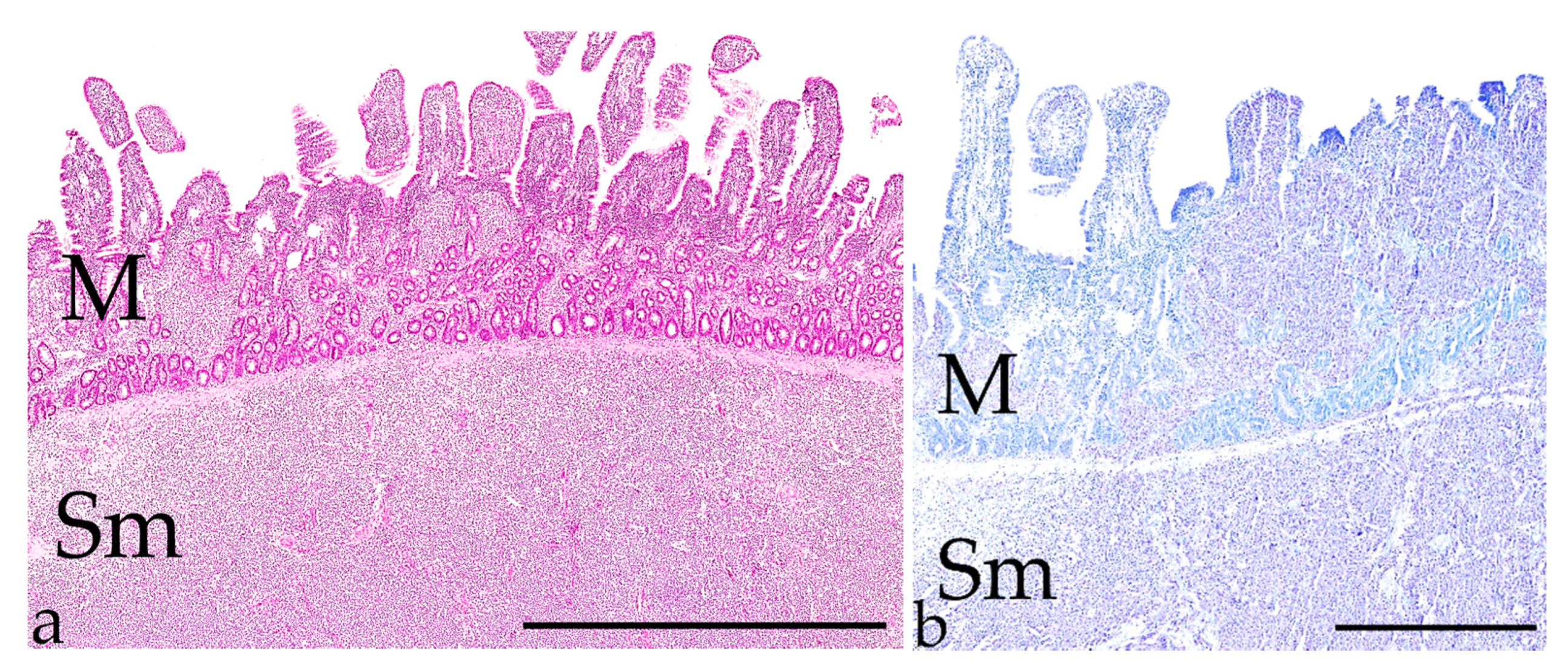
| Antigen (Clone) | Species | Supplier | Dilution | Pre-Treatment |
|---|---|---|---|---|
| CD3 (F7.2.38) | mouse | Dako 1 #M7254 | 1:100 | EDTA buffer |
| CD79a (HM57) | mouse | BioRad 2 #MCA2538GA | 1:3000 | EDTA buffer |
| CD20 | rabbit | Epria 3 #RB-9013-P1 | 1:100 | EDTA buffer |
| cKit/CD117 | rabbit | Dako 1 #A4502 | 1:150 | EDTA buffer |
| Smooth muscle Alpha actin (1A4) | mouse | Dako 1 #M0851 | 1:100 | target retrieval solution buffer |
| Glial fibrillary protein | rabbit | Dako 1 #Z0334 | 1:300 | EDTA buffer |
| Group | Breed | Age (Years) | Sex |
|---|---|---|---|
| B-cell lymphoma (n = 6) | 4 DSH, 1 mix, 1 Somali | 4–16 | 1 m, 1 mc, 1 f, 3 fs |
| T-cell lymphoma (n = 5) | 5 DSH | 6–14 | 3 mc, 2 fs |
| Carcinoma (n = 5) | 4 DSH, 1 Russ. blue | 8–14 | 1 m, 4 mc |
| Controls (n = 5) | 3 DSH, 1 BSH, 1 Russ. blue | 1–13 | 2 m, 3 mc |
| Site | Breed | Age (Years) | Sex |
|---|---|---|---|
| Stomach (n = 118) | 74 DSH, 8 Maine coon, 7 BSH, 6 mix, 3 Chartreux, 3 Persian, 3 Russ. blue, 3 Siamese, 3 Thai, 2 Burmese, 2 Norw. forest cat, 4 others | 1–17 median: 10 | 8 m, 48 mc, 10 f, 52 fs |
| Small intestine (n = 310) | 233 DSH, 27 mix, 9 Maine coon, 9 Norw. forest cat, 6 Persian, 4 BSH, 4 Siamese, 4 Turkish Angora, 3 Bengal, 3 ELH, 2 ASH, 6 others | 1–18 median: 11 | 22 m, 157 mc, 23 f, 108 fs |
| Large intestine (n = 44) | 32 DSH, 4 BSH, 3 mix, 2 Maine coon, 3 others | 1–15 median: 10 | 2 m, 24 mc, 2 f, 16 fs |
| Intestine NOS (n = 182) | 123 DSH, 20 mix, 11 BSH, 5 Maine coon, 3 ELH, 2 Bengal, 2 DLH, 2 Siamese, 14 others | 1–16 median: 10 | 27 m, 76 mc, 18 f, 61 fs |
| Intestine and stomach (n = 25) | 15 DSH, 2 BSH, 2 mix, 6 others | 4–14 median: 11 | 1 m, 10 mc, 1 f, 13 fs |
| Site | Breed | Age (Years) | Sex | Morphology |
|---|---|---|---|---|
| Stomach (n = 3) | 1 Bengal, 1 Norw. forest cat, 1 Siberian | 9–12 median: 10 | 1 mc, 2 fs | 2 tubular, 1 undiff. |
| Small intestine (n = 39) | 26 DSH, 5 mix, 1 Balinese, 1 Bengal, 1 Birman, 1 BSH, 1 ELH, 1 Oriental, 1 Russ. blue, 1 Siamese | 5–19 median: 11 | 7 m, 20 mc, 3 f, 9 fs | 25 acinar (2× bone metaplasia), 4 mucinous, 10 undiff. |
| Large intestine (n = 71) | 47 DSH, 8 mix, 6 BSH, 3 Maine coon, 3 Persian, 2 Chartreux, 1 BLH,1 Norw. forest cat | 3–23 median: 11 | 5 m, 33 mc, 12 f, 21 fs | 54 acinar (3× bone metaplasia), 9 undiff., 8 mucinous |
| Intestine NOS (n = 9) | 6 DSH, 1 Balinese, 1 Persian, 1 Sphynx | 7–17 median: 12 | 6 mc, 3 fs | 5 acinar, 3 undiff., 1 mucinous |
| Tumour | Site | Breed | Age (Years) | Sex |
|---|---|---|---|---|
| Leiomyosarcoma (n = 8) | 5 small intestine, 1 large intestine, 1 stomach, 1 intestine NOS | 7 DSH, 1 Cornish Rex | 2–15 | 4 mc, 4 fs |
| Haemangiosarcoma (n = 4) | 2 large intestine 2 intestine NOS | 3 DSH, 1 Persian | 5–10 | 1 mc, 3 fs |
| Leiomyoma (n = 1) | small intestine | DSH | 13 | fs |
| Neurofibrosarcoma (n = 1) | large intestine | DSH | 7 | fs |
| Spindle cell sarcoma NFD (n = 12) | 6 large intestine, 3 small intestine, 3 stomach | 7 DSH, 3 BSH, 2 mix | 5–16 | 2 m, 5 mc, 3 f, 2 fs |
| Pleomorphic sarcoma NOS (n = 3) | 3 intestine NOS | 3 DSH | 12–18 | 1 mc, 1 f, 1 fs |
| Site | Breed | Age (Years) | Sex |
|---|---|---|---|
| Stomach (n = 3) | 1 BSH, 1 DSH, 1 mix | 7–14 | 1 mc, 2 fs |
| Small intestine (n = 8) | 8 DSH | 11–16 | 1 m, 5 mc, 2 fs |
| Large intestine (n = 12) | 5 DSH, 2 Chartreux, 2 Maine coon, 2 mix, 1 Norw. forest cat | 1–15 | 8 mc, 1 f, 3 fs |
| Case No | Sex | Age | Cellular Differentiation | Mitotic Figures/hpf | cKit Expression Pattern |
|---|---|---|---|---|---|
| 1 | mc | 11 | moderate | 3 | diffuse |
| 2 | fs | 11 | good | 1 | diffuse + stippled |
| 3 | fs | 13 | sclerosing | 1 | diffuse |
| 4 | fs | 11 | good | 1 | diffuse + stippled |
| 5 | mc | 10 | pleomorphic | 10 | diffuse |
| 6 | mc | 9 | pleomorphic | 2 | diffuse |
| 7 | mc | 16 | poor (histiocytic) | 1 | diffuse + stippled |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kehl, A.; Törner, K.; Jordan, A.; Lorenz, M.; Schwittlick, U.; Conrad, D.; Steiger, K.; Schusser, B.; Aupperle-Lellbach, H. Pathological Findings in Gastrointestinal Neoplasms and Polyps in 860 Cats and a Pilot Study on miRNA Analyses. Vet. Sci. 2022, 9, 477. https://doi.org/10.3390/vetsci9090477
Kehl A, Törner K, Jordan A, Lorenz M, Schwittlick U, Conrad D, Steiger K, Schusser B, Aupperle-Lellbach H. Pathological Findings in Gastrointestinal Neoplasms and Polyps in 860 Cats and a Pilot Study on miRNA Analyses. Veterinary Sciences. 2022; 9(9):477. https://doi.org/10.3390/vetsci9090477
Chicago/Turabian StyleKehl, Alexandra, Katrin Törner, Annemarie Jordan, Mareike Lorenz, Ulrike Schwittlick, David Conrad, Katja Steiger, Benjamin Schusser, and Heike Aupperle-Lellbach. 2022. "Pathological Findings in Gastrointestinal Neoplasms and Polyps in 860 Cats and a Pilot Study on miRNA Analyses" Veterinary Sciences 9, no. 9: 477. https://doi.org/10.3390/vetsci9090477
APA StyleKehl, A., Törner, K., Jordan, A., Lorenz, M., Schwittlick, U., Conrad, D., Steiger, K., Schusser, B., & Aupperle-Lellbach, H. (2022). Pathological Findings in Gastrointestinal Neoplasms and Polyps in 860 Cats and a Pilot Study on miRNA Analyses. Veterinary Sciences, 9(9), 477. https://doi.org/10.3390/vetsci9090477








