Nasal Lymphoma with Low Mitotic Index in Three Cats Treated with Chlorambucil and Prednisolone
Abstract
:Simple summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Cases
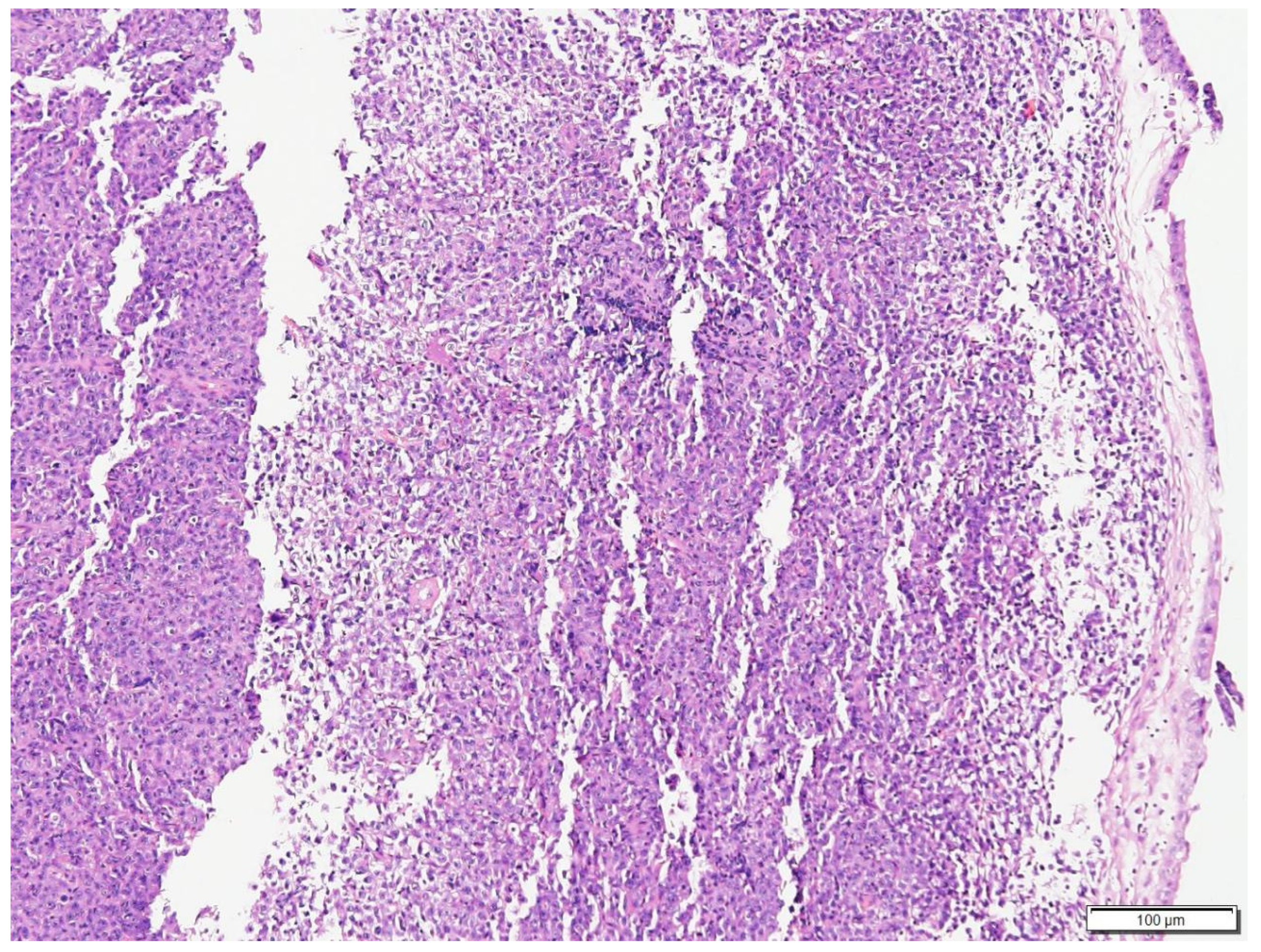
2.1.1. Case N.1
2.1.2. Case N.2
2.1.3. Case N.3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferguson, S.; Smith, K.C. A Retrospective Study of More than 400 Feline Nasal Biopsy Samples in the UK (2006–2013). J. Feline Med. Surg. 2020, 22, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.M.; Bradley, K. Investigation of Nasal Disease in the Cat—A Retrospective Study of 77 Cases. J. Feline Med. Surg. 2004, 6, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Mukaratirwa, S.; van der Linde-Sipman, J. Feline Nasal and Paranasal Sinus Tumours: Clinicopathological Study, Histomorphological Description and Diagnostic Immunohistochemistry of 123 Cases. J. Feline Med. Surg. 2001, 3, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Haney, S.M.; Beaver, L. Survival Analysis of 97 Cats with Nasal Lymphoma: A Multi-Institutional Retrospective Study (1986–2006). J. Vet. Intern. Med. 2009, 23, 287–294. [Google Scholar] [CrossRef]
- Little, L.; Patel, R. Nasal and Nasopharyngeal Lymphoma in Cats: 50 Cases (1989–2005). Vet. Pathol. 2007, 44, 885–892. [Google Scholar] [CrossRef]
- Day, M.J.; Henderson, S.M. An Immunohistochemical Investigation of 18 Cases of Feline Nasal Lymphoma. J. Comp. Pathol. 2004, 130, 152–161. [Google Scholar] [CrossRef]
- Santagostino, S.F.; Mortellaro, C.M. Feline Upper Respiratory Tract Lymphoma. Vet. Pathol. 2015, 52, 250–259. [Google Scholar] [CrossRef]
- Meier, V.S.; Beatrice, L. Outcome and Failure Patterns of Localized Sinonasal Lymphoma in Cats Treated with First-line Single-modality Radiation Therapy: A Retrospective Study. Vet. Comp. Oncol. 2019, 17, 528–536. [Google Scholar] [CrossRef]
- Taylor, S.S.; Goodfellow, M.R. Feline Extranodal Lymphoma: Response to Chemotherapy and Survival in 110 Cats. J. Small Anim. Pract. 2009, 50, 584–592. [Google Scholar] [CrossRef]
- Teske, E.; van Straten, G. Chemotherapy with Cyclophosphamide, Vincristine, and Prednisolone (COP) in Cats with Malignant Lymphoma: New Results with an Old Protocol. J. Vet. Intern. Med. 2002, 16, 179–186. [Google Scholar] [CrossRef]
- Cunha, S.C.d.S.; Silva, F.B. Retrospective Study of Adverse Events of Chemotherapy in Cats. Acta Sci. Vet. 2018, 46, 12. [Google Scholar] [CrossRef]
- Paulin, M.v.; Couronné, L. Feline Low-Grade Alimentary Lymphoma: An Emerging Entity and a Potential Animal Model for Human Disease. BMC Vet. Res. 2018, 14, 306. [Google Scholar] [CrossRef]
- Stein, T.J.; Pellin, M. Treatment of Feline Gastrointestinal Small-Cell Lymphoma with Chlorambucil and Glucocorticoids. J. Am. Anim. Hosp. Assoc. 2010, 46, 413–417. [Google Scholar] [CrossRef]
- Takahashi, K.; Baba, T. Long-Term Management of a Cat with Nasopharyngeal Lymphoma by Chlorambucil. Open Vet. J. 2021, 11, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Myint, M.S. Classification of Canine Malignant Lymphomas According to the World Health Organization Criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Kass, P.H. Canine Lymphomas. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Freiche, V.; Cordonnier, N. Feline Low-Grade Intestinal T Cell Lymphoma: A Unique Natural Model of Human Indolent T Cell Lymphoproliferative Disorder of the Gastrointestinal Tract. Lab. Investig. 2021, 101, 794–804. [Google Scholar] [CrossRef]
- Adams, W.M.; Kleiter, M.M. Prognostic Significance of Tumor Histology and Computed Tomographic Staging for Radiation Treatment Response of Canine Nasal Tumors. Vet. Radiol. Ultrasound 2009, 50, 330–335. [Google Scholar] [CrossRef]
- Moore, P.F.; Rodriguez-Bertos, A. Feline Gastrointestinal Lymphoma. Vet. Pathol. 2012, 49, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, F.; Calam, A.E. Feline Mediastinal Lymphoma: A Retrospective Study of Signalment, Retroviral Status, Response to Chemotherapy and Prognostic Indicators. J. Feline Med. Surg. 2014, 16, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Wolfesberger, B.; Skor, O. Does Categorisation of Lymphoma Subtypes According to the World Health Organization Classification Predict Clinical Outcome in Cats? J. Feline Med. Surg. 2017, 19, 897–906. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, K.W.L.; Beatty, J.A.; Tse, M.P.Y.; Giuliano, A. Nasal Lymphoma with Low Mitotic Index in Three Cats Treated with Chlorambucil and Prednisolone. Vet. Sci. 2022, 9, 472. https://doi.org/10.3390/vetsci9090472
Ng KWL, Beatty JA, Tse MPY, Giuliano A. Nasal Lymphoma with Low Mitotic Index in Three Cats Treated with Chlorambucil and Prednisolone. Veterinary Sciences. 2022; 9(9):472. https://doi.org/10.3390/vetsci9090472
Chicago/Turabian StyleNg, Karen W. L., Julia A. Beatty, May P. Y. Tse, and Antonio Giuliano. 2022. "Nasal Lymphoma with Low Mitotic Index in Three Cats Treated with Chlorambucil and Prednisolone" Veterinary Sciences 9, no. 9: 472. https://doi.org/10.3390/vetsci9090472
APA StyleNg, K. W. L., Beatty, J. A., Tse, M. P. Y., & Giuliano, A. (2022). Nasal Lymphoma with Low Mitotic Index in Three Cats Treated with Chlorambucil and Prednisolone. Veterinary Sciences, 9(9), 472. https://doi.org/10.3390/vetsci9090472






