Study on NGF and VEGF during the Equine Perinatal Period—Part 2: Foals Affected by Neonatal Encephalopathy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Clinical Data and Sample Collection
2.3. Measurement of NGF and VEGF by ELISA
2.4. Measurement of Thyroid Hormones
2.5. Placental Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Population Characterization
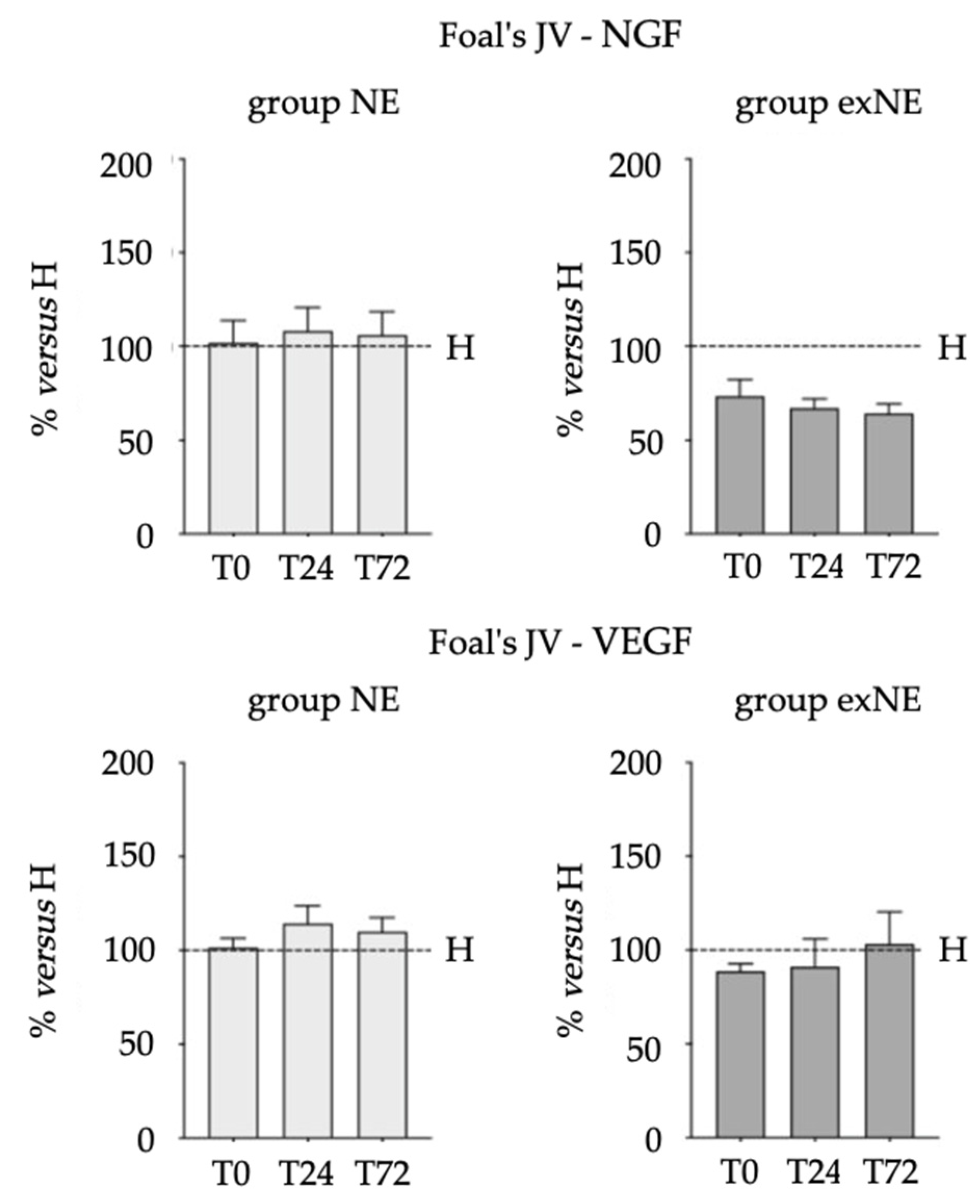
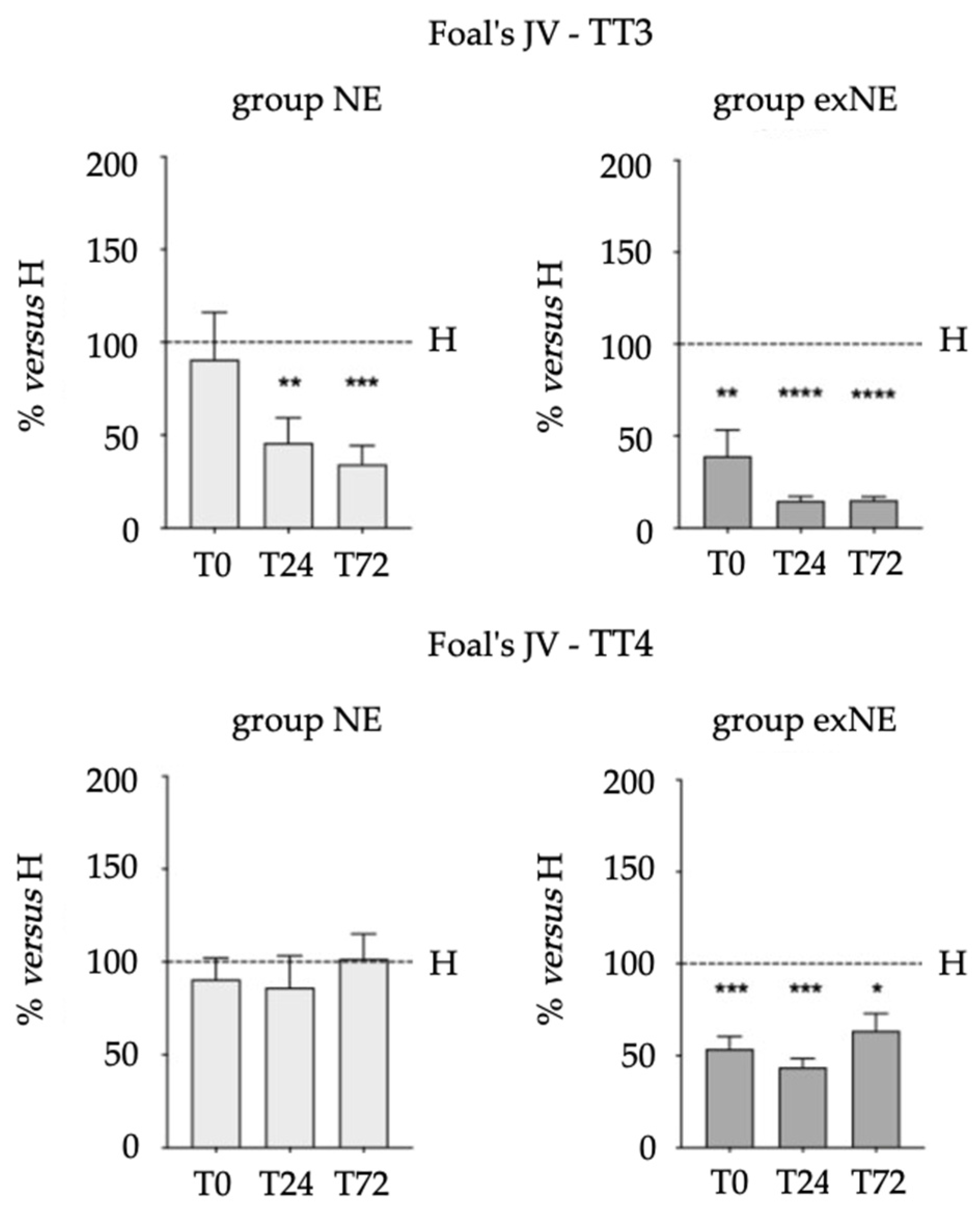
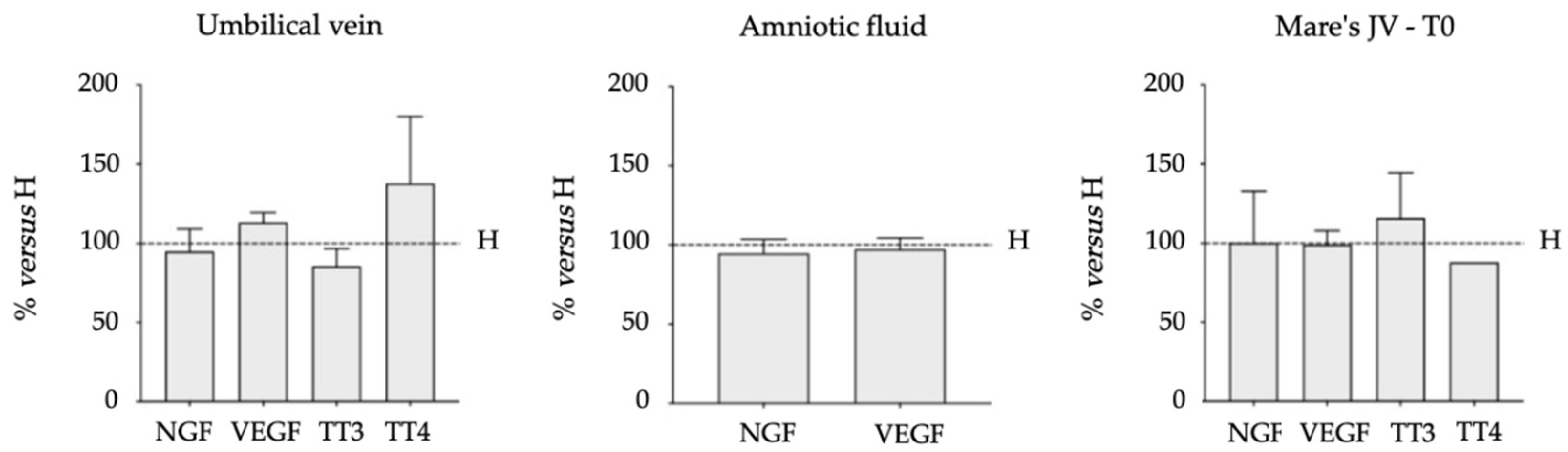
3.2. Biomarkers (NGF, VEGF, TT3 and TT4) in Biological Fluids
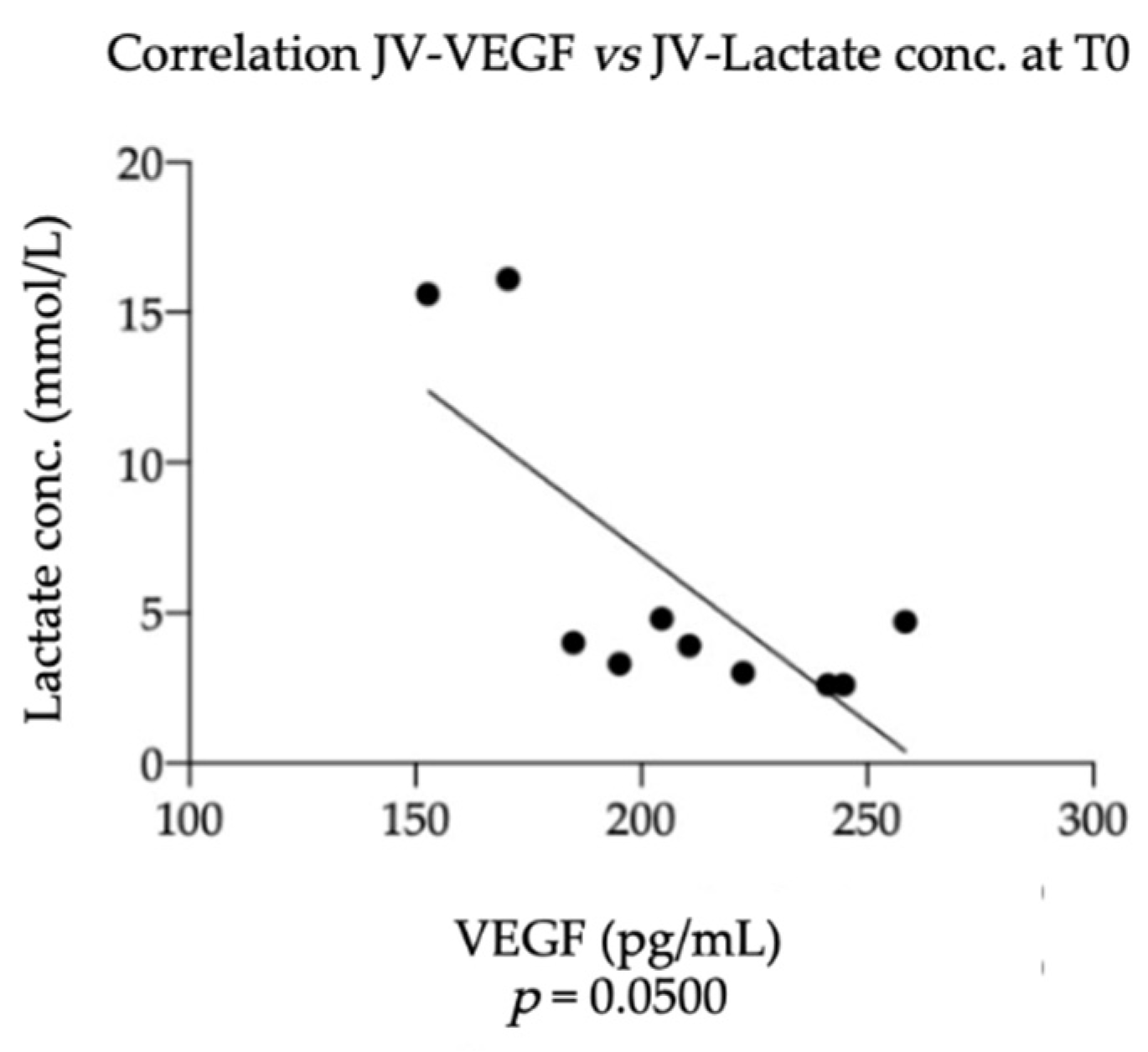
3.3. Equine NGF/VEGF and Clinical Data
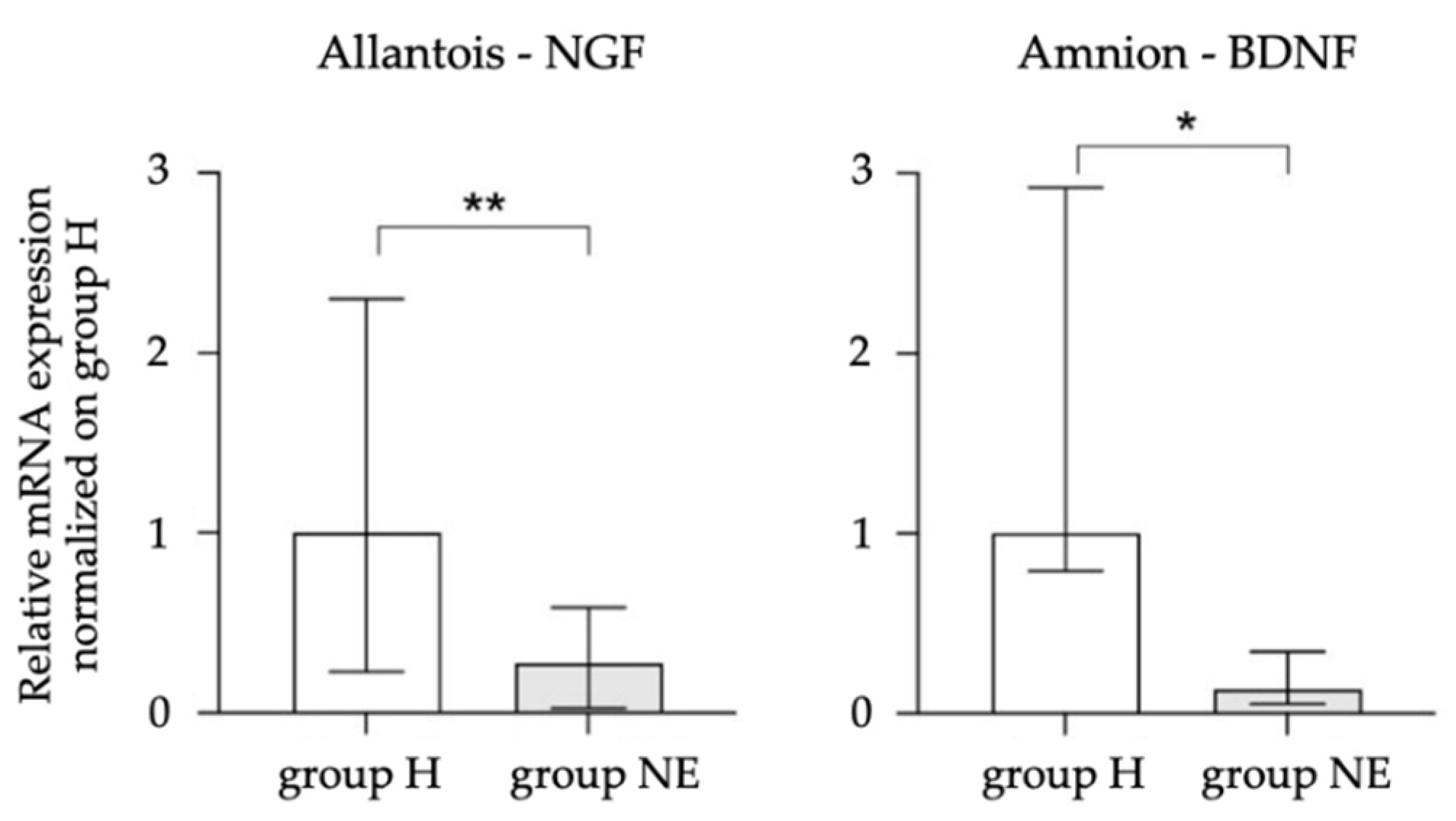
3.4. Equine NGF, VEGF, and BDNF in the Placenta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galvin, N.; Collins, D. Perinatal asphyxia syndrome in the foal: Review and a case report. Ir. Vet. J. 2004, 57, 707–714. [Google Scholar] [CrossRef] [PubMed]
- MacKay, R.J. Neurologic disorders of neonatal foals. Vet. Clin. Equine 2005, 21, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Dickey, E.J.; Long, S.N.; Hunt, R.W. Hypoxic ischemic encephalopathy—What can we learn from humans? J. Vet. Intern. Med. 2011, 25, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Wilkins, P.A.; Bain, F.T.; Brockus, C. Neonatal encephalopathy in foals. Compend. Contin. Educ. Vet. 2011, 33, E1–E10. [Google Scholar]
- Ringger, N.C.; Giguere, S.; Morresey, P.R.; Yang, C.; Shaw, G. Biomarkers of brain injury in foals with hypoxic-ischemic encephalopathy. J. Vet. Intern. Med. 2011, 25, 132–137. [Google Scholar] [CrossRef]
- Diesch, T.J.; Mellor, D.J. Birth transitions: Pathophysiology, the onset of consciousness and possible implications for neonatal maladjustment syndrome in the foal. Equine Vet. J. 2013, 45, 656–660. [Google Scholar] [CrossRef]
- Pirrone, A.; Panzani, S.; Govoni, N.; Castagnetti, C.; Veronesi, M.C. Thyroid hormone concentrations in foals affected by perinatal asphyxia syndrome. Theriogenology 2013, 80, 624–629. [Google Scholar] [CrossRef]
- Mariella, J.; Isani, G.; Andreani, G.; Freccero, F.; Carpenè, E.; Castagnetti, C. Total plasma magnesium in healthy and critically ill foals. Theriogenology 2016, 85, 180–185. [Google Scholar] [CrossRef]
- Gold, J.R. Perinatal asphyxia syndrome. Equine Vet. Educ. 2017, 29, 158–164. [Google Scholar] [CrossRef]
- Lyle-Dugas, J.; Giguere, S.; Mallicote, M.F.; MacKay, R.J.; Sanchez, L.C. Factors associated with outcome in 94 hospitalised foals diagnosed with neonatal encephalopathy. Equine Vet. J. 2017, 49, 207–210. [Google Scholar] [CrossRef]
- Wong, D.M.; Jeffery, N.; Hepworth-Warren, K.L.; Wiechert, S.A.; Miles, K. Magnetic resonance imaging of presumptive neonatal encephalopathy in a foal. Equine Vet. Educ. 2017, 29, 534–538. [Google Scholar] [CrossRef]
- Toribio, R.E. Equine neonatal encephalopathy: Facts, evidence, and opinions. Vet. Clin. Equine 2019, 35, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M. Practical management and treatment of foals with neonatal encephalopathy/neonatal maladjustment syndrome in an ICU setting. Equine Vet. Educ. 2022. [Google Scholar] [CrossRef]
- Santschi, E.M.; Vaala, W.E. Identification of the high-risk pregnancy. In Equine Reproduction; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 5–15. [Google Scholar]
- Vaala, W.E.; Sertich, P.L. Management strategies for mares at risk for periparturient complications. Vet. Clin. N. Am. Equine Pract. 1994, 10, 237–265. [Google Scholar] [CrossRef]
- Giles, R.C.; Donahue, J.M.; Hong, C.B.; Tuttle, P.A.; Petrites-Murphy, M.B.; Poonacha, K.B.; Roberts, A.W.; Tramontin, R.R.; Smith, B.; Swerczek, T.W. Causes of abortion, stillbirth, and perinatal death in horses: 3527 cases (1986–1991). J. Am. Vet. Med. Assoc. 1993, 203, 1170–1175. [Google Scholar] [PubMed]
- Laugier, C.; Foucher, N.; Sevin, C.; Leon, A.; Tapprest, J. A 24-year retrospective study of equine abortion in Normandy (France). J. Equine Vet. Sci. 2011, 31, 116–123. [Google Scholar] [CrossRef]
- Ellero, N.; Lanci, A.; Ferlizza, E.; Andreani, G.; Mariella, J.; Isani, G.; Castagnetti, C. Activities of matrix metalloproteinase-2 and-9 in amniotic fluid at parturition in mares with normal and high-risk pregnancy. Theriogenology 2021, 172, 116–122. [Google Scholar] [CrossRef]
- Chouthai, N.S.; Sampers, J.; Desai, N.; Smith, G.M. Changes in neurotrophin levels in umbilical cord blood from infants with different gestational ages and clinical conditions. Pediatr. Res. 2003, 53, 965–969. [Google Scholar] [CrossRef]
- Ruiz de Almodovar, C.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 2009, 89, 607–648. [Google Scholar] [CrossRef]
- Silverman, W.F.; Krum, J.M.; Mani, N.; Rosenstein, J.M. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience 1999, 90, 1529–1541. [Google Scholar] [CrossRef]
- Mani, N.; Khaibullina, A.; Krum, J.M.; Rosenstein, J.M. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: Receptor mediation and signal transduction pathways. Exp. Neurol. 2005, 192, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Ellero, N.; Lanci, A.; Baldassarro, V.A.; Alastra, G.; Mariella, J.; Cescatti, M.; Giardino, L.; Castagnetti, C. Study on NGF and VEGF during the Equine Perinatal Period—Part 1: Healthy Foals Born from Normal Pregnancy and Parturition. Vet. Sci. 2022, 9, 451. [Google Scholar] [CrossRef]
- Toribio, R.E. Endocrine dysregulation in critically ill foals and horses. Vet. Clin. Equine 2011, 27, 35–47. [Google Scholar] [CrossRef]
- Himler, M.; Cattaneo, A.; Barsnick, R.J.I.M.; Hurcombe, S.D.A.; Rathgeber, R.; Toribio, R.E. Thyroid hormones in healthy, sick non-septic and septic foals. J. Vet. Intern. Med. 2010, 24, 784. [Google Scholar]
- Breuhaus, B.A.; LaFevers, D.H. Thyroid function in normal, sick and premature Foals. J. Vet. Intern. Med. 2005, 19, 445. [Google Scholar]
- Frazer, G.S.; Perkins, N.R.; Embertson, R.M. Normal parturition and evaluation of the mare in dystocia. Equine Vet. Educ. 1999, 11, 41–46. [Google Scholar] [CrossRef]
- Lanci, A.; Perina, F.; Donadoni, A.; Castagnetti, C.; Mariella, J. Dystocia in the Standardbred mare: A retrospective study from 2004 to 2020. Animals 2022, 12, 1486. [Google Scholar] [CrossRef]
- Koterba, A.M. Management of the intensive care unit: Levels of care, quality control and care after discharge. In Equine Clinical Neonatology; Lea and Febiger: Philadelphia, PA, USA, 1990; pp. 769–778. [Google Scholar]
- Knottenbelt, D.C.; Holdstock, N.; Madigan, J.E. Equine Neonatology Medicine and Surgery; Saunders: Edinburgh, UK, 2004; pp. 155–363. [Google Scholar]
- Vaala, W.E.; House, J.K.; Madigan, J.E. Initial management and physical examination of the neonate. In Large Animal Internal Medicine; Mosby St. Louis: Kirkwood, MO, USA, 2002; pp. 277–293. [Google Scholar]
- Lopez-Rodriguez, M.F.; Cymbaluk, N.F.; Epp, T.; Laarveld, B.; Thrasher, M.; Card, C.E. A field study of serum, colostrum, milk iodine, and thyroid hormone concentrations in postpartum draft mares and foals. J. Equine Vet. Sci. 2020, 90, 103018. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, M.F.; Cymbaluk, N.; Epp, T.; Laarveld, B.; Recalde, E.C.S.; Simko, E.; Card, C. Effects of the glucosinolate sinigrin in combination with a noniodine supplemented diet on serum iodine and thyroid hormone concentrations in nonpregnant mares. J. Equine Vet. Sci. 2020, 91, 103110. [Google Scholar] [CrossRef]
- Harvey, J.W. Normal hematological values. In Equine Clinical Neonatology; Koterba, A.M., Drummond, W.H., Kosch, P.C., Eds.; Lea and Febiger: Philadelphia, PA, USA, 1990; pp. 561–570. [Google Scholar]
- Bauer, J.E.; Harvey, J.W.; Asquith, R.L.; McNulty, P.K.; Kivipelto, J.A.N. Clinical chemistry reference values of foals during the first year of life. Equine Vet. J. 1984, 16, 361–363. [Google Scholar] [CrossRef]
- Stoneham, S.J.; Palmer, L.; Cash, R.; Rossdale, P.D. Measurement of serum amyloid A in the neonatal foal using a latex agglutination immunoturbidimetric assay: Determination of the normal range, variation with age and response to disease. Equine Vet. J. 2001, 33, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, A.; Mariella, J.; Gentilini, F.; Castagnetti, C. Amniotic fluid and blood lactate concentrations in mares and foals in the early postpartum period. Theriogenology 2012, 78, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Tejero, E.; Estepa, J.C.; Lopez, I.; Mayer-Valor, R.; Rodriguez, M. Arterial blood gases and acid-base balance in healthy young and aged horses. Equine Vet. J. 1998, 30, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Strickland, T.; Molloy, E.J. Neonatal encephalopathy: Need for recognition of multiple etiologies for optimal management. Front. Pediatr. 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, L.W.; Rossdale, P.D. Convulsive and allied syndromes in newborn foals. Vet. Rec. 1957, 69, 1277–1289. [Google Scholar]
- Madigan, J.E.; Haggett, E.F.; Pickles, K.J.; Conley, A.; Stanley, S.; Moeller, B.; Toth, B.; Aleman, M. Allopregnanolone infusion induced neurobehavioural alterations in a neonatal foal: Is this a clue to the pathogenesis of neonatal maladjustment syndrome? Equine Vet. J. 2012, 44, 109–112. [Google Scholar] [CrossRef]
- Ferriero, D.M. Neonatal brain injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef]
- Axon, J.E.; Palmer, J.E. Clinical pathology of the foal. Vet. Clin. N. Am. Equine Prac. 2008, 24, 357–385. [Google Scholar] [CrossRef]
- Pirrone, A.; Antonelli, C.; Mariella, J.; Castagnetti, C. Gross placental morphology and foal serum biochemistry as predictors of foal health. Theriogenology 2014, 81, 1293–1299. [Google Scholar] [CrossRef]
- Chiba, A.; Aoki, T.; Itoh, M.; Yamagishi, N.; Shibano, K. Hematological and blood biochemical characteristics of newborn heavy draft foals after dystocia. J. Equine Vet. Sci. 2017, 50, 69–75. [Google Scholar] [CrossRef]
- Himler, M.; Hurcombe, S.D.A.; Griffin, A.; Barsnick, R.J.; Rathgeber, R.A.; MacGillivray, K.C.; Toribio, R.E. Presumptive nonthyroidal illness syndrome in critically ill foals. Equine Vet. J. 2012, 44, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Economidou, F.; Douka, E.; Tzanela, M.; Nanas, S.; Kotanidou, A. Thyroid function during critical illness. Hormones 2011, 10, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Malamitsi-Puchner, A.; Nikolaou, K.E.; Economou, E.; Boutsikou, M.; Boutsikou, T.; Kyriakakou, M.; Puchner, K.P.; Hassiakos, D. Intrauterine growth restriction and circulating neurotrophin levels at term. Early Hum. Dev. 2007, 83, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Kilari, A.; Mehendale, S.; Pisal, H.; Panchanadikar, T.; Kale, A.; Joshi, S. Nerve growth factor, birth outcome and pre-eclampsia. Int. J. Dev. Neurosci. 2011, 29, 71–75. [Google Scholar] [CrossRef]
- Dhobale, M.; Mehendale, S.; Pisal, H.; Nimbargi, V.; Joshi, S. Reduced maternal and cord nerve growth factor levels in preterm deliveries. Int. J. Dev. Neurosci. 2012, 30, 99–103. [Google Scholar] [CrossRef]
- Chiaretti, A.; Antonelli, A.; Riccardi, R.; Genovese, O.; Pezzotti, P.; Di Rocco, C.; Tortorolo, L.; Piedimonte, G. Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur. J. Paediatr. Neurol. 2008, 12, 195–204. [Google Scholar] [CrossRef]
- Castagnetti, C.; Pirrone, A.; Mariella, J.; Mari, G. Venous blood lactate evaluation in equine neonatal intensive care. Theriogenology 2010, 73, 343–357. [Google Scholar] [CrossRef]
- Bogetti, M.E.; Devoto, V.M.P.; Rapacioli, M.; Flores, V.; de Plazas, S.F. NGF, TrkA-P and neuroprotection after a hypoxic event in the developing central nervous system. Int. J. Dev. Neurosci. 2018, 71, 111–121. [Google Scholar] [CrossRef]
- Chiaretti, A.; Genovese, O.; Riccardi, R.; Di Rocco, C.; Di Giuda, D.; Mariotti, P.; Pulitan, S.; Piastra, M.; Polidori, G.; Colofati, G.S.; et al. Intraventricular nerve growth factor infusion: A possible treatment for neurological deficits following hypoxic–ischemic brain injury in infants. Neurol. Res. 2005, 27, 741–746. [Google Scholar] [CrossRef]
- Chiaretti, A.; Antonelli, A.; Genovese, O.; Fernandez, E.; Giuda, D.; Mariotti, P.; Riccardi, R. Intraventricular nerve growth factor infusion improves cerebral blood flow and stimulates doublecortin expression in two infants with hypoxic–ischemic brain injury. Neurol. Res. 2008, 30, 223–228. [Google Scholar] [CrossRef]
- Walker, P.; Weil, N.L.; Weichsel, M.E., Jr.; Fischer, D.A. Effect of thyroxine on nerve growth factor concentration in neonatal mouse brain. Life Sci. 1981, 28, 1777–1787. [Google Scholar] [CrossRef]
- Figueiredo, B.C.; Almazan, G.; Ma, Y.; Tetzlaff, W.; Miller, F.D.; Cuello, A.C. Gene expression in the developing cerebellum during perinatal hypo-and hyperthyroidism. Mol. Brain Res. 1993, 17, 258–268. [Google Scholar] [CrossRef]
- Calzà, L.; Giardino, L.; Aloe, L. Thyroid hormone regulates NGF content and p75LNGFR expression in the basal forebrain of adult rats. Exp. Neurol. 1997, 143, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Calzà, L.; Giardino, L.; Aloe, L. NGF content and expression in the rat pituitary gland and regulation by thyroid hormone. Mol. Brain Res. 1997, 51, 60–68. [Google Scholar] [CrossRef]
- Walker, P.; Weichsel, M.E., Jr.; Fisher, D.A.; Guo, S.M.; Fisher, D.A. Thyroxine increases nerve growth factor concentration in adult mouse brain. Science 1979, 204, 427–429. [Google Scholar] [CrossRef]
- Oh, J.D.; Butcher, L.L.; Woolf, N.J. Thyroid hormone modulates the development of cholinergic terminal fields in the rat forebrain: Relation to nerve growth factor receptor. Dev. Brain Res. 1991, 59, 133–142. [Google Scholar] [CrossRef]
- Giordano, T.; Pan, J.B.; Casuto, D.; Watanabe, S.; Arneric, S.P. Thyroid hormone regulation of NGF, NT-3 and BDNF RNA in the adult rat brain. Mol. Brain Res. 1992, 16, 239–245. [Google Scholar] [CrossRef]
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181. [Google Scholar] [CrossRef]
- Nico, B.; Mangieri, D.; Benagiano, V.; Crivellato, E.; Ribatti, D. Nerve growth factor as an angiogenic factor. Microvasc. Res. 2008, 75, 135–141. [Google Scholar] [CrossRef]
- Allen, W.R.; Gower, S.; Wilsher, S. Immunohistochemical localization of vascular endothelial growth factor (VEGF) and its two receptors (Flt-I and KDR) in the endometrium and placenta of the mare during the oestrous cycle and pregnancy. Reprod. Domest. Anim. 2007, 42, 516–526. [Google Scholar] [CrossRef]
- Lanci, A.; Ingrà, L.; Dondi, F.; Tomasello, F.; Teti, G.; Mariella, J.; Falconi, M.; Castagnetti, C. Morphological study of equine amniotic compartment. Theriogenology 2022, 177, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Kawamura, N.; Fukuda, J.; Kumagai, J.; Hsueh, A.J.; Tanaka, T. Regulation of preimplantation embryo development by brain-derived neurotrophic factor. Dev. Biol. 2007, 311, 147–158. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.; Ceccanti, M.; Petrella, C.; Greco, A.; Tirassa, P.; Rosso, P.; Ralli, M.; Ferraguti, G.; Fiore, M.; Messina, M.P. Role of neurotrophins in pregnancy, delivery and postpartum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Tatsumi, K.; Kondoh, E.; Chigusa, Y.; Mogami, H.; Fujii, T.; Yura, S.; Kakui, K.; Konishi, I. Differential expression and the anti-apoptotic effect of human placental neurotrophins and their receptors. Placenta 2011, 32, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, P.; Barrientos, G.L.; Tirado González, I.; Cohen, M.; Moschansky, P.; Peters, E.M.; Klapp, B.F.; Rose, M.; Tometten, M.; Blois, S.M. Balanced levels of nerve growth factor are required for normal pregnancy progression. Reproduction 2014, 148, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cassens, C.; Kleene, R.; Xiao, M.F.; Friedrich, C.; Dityateva, G.; Schafer-Nielsen, C.; Schachner, M. Binding of the receptor tyrosine kinase TrkB to the neural cell adhesion molecule (NCAM) regulates phosphorylation of NCAM and NCAM-dependent neurite outgrowth. J. Biol. Chem. 2010, 285, 28959–28967. [Google Scholar] [CrossRef]
- Nakamura, K.; Martin, K.C.; Jackson, J.K.; Beppu, K.; Woo, C.W.; Thiele, C.J. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006, 66, 4249–4255. [Google Scholar] [CrossRef]
- Wang, L.H.; Paden, A.J.; Johnson, E.M. Mixed-lineage kinase inhibitors require the activation of trk receptors to maintain long-term neuronal trophism and survival. J. Pharmacol. Exp. Ther. 2005, 312, 1007–1019. [Google Scholar] [CrossRef]
- Tervonen, T.A.; Ajamian, F.; De Wit, J.; Verhaagen, J.; Castrén, E.; Castrén, M. Overexpression of a truncated TrkB isoform increases the proliferation of neural progenitors. Eur. J. Neurosci. 2006, 24, 1277–1285. [Google Scholar] [CrossRef]

| Genes | Primer Sequences (5′ -> 3′) |
|---|---|
| NGF Nerve Growth Factor | Forward: GGGCCCATTAACGGCTTTTC Reverse: CATTGCTCTCTGTGTGGGGT |
| P75NTR p75 Neurotrophin Receptor | Forward: GAGCCAACCAGACTGTGTGT Reverse: GGTAGTAGCCATAGGCGCAG |
| TRKA Tropomyosin Receptor Kinase A | Forward: GGAGCTGAGAAACCTCACCAT Reverse: GCACAGGAACAGTCCAGAGG |
| VEGF Vascular Endothlial Growth Factor | Forward: AACGACGAGGGCCTAGAGT Reverse: CAAGGCCCACAGGGATTTTCT |
| KDR Kinase Insert Domain Receptor | Forward: GATGACAACCAGACGGACAGT Reverse: TTTTGCTGGGCATCAGTCCA |
| FLT1 Fms Related Receptor Tyrosine Kinase 1 | Forward: CTGGCATCCCTGTAACCACA Reverse: AGGGTGCTAGCCGTCTTATTC |
| BDNF Brain Derived Neurotrophic Factor | Forward: CATGTCTATGAGGGTCCGGC Reverse: CATGTCCACTGCCGTCTTCT |
| TRKB Tropomyosin Receptor Kinase B | Forward: CGGGAACACCTCTCGGTCTA Reverse: CTGGACCAACACCTTGTCTTGA |
| ACTB Beta Actin | Forward: TCCCCCCTGGAGAAGAGCTACGAG Reverse: TTTCGTGGATGCCACAGGAT |
| Breed | Age (Years) | Parity | US Findings | Cervical Swab | Prepartum Treatment (Y/N) | Gest. Length (Days) | Dystocia (Y/N) | Stage II Labor Length (min) | Placenta/Foal Weight Ratio (%) | Macroscopic Placenta Evaluation | Histopathologic Placenta Evaluation | Mare’s Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SB (n = 10) QH (n = 1) WB (n = 1) SD (n = 1) | 10 ± 3.7 (4–16) | 2 ± 1.9 (1–7) | Normal (n = 9) Increased CTUP (n = 4) | Neg (n = 4) NA (n = 9) | Y (n = 4) N (n = 9) | 337 ± 10.3 (326–359) | Y (n = 5) N (n = 8) | 19 ± 13.5 (7–55) | 11.1 ± 2.4 (8.2–16.7) | Villous hypoplasia (n = 4) Extensive edema (n = 3) Meconium-stained amnion (n = 1) Normal (n = 5) | Chorionic epithelium hypoplasia (n = 5) Severe edema (n = 3) Normal (n = 5) | Placental insufficiency (n = 8) Dystocia (n = 4) Apparently normal (n = 1) |
| Breed | Sex | Weight (kg) | Apgar Score (0–10) | Clinical Signs of NE | Organ Dysfunctions Associated with NE | Care Level (1–3) | Hosp. Length (Days) | Outcome |
|---|---|---|---|---|---|---|---|---|
| SB (n = 10) QH (n = 1) WB (n = 1) SD (n = 1) | Males (n = 10) Females (n = 3) | 45 ± 9.7 (17–55) | 7 ± 2.4 (1–10) | Depression (n = 7); severe and prolonged dysphagia (n = 7); hypoventilation (n = 5); lateral recumbency (n = 4); lack of affinity with the mare (n = 4), lack of suckle reflex (n = 4); hypothermia (n = 2); weakness (n = 2); disorientation (n = 1); tongue protrusion (n = 1) | Cardiovascular (n = 1) Respiratory (n = 2) Gastrointestinal (n = 5) Renal (n = 2) Metabolic (n = 2) None (n = 6) | Level 1 (n = 7) Level 2 (n = 4) Level 3 (n = 2) | 12 ± 8.6 (4–30) | Sv (n = 12) NSv (n = 1) |
| Breed | Sex | Age at Adm. (Hours) | Weight (kg) | Mare’s History | Clinical Signs of NE | Organ Dysfunctions Associated with NE | Care Level (1–3) | Hosp. Length (Days) | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) | Parity | Gest Length (Days) | Other | |||||||||
| SB (n = 10) QH (n = 6) SD (n = 3) AH (n = 1) | Males (n = 12) Females (n = 8) | 10 ± 6.4 (1–24) | 42 ± 8 (17–56) | 12 ± 5.2 (4–21) | 3 ± 1.7 (1–6) | 334 ± 12.5 (320–357) | Dystocia (n = 9) NA (n = 9) Placental insuff. (n = 2) | Depression (n = 13); lack of suckle reflex (n = 12); hypoventilation (n = 11); lack of affinity with the mare (n = 10); lateral recumbency (n = 6); tongue protrusion (n = 6); coma (n = 4); severe and prolonged dysphagia (n = 4); hyperexcitability (n = 4); head pressing (n = 3); convulsions (n = 2); barking (n = 2); chewing movements (n = 2); wandering (n = 2); hypothermia (n = 1); weakness (n = 1); facial hemiplegia (n = 1); sialorrhea (n = 1); tremors (n = 1); anisocoria (n = 1) | Cardiovascular (n = 9) Respiratory (n = 11) Gastrointestinal (n = 8) Renal (n = 9) Metabolic (n = 4) None (n = 5) | Level 1 (n = 2) Level 2 (n = 10) Level 3 (n = 8) | 11 ± 5 (4–20) | Sv (n = 15) NSv (n = 5) |
| a. NE | T0 | T24 | T72 |
|---|---|---|---|
| NGF (ng/mL) | 114.9 ± 47.9 (54.6–204.8) (n = 13) | 93.0 ± 37.7 *1 (39.8–181.2) (n = 12) | 97.5 ± 39.8 (46.5–177.4) (n = 12) |
| VEGF (pg/mL) | 199.3 ± 34.9 (150.2–258.4) (n = 13) | 188.6 ± 54.8 (92.1–261.8) (n = 12) | 179.8 ± 43.7 *2 (95.9–231.1) (n = 12) |
| TT3 (ng/dL) | 850.0 ± 826.4 (140.0–2172.0) (n = 12) | 867.5 ± 830.9 (162.0–2324.0) (n = 11) | 638.7 ± 618.8 (78.6–2008.0) (n = 11) |
| TT4 (nmol/L) | 511.4 ± 220.0 (220.0–860.0) (n = 12) | 386.0 ± 250.8 (123.0–776.0) (n = 11) | 227.1 ± 98.5 ***3 (66.0–440.0) (n = 11) |
| b. NE | Amniotic fluid | Umbilical cord vein | Mare’s jugular vein (TP) |
| NGF (ng/mL) | 139.4 ± 40.2 (61.2–201.9) (n = 10) | 127.5 ± 57.0 (65.5–214.5) (n = 9) | 102.2 ± 74.4 (43.5–193.4) (n = 5) |
| VEGF (pg/mL) | 264.0 ± 48.4 (138.9–328.4) (n = 10) | 218.1 ± 33.4 (151.5–261.8) (n = 8) | 132.8 ± 26.5 (106.0–161.6) (n = 5) |
| TT3 (ng/dL) | NA | 350.3 ± 145.0 (110.0–540.0) (n = 10) | 69.3 ± 34.1 (40.0–118.0) (n = 4) |
| TT4 (nmol/L) | NA | 501.4 ± 220.2 (221.0–800.0) (n = 10) | 18.7 ± 11.5 (12.9–36.0) (n = 4) |
| c. exNE | T0 | T24 | T72 |
| NGF (ng/mL) | 82.6 ± 36.8 (56.8–176.9) (n = 13) | 57.6 ± 14.6 *4 (34.6–77.7) (n = 12) | 59.0 ± 14.0 (42.8–84.7) (n = 9) |
| VEGF (pg/mL) | 174.6 ± 15.0 (165.4–197.0) (n = 4) | 150.6 ± 48.3 (88.3–204.6) (n = 4) | 169.2 ± 56.4 (89.6–222.2) (n = 4) |
| TT3 (ng/dL) | 368.1 ± 470.0 (60.3–1856.0) (n = 13) | 279.8 ± 151.3 (99.7–552.0) (n = 13) | 279.0 ± 111.4 (157.0–505.0) (n = 10) |
| TT4 (nmol/L) | 302.5 ± 136.7 (57.8–500.0) (n = 13) | 196.1 ± 75.2 **5 (56.1–292.0) (n = 13) | 142.0 ± 65.8 **6 (46.6–282.0) (n = 10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellero, N.; Lanci, A.; Baldassarro, V.A.; Alastra, G.; Mariella, J.; Cescatti, M.; Castagnetti, C.; Giardino, L. Study on NGF and VEGF during the Equine Perinatal Period—Part 2: Foals Affected by Neonatal Encephalopathy. Vet. Sci. 2022, 9, 459. https://doi.org/10.3390/vetsci9090459
Ellero N, Lanci A, Baldassarro VA, Alastra G, Mariella J, Cescatti M, Castagnetti C, Giardino L. Study on NGF and VEGF during the Equine Perinatal Period—Part 2: Foals Affected by Neonatal Encephalopathy. Veterinary Sciences. 2022; 9(9):459. https://doi.org/10.3390/vetsci9090459
Chicago/Turabian StyleEllero, Nicola, Aliai Lanci, Vito Antonio Baldassarro, Giuseppe Alastra, Jole Mariella, Maura Cescatti, Carolina Castagnetti, and Luciana Giardino. 2022. "Study on NGF and VEGF during the Equine Perinatal Period—Part 2: Foals Affected by Neonatal Encephalopathy" Veterinary Sciences 9, no. 9: 459. https://doi.org/10.3390/vetsci9090459
APA StyleEllero, N., Lanci, A., Baldassarro, V. A., Alastra, G., Mariella, J., Cescatti, M., Castagnetti, C., & Giardino, L. (2022). Study on NGF and VEGF during the Equine Perinatal Period—Part 2: Foals Affected by Neonatal Encephalopathy. Veterinary Sciences, 9(9), 459. https://doi.org/10.3390/vetsci9090459








