2D-SWE of the Metacarpophalangeal Joint Capsule in Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caron, J.P. Synovial Joint Biology and Pathobiology Equine Surgery, 4th ed.; Auer, J.A., Stick, J.A., Eds.; Elsevier: Saint Luois, MI, USA, 2012; pp. 1096–1114. [Google Scholar]
- Wijesekera, N.T.; Calder, J.D.; Lee, J.C. Imaging in the assessment and management of Achilles tendinopathy and paratendinitis. In Seminars in Musculoskeletal Radiology; Thieme Medical Publishers: New York, NY, USA, 2011; Volume 15, pp. 89–100. [Google Scholar]
- Aubry, S.; Nueffer, J.-P.; Tanter, M.; Becce, F.; Vidal, C.; Michel, F. Viscoelasticity in Achilles Tendonopathy: Quantitative Assessment by Using Real-time Shear-Wave Elastography. Radiology 2014, 274, 821–829. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Kawcak, C.; Baxter, G.M.; Goodrich, L.R.; Valberg, S.J. Principles of Musculoskeletal Disease: Joint injuries and disease and osteoarthritis. In Adams and Stashak’s Lameness in Horses, 7th ed.; Baxter, G.M., Ed.; John Wiley and Sons: Oxford, UK, 2020; pp. 801–874. [Google Scholar]
- McIlwraith, C.W. Traumatic arthritis and posttraumatic osteoarthritis in the horse. In Joint Disease in the Horse, 2nd ed.; McIlwraith, C.W., Frisbie, D.D., van Weeren, P.R., Eds.; Elsevier: Saint Louis, MI, USA, 2016; pp. 33–48. [Google Scholar]
- Chen, X.-M.; Cui, L.-G.; He, P.; Shen, W.-W.; Qian, Y.-J.; Wang, J.-R. Shear Wave Elastographic Characterization of normal and torn Achilles tendons: A pilot study. J Ultrasound Med. 2013, 32, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Pickerell, D.M. Elastography: Imaging of tomorrow? J. Diagnostic Med. Sonogr. 2010, 26, 109–113. [Google Scholar] [CrossRef]
- Lin, T.W.; Cardenas, L.; Soslowsky, L.J. Biomechanics of tendon injury and repair. J. Biomech. 2004, 37, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Dirrichs, T.; Schrading, S.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Quack, V. Shear Wave Elastography (SWE) of Asymptomatic Achilles Tendons: A Comparison between Semiprofessional Athletes and the Nonathletic General Population. Acad. Radiol. 2019, 26, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Doherty, J.R.; Trahey, G.E.; Nightingale, K.R.; Palmeri, M.L. Acoustic radiation force elasticity imaging in diagnostic ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 685–701. [Google Scholar] [CrossRef]
- Athanasiou, A.; Tardivon, A.; Tanter, M.; Sigal-Zafrani, B.; Bercoff, J.; Deffieux, T.; Gennisson, J.-L.; Fink, M.; Neuenschwander, S. Breast lesions: Quantitative elastography with supersonic shear imaging—preliminary results. Radiology 2010, 256, 297–303. [Google Scholar] [CrossRef]
- Tanter, M.; Bercoff, J.; Athanasiou, A.; Deffieux, T.; Gennisson, J.-L.; Montaldo, G.; Muller, M.; Tardivon, A.; Fink, M. Quantitative assessment of breast lesion viscoelasticity: Initial clinical results using supersonic shear imaging. Ultrasound Med. Biol. 2008, 34, 1373–1386. [Google Scholar] [CrossRef]
- Bavu, É.; Gennisson, J.-L.; Couade, M.; Bercoff, J.; Mallet, V.; Fink, M.; Badel, A.; Vallet-Pichard, A.; Nalpas, B.; Tanter, M.; et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: A clinical study on 113 hepatitis C virus patients. Ultrasound Med. Biol. 2011, 37, 1361–1373. [Google Scholar] [CrossRef]
- Ragazzoni, F.; Deandrea, M.; Mormile, A.; Ramunni, M.J.; Garino, F.; Magliona, G.; Motta, M.; Torchio, B.; Garberoglio, R.; Limone, P. High diagnostic accuracy and interobserver reliability of real-time elastography in the evaluation of thyroid nodules. Ultrasound Med. Biol. 2012, 38, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Straticò, P.; Guerri, G.; Palozzo, A.; Di Francesco, P.; Vignoli, M.; Varasano, V.; Petrizzi, L. Elastosonographic features of the metacarpophalangeal joint capsule in horses. BMC Vet. Res. 2021, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Nukada, T.; Kato, T.; Kuroda, T.; Kotoyori, Y.; Fukuda, K.; Kasashima, Y. The use of sonoelastography to assess the recovery of stiffness after equine superficial digital flexor tendon injuries: A preliminary prospective longitudinal study of the healing process. Equine Vet. J. 2017, 49, 590–595. [Google Scholar] [CrossRef]
- Drakonaki, E.E.; Allen, G.M.; Wilson, D.J. Ultrasound elastography for musculoskeletal applications. Br. J. Radiol. 2012, 85, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Piccionello, A.P.; Busoni, V.; Salvaggio, A.; Bonazzi, M.; Bergamino, C.; Volta, A.; Serrani, D. Sonoelastographic Features of the Patellar Ligament in Clinically Normal Dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 279–284. [Google Scholar] [CrossRef] [PubMed]
- El Badry, A.; Ghieda, U.; El Khouly, R.; Elreweny, E.A. Evaluation of sonoelastography in Achilles tendon of healthy volunteers and patients with symptomatic Achilles tendon. Egypt. J. Radiol. Nucl. Med. 2018, 49, 119–127. [Google Scholar] [CrossRef]
- Lustgarten, M.; Redding, W.R.; Labens, R.; Morgan, M.; Davis, W.; Seiler, G.S. Elastographic characteristics of the metacarpal tendons in horses without clinical evidence of tendon injury. Vet. Radiol. Ultrasound 2014, 55, 92–101. [Google Scholar] [CrossRef]
- Lustgarten, M.; Redding, W.R.; Labens, R.; Davis, W.; Daniel, T.M.; Griffith, E.; Seiler, G.S. Elastographic evaluation of naturally occuring tendon and ligament injuries of the equine distal limb. Vet. Radiol. Ultrasound 2015, 56, 670–679. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, W.S.; Park, G.Y.; Wang, T.G.; Lew, H.L. Musculoskeletal Sonoelastography: A Focused Review of its Diagnostic Applications for Evaluating Tendons and Fascia. J. Med. Ultrasound 2012, 20, 79–86. [Google Scholar] [CrossRef]
- Del Signore, F.; De Dominicis, S.; Mastromatteo, G.; Simeoni, F.; Scapolo, P.A.; Tamburro, R.; Vignoli, M. Sonoelastography of Normal Canine Common Calcaneal Tendon: Preliminary Results. Vet. Comp. Orthop. Traumatol. 2021, 34, 200–205. [Google Scholar] [CrossRef]
- Winn, N.; Lalam, R.; Cassar-Pullicino, V. Sonoelastography in the musculoskeletal system: Current role and future directions. World J. Radiol. 2016, 8, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Sadeghi, S.; Bader, D.A.; Cortes, D.H. Ultrasound Shear Wave Elastography of the Elbow Ulnar Collateral Ligament: Reliability Test and a Preliminary Case Study in a Baseball Pitcher. J. Eng. Sci. Med. Diagn. Ther. 2017, 1, 011004. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Jeong, W.K. Current status of musculoskeletal application of shear wave elastography. Ultrasonography 2017, 36, 185–197. [Google Scholar] [CrossRef]
- Bassage, L.H., II; Ross, M.W. Diagnostic analgesia. In Diagnosis and Management of Lameness in the Horse; Elsevier: Amsterdam, The Netherlands, 2003; pp. 93–124. [Google Scholar]
- Vanderperren, K.; Saunders, J.H. Diagnostic imaging of the equine fetlock region using radiography and ultrasonography. Part 1: Soft tissues. Vet. J. 2009, 181, 111–122. [Google Scholar] [CrossRef]
- Lacitignola, L.; Imperante, A.; Staffieri, F.; De Siena, R.; De Luca, P.; Muci, A.; Crovace, A. Assessment of Intra-and Inter-observer measurement variability in a radiographic metacarpophalangeal joint osteophytosis scoring system for the horse. Vet. Sci. 2020, 7, 39. [Google Scholar] [CrossRef]
- Yamada, A.L.M.; Pinheiro, M.; Marsiglia, M.F.; Hagen, S.C.F.; Baccarin, R.Y.A.; da Silva, L.C.L.C. Ultrasound and clinical findings in the metacarpophalangeal joint assessment of show jumping horses in training. J. Vet. Sci. 2020, 21, e21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 31 May 2022).
- Drakonaki, E.E.; Allen, G.M.; Wilson, D.J. Real-time ultrasound elastography of the normal Achilles tendon: Reproducibility and pattern description. Clin. Radiol. 2009, 64, 1196–1202. [Google Scholar] [CrossRef]
- Barr, R.G.; Nakashima, K.; Amy, D.; Cosgrove, D.; Farrokh, A.; Schafer, F.; Bamber, J.C.; Castera, L.; Choi, B.I.; Chou, Y.H.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: Breast. Ultrasound Med. Biol. 2015, 41, 1148–1160. [Google Scholar] [CrossRef] [Green Version]
- Secchi, V.; Masala, G.; Corda, A.; Corda, F.; Potop, E.; Fernandez, A.B.; Parpaglia, M.L.P.; Passino, E.S. Strain elastography of injured equine superficial digital flexor tendons: A reliability study of manual measurements. Animals 2021, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Zhang, Z. Effects of precompression on elasticity imaging of the breast: Development of a clinically useful semiquantitative method of precompression assessment. J. Ultrasound Med. 2012, 31, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.C.; Baumer, T.G.; Bey, M.J.; Van Holsbeeck, M.T. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2018, 38, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, P.; Gennisson, J.-L.; Podda, A.; Alilet, M.; Carrié, M.; Aubry, S. Artifacts and Technical Restrictions in 2D Shear Wave Elastography. Ultraschall Med.-Eur. J. Ultrasound 2020, 41, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Aubry, S.; Risson, J.R.; Kastler, A.; Barbier-Brion, B.; Siliman, G.; Runge, M.; Kastler, B. Biomechanical properties of the calcaneal tendon in vivo assessed by transient shear wave elastography. Skelet. Radiol. 2013, 42, 1143–1150. [Google Scholar] [CrossRef]
- Mancini, M.; Megna, A.S.; Ragucci, M.; De Luca, M.; Marsilia, G.M.; Nardone, G.; Coccoli, P.; Prinster, A.; Mannelli, L.; Vergara, E.; et al. Reproducibility of shear wave elastography (SWE) in patients with chronic liver disease. PLoS ONE 2017, 12, e0185391. [Google Scholar] [CrossRef]
- Ferraioli, G.; Tinelli, C.; Dal Bello, B.; Zicchetti, M.; Filice, G.; Filice, C.; on behalf of the Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: A pilot study. Hepatology 2012, 56, 2125–2133. [Google Scholar] [CrossRef]
- Suh, C.H.; Kim, S.Y.; Kim, K.W.; Lim, Y.-S.; Lee, S.J.; Lee, M.-G.; Lee, J.; Lee, S.-G.; Yu, E. Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology 2014, 271, 895–900. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Arıbas, B.K.; Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am. J. Roentgenol. 2011, 197, 532. [Google Scholar] [CrossRef]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Rath, B.; Betsch, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for Monitoring of Treatment of Tendinopathies: A Double-blinded, Longitudinal Clinical Study. Acad. Radiol. 2018, 25, 265–272. [Google Scholar] [CrossRef]
- Petrescu, P.H.; Izvernariu, D.A.; Iancu, C.; Dinu, G.O.; Crişan, D.; Popescu, S.A.; Şirli, R.L.D.; Nistor, B.M.; Răuţia, I.C.; Lăzureanu, D.C.; et al. Evaluation of normal and pathological Achilles tendon by real-time shear wave elastography. Rom. J. Morphol. Embryol. 2016, 57, 785–790. [Google Scholar] [PubMed]
- Fu, S.; Cui, L.; He, X.; Sun, Y. Elastic characteristics of the normal Achilles tendon assessed by virtual touch imaging quantification shear wave elastography. J. Ultrasound Med. 2016, 35, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Baumer, T.G.; Dischler, J.; Davis, L.; Labyed, Y.; Siegal, D.S.; Van Holsbeeck, M.; Moutzouros, V.; Bey, M.J. Effects of age and pathology on shear wave speed of the human rotator cuff. J. Orthop. Res. 2018, 36, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Washburn, N.; Onishi, K.; Wang, J.H.C. Ultrasound elastography and ultrasound tissue characterisation for tendon evaluation. J. Orthop. Transl. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Rominger, M.B.; Kälin, P.; Mastalerz, M.; Martini, K.; Klingmüller, V.; Sanabria, S.; Frauenfelder, T. Influencing Factors of 2D Shear Wave Elastography of the Muscle—An Ex Vivo Animal Study. Ultrasound Int. Open 2018, 4, E54–E60. [Google Scholar] [CrossRef]
- Tamura, N.; Kuroda, T.; Kotoyori, Y.; Fukuda, K.; Nukada, T.; Kato, T.; Kuwano, A.; Kasashima, Y. Application of sonoelastography for evaluating the stiffness of equine superficial digital flexor tendon during healing. Vet. Rec. 2017, 180, 120. [Google Scholar] [CrossRef]
- Ehrle, A.; Lilge, S.; Clegg, P.D.; Maddox, T.W. Equine flexor tendon imaging part 1: Recent developments in ultrasonography, with focus on the superficial digital flexor tendon. Vet. J. 2021, 278, 105764. [Google Scholar] [CrossRef] [PubMed]
- Youk, J.H.; Gweon, H.M.; Son, E.J. Shear-wave elastography in breast ultrasonography: The state of the art. Ultrasonography 2017, 36, 300–309. [Google Scholar] [CrossRef]
- Klauser, A.S.; Miyamoto, H.; Tamegger, M.; Faschingbauer, R.; Moriggl, B.; Klima, G.; Feuchtner, G.M.; Kastlunger, M.; Jaschke, W.R. Achilles Tendon Assessed With Sonoelastography: Histologic agreement. Radiology 2013, 267, 837–842. [Google Scholar] [CrossRef]
- Ooi, C.C.; Schneider, M.E.; Malliaras, P.; Chadwick, M.; Connell, D.A. Diagnostic Performance of Axial-Strain Sonoelastography in Confirming Clinically Diagnosed Achilles Tendinopathy: Comparison with B-Mode Ultrasound and Color Doppler Imaging. Ultrasound Med. Biol. 2015, 41, 15–25. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamaguchi, S.; Sasho, T.; Fukawa, T.; Akatsu, Y.; Akagi, R.; Yamaguchi, T.; Takahashi, K.; Nagashima, K.; Takahashi, K. Quantitative US Elastography Can Be Used to Quantify Mechanical and Histologic Tendon Healing in a Rabbit Model of Achilles Tendon Transection. Radiology 2017, 283, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Serrani, D.; Volta, A.; Cingolani, F.; Pennasilico, L.; Di Bella, C.; Bonazzi, M.; Salvaggio, A.; Piccionello, A.P. Serial ultrasonographic and real-time elastosonographic assessment of the ovine common calcaneal tendon, after an experimentally induced tendinopathy. Vet. Sci. 2021, 8, 54. [Google Scholar] [CrossRef]
- Haen, T.X.; Roux, A.; Soubeyrand, M.; Laporte, S. Shear waves elastography for assessment of human Achilles tendon’s biomechanical properties: An experimental study. J. Mech. Behav. Biomed. Mater. 2017, 69, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Peltz, C.D.; Haladik, J.A.; Divine, G.; Siegal, D.; van Holsbeeck, M.; Bey, M.J. ShearWave elastography: Repeatability for measurement of tendon stiffness. Skelet. Radiol. 2013, 42, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Bejder, L.; Quack, V.; Schrading, S.; Dirrichs, T.; Tingart, M.; Kuhl, C.; Betsch, M. Shear Wave Elastography (SWE) for the Evaluation of Patients with Plantar Fasciitis. Acad. Radiol. 2020, 27, 363–370. [Google Scholar] [CrossRef]
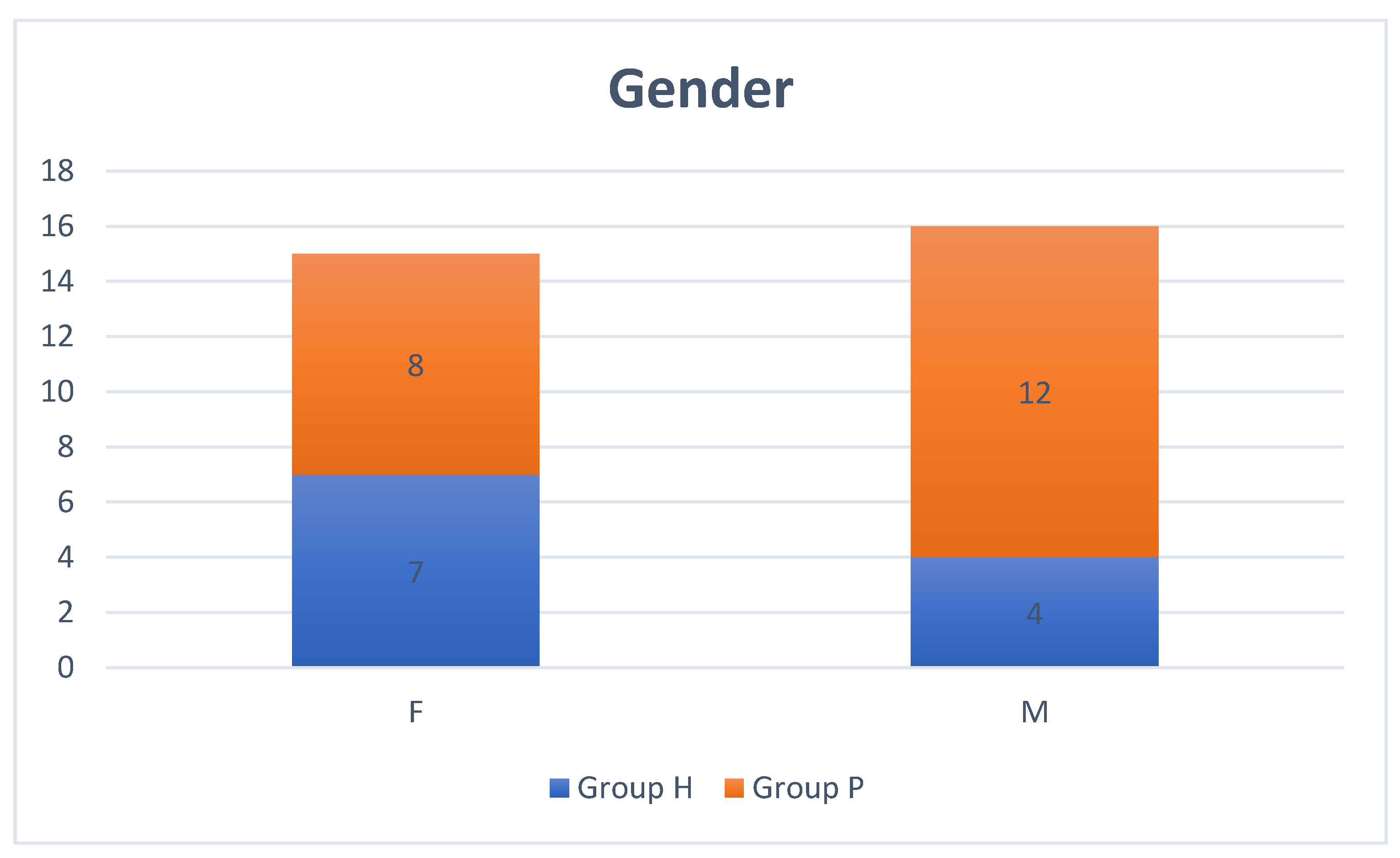
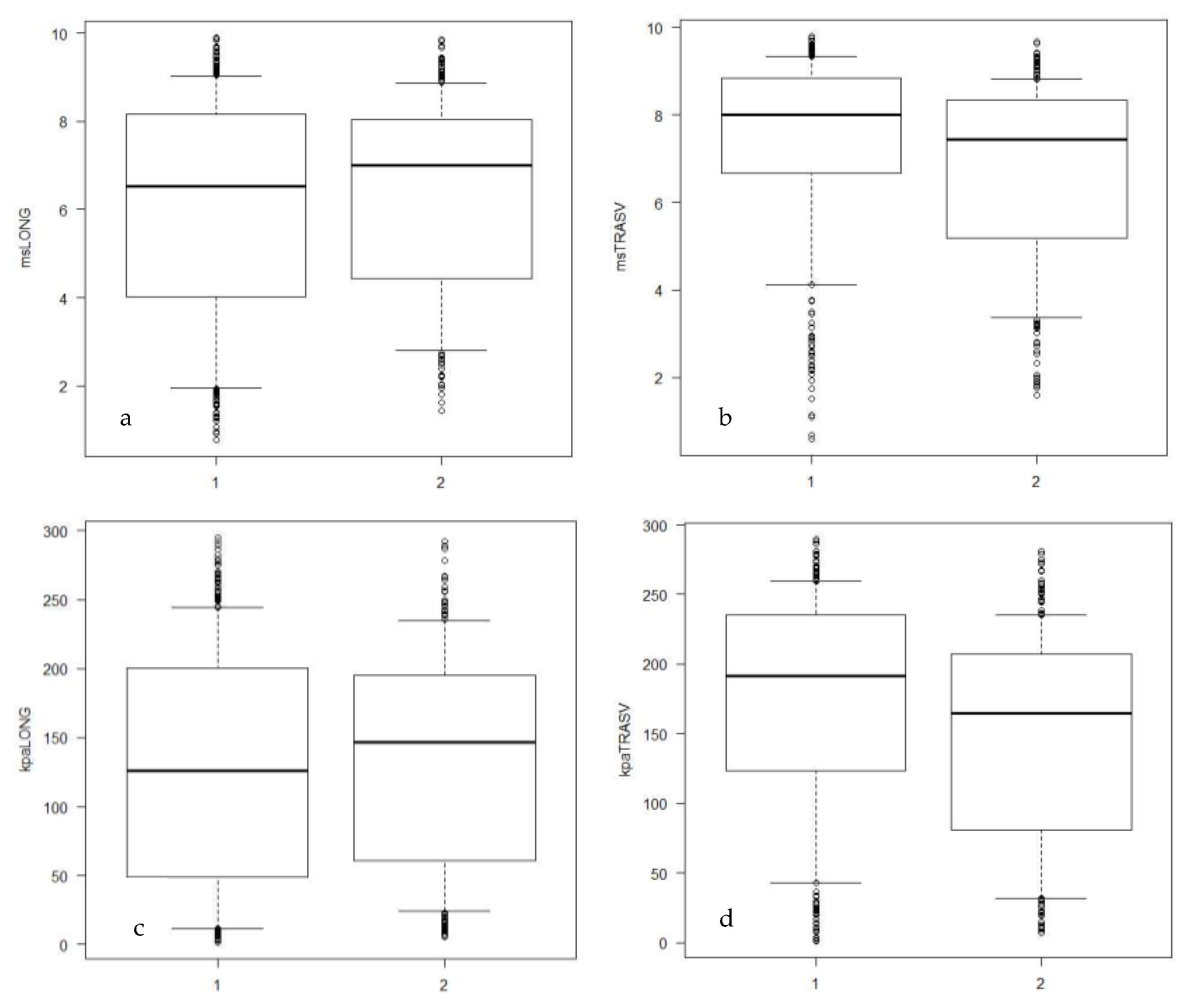
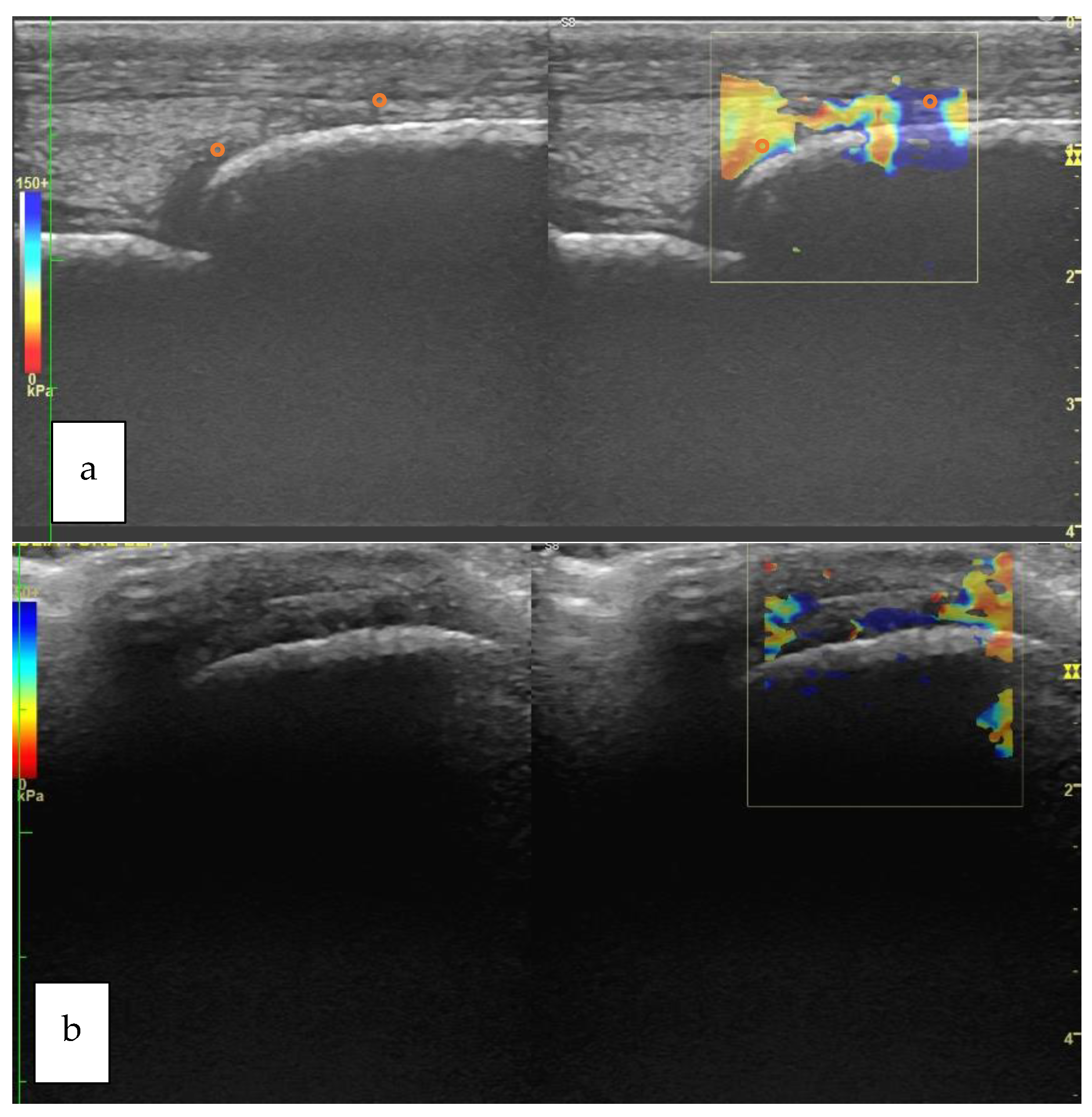

| Variable | Group H | Group P |
|---|---|---|
| Left | 1.05 a | 1.44 b |
| Right | 1.18 a | 1.47 b |
| Variable | Group H | Group P | ||
|---|---|---|---|---|
| Longitudinal | Transverse | Longitudinal | Transverse | |
| m/s | 6.53 (0.78–9.9) a | 8 (0.6–9.8) b | 7.01 (1.45–9.86) a | 7.43 (1.61–9.68) c |
| kPa | 125.86 (1.59–295.04) a | 191.56 (1.11–289.63) b | 146.36 (5.65–292.37) a | 164.64 (7.1–280.75) c |
| Group H | Left Limb | Right Limb | ||
| Longitudinal | Transverse | Longitudinal | Transverse | |
| m/s | 6.5 (0.78–9.9) | 8 (1.93–9.67) | 6.69 (0.96–9.83) | 8.04 (0.6–9.8) |
| kPa | 125.75 (1.59–295.04) | 191.55 (10.66–281.28) | 128.77 (3.38–289.51) | 190.84 (1.11–289.63) |
| Group P | Left Limb | Right Limb | ||
| Longitudinal | Transverse | Longitudinal | Transverse | |
| m/s | 7.02 (2.4–9.86) | 7.9 (1.91–9.43) | 6.99 (1.45–9.25) | 6.31 (1.61–9.68) |
| kPa | 146.36 (15.5–292.37 | 187.59 (10.83–271.6) | 143.84 (5.65256.03) | 121.73 (7.1–280.75) |
| Variable | Not Affected Limb of Group P | Affected Limb of Group P | ||
|---|---|---|---|---|
| Longitudinal | Transverse | Longitudinal | Transverse | |
| m/s | 6.94 (1.29–9.3) a | 7.65 (0.6–9.28) c | 7.09 (1.45–9.86) b | 7.43 (1.61–9.68) c |
| kPa | 143.72 (4.47–256.61) a | 175.34 (1.11–258.91) c | 149.8 (5.65–292.37) b | 164.64 (7.1–280.75) c |
| AUC | 95%CI | Cut-off | ||||
|---|---|---|---|---|---|---|
| Longitudinal | Transverse | Longitudinal | Transverse | Longitudinal | Transverse | |
| m/s | 0.517 | 0.616 | 0.4732–0.5609 | 0.571–0.661 | 6.61 | 7.55 |
| kPa | 0.518 | 0.61 | 0.4741–0.5618 | 0.565–0.655 | 130.75 | 184.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerri, G.; Palozzo, A.; Straticò, P.; Varasano, V.; Celani, G.; Di Francesco, P.; Vignoli, M.; Petrizzi, L. 2D-SWE of the Metacarpophalangeal Joint Capsule in Horses. Vet. Sci. 2022, 9, 478. https://doi.org/10.3390/vetsci9090478
Guerri G, Palozzo A, Straticò P, Varasano V, Celani G, Di Francesco P, Vignoli M, Petrizzi L. 2D-SWE of the Metacarpophalangeal Joint Capsule in Horses. Veterinary Sciences. 2022; 9(9):478. https://doi.org/10.3390/vetsci9090478
Chicago/Turabian StyleGuerri, Giulia, Adriana Palozzo, Paola Straticò, Vincenzo Varasano, Gianluca Celani, Paola Di Francesco, Massimo Vignoli, and Lucio Petrizzi. 2022. "2D-SWE of the Metacarpophalangeal Joint Capsule in Horses" Veterinary Sciences 9, no. 9: 478. https://doi.org/10.3390/vetsci9090478
APA StyleGuerri, G., Palozzo, A., Straticò, P., Varasano, V., Celani, G., Di Francesco, P., Vignoli, M., & Petrizzi, L. (2022). 2D-SWE of the Metacarpophalangeal Joint Capsule in Horses. Veterinary Sciences, 9(9), 478. https://doi.org/10.3390/vetsci9090478










