Development and Evaluation of NanoPCR for the Detection of Goose Parvovirus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Clinical Samples
2.2. The Extraction of DNA/RNA from the Samples
2.3. Primer Design and Plasmid Construction
2.4. Optimization of the Reaction Conditions of nanoPCR
2.5. Sensitivity, Specificity, and Reproducibility Tests of nanoPCR
2.6. Detection of GPV in Clinical Samples by nanoPCR
3. Results
3.1. Optimization of nanoPCR
3.2. Sensitivity of nanoPCR
3.3. Specificity of nanoPCR
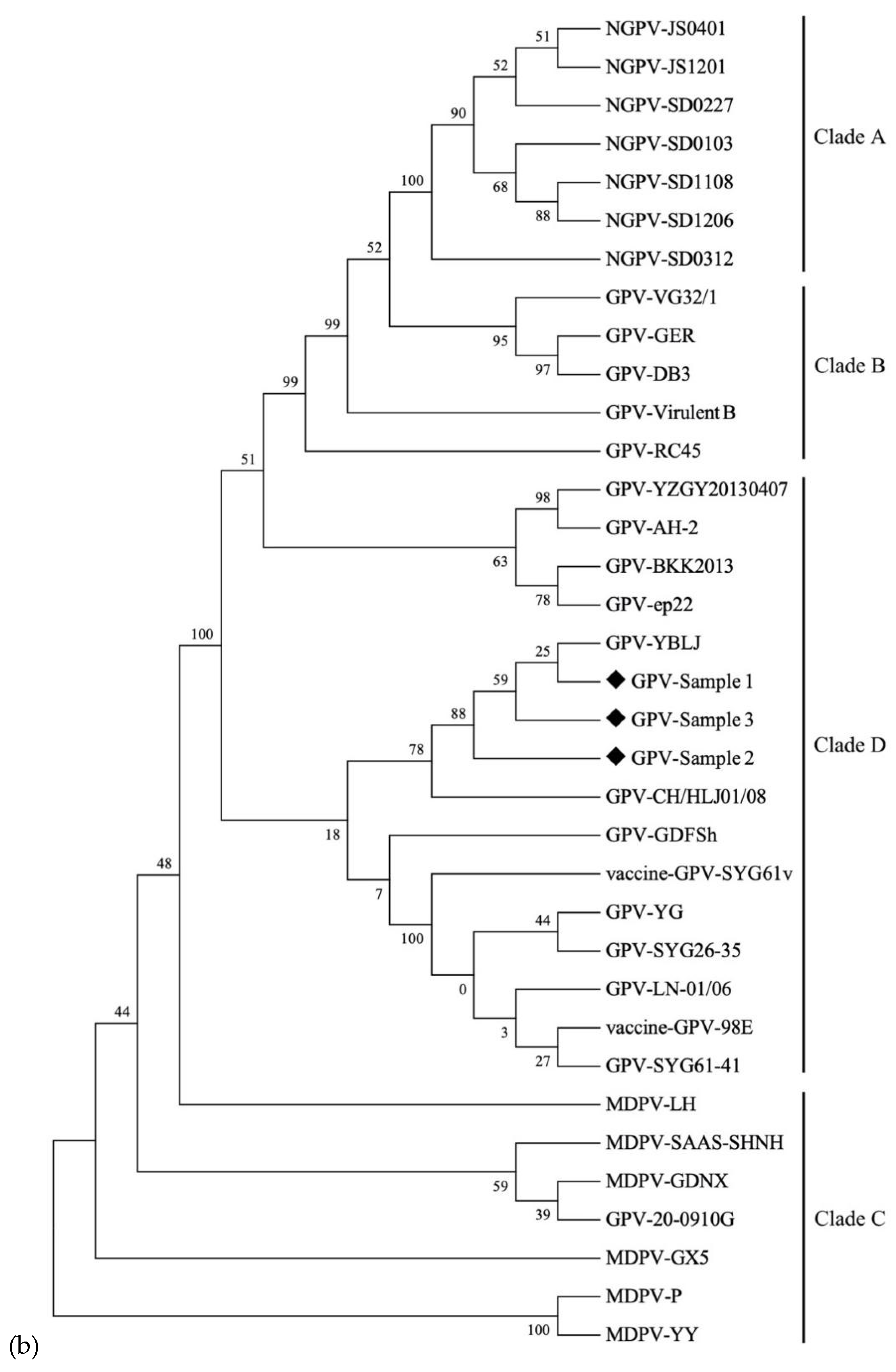
3.4. GPV Detection in Clinical Samples and Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, D. Recommendation of GPV. Vet. Sci. China 1962, 8, 19–20. [Google Scholar]
- Brown, K.E.; Green, S.W.; Young, N.S. Goose parvovirus—An autonomous member of the dependovirus genus? Virology 1995, 210, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, F.; Chen, S.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Sun, K.; Yang, Q.; Wu, Y.; et al. Identification of 2′-5′-Oligoadenylate Synthetase-Like Gene in Goose: Gene Structure, Expression Patterns, and Antiviral Activity Against Newcastle Disease Virus. J. Interf. Cytok. Res. 2016, 36, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.M. Establishment and Application of Fluorescence Quantitative PCR for Detecting Goose Parvovirus and Comparing and Analyzing of the VP3 Sequence of Goose Parvovirus. Master’s thesis, Shandong Agricultural University, Shandong, China, 2007. [Google Scholar]
- Jansson, D.S.; Feinstein, R.; Kardi, V.; Mató, T.; Palya, V. Epidemiologic investigation of an outbreak of goose parvovirus infection in Sweden. Avian Dis. 2007, 51, 609–613. [Google Scholar] [CrossRef]
- Kozdruń, W.; Woźniakowski, G.; Samorek-Salamonowicz, E.; Czekaj, H. Viral infections in goose flocks in Poland. Pol. J. Vet. Sci. 2012, 15, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Y.; Dou, Y.; Zheng, X.; Diao, Y. Evidence for Vertical Transmission of Novel Duck-Origin Goose Parvovirus-Related Parvovirus. Transbound. Emerg. Dis. 2016, 63, 243–247. [Google Scholar] [CrossRef]
- Christensen, J.; Tattersall, P. Parvovirus initiator protein NS1 and RPA coordinate replication fork progression in a reconstituted DNA replication system. J. Virol. 2002, 76, 6518–6531. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Christensen, J.; Nüesch, J.P.; Tattersall, P. The NS1 polypeptide of the murine parvovirus minute virus of mice binds to DNA sequences containing the motif [ACCA]2-3. J. Virol. 1995, 69, 1652–1660. [Google Scholar] [CrossRef]
- Chipman, P.R.; Agbandje-McKenna, M.; Kajigaya, S.; Brown, K.E.; Young, N.S.; Baker, T.S.; Rossmann, M.G. Cryo-electron microscopy studies of empty capsids of human parvovirus B19 complexed with its cellular receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 7502–7506. [Google Scholar] [CrossRef]
- Summerford, C.; Samulski, R.J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998, 72, 1438–1445. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, M.; Zhang, D.; Guo, D.; Liu, C.; Zhi, H.; Wang, X.; Li, G.; Li, N.; et al. Development and evaluation of a VP3-ELISA for the detection of goose and Muscovy duck parvovirus antibodies. J. Virol. Methods 2010, 163, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Zádori, Z.; Stefancsik, R.; Rauch, T.; Kisary, J. Analysis of the complete nucleotide sequences of goose and muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2. Virology 1995, 212, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Li, B.C.; Qi, Y.; Ma, B.; Wang, J.W. Comparing and analyzing of the VP3 gene sequence of different goose parvovirus strains. J. Prev. Vet. Med. China 2003, 6, 31–34. [Google Scholar]
- Ali, Z.; Jin, G.; Hu, Z.; Wang, Z.; Khan, M.A.; Dai, J.; Tang, Y. A Review on NanoPCR: History, Mechanism and Applications. J. Nanosci. Nanotechnol. 2018, 18, 8029–8046. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pan, D.; Fan, C.; Chen, J.; Huang, K.; Wang, D.; Zhang, H.; Li, Y.; Feng, G.; Liang, P.; et al. Gold nanoparticles for high-throughput genotyping of long-range haplotypes. Nat. Nanotechnol. 2011, 6, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Powell, A.C.; Paciotti, G.F.; Libutti, S.K. Colloidal gold: A novel nanoparticle for targeted cancer therapeutics. Methods Mol. Biol. 2010, 624, 375–384. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, Y. Mechanism studies on nanoPCR and applications of gold nanoparticles in genetic analysis. ACS Appl. Mater. Interfaces 2013, 5, 6276–6284. [Google Scholar] [CrossRef]
- Pan, D.; Mi, L.; Huang, Q.; Hu, J.; Fan, C. Genetic analysis with nanoPCR. Integr. Biol. 2012, 4, 1155–1163. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Z.; Ma, X.; Liu, J.; Cui, S. A sensitive and specific nanoparticle-assisted PCR assay for rapid detection of porcine parvovirus. Lett. Appl. Microbiol. 2014, 58, 163–167. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, Z.; Li, J.; Li, Z.; Wang, C.; Wang, J.; Guo, L. Development of a nanoparticle-assisted PCR assay for detection of bovine respiratory syncytial virus. BMC Vet. Res. 2019, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.; Liu, R.; Wei, W.; Ding, C. Development of a sensitive and specific nanoparticle-assisted PCR assay for detecting HPV-16 and HPV-18 DNA. J. Med. Virol. 2020, 92, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.H.; Zuo, Y.Z.; Yang, Z.; Pei, L.H. The development of an indirect ELISA for the detection of antibodies to goose parvovirus in blood serum. Lett. Appl. Microbiol. 2013, 57, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, C.; Li, G.; Yu, H.; Jiang, Y.; Yan, L.; Meng, C.; Zhou, Y.; Tong, G.; Liu, G. Rapid diagnosis of goose viral infections by multiplex PCR. J. Virol. Methods 2013, 191, 101–104. [Google Scholar] [CrossRef]
- Yang, J.L.; Cheng, A.C.; Wang, M.S.; Pan, K.C.; Li, M.; Guo, Y.F.; Li, C.F.; Zhu, D.K.; Chen, X.Y. Development of a fluorescent quantitative real-time polymerase chain reaction assay for the detection of Goose parvovirus in vivo. Virol. J. 2009, 6, 142. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, Z.X.; Gao, X.; Li, Z.X.; Wang, M.Q.; Liu, J.Y.; Lu, C.; Liang, W.F.; Yu, L.Z.; Wang, Y. Establishment and clinical application of TaqMan fluorescent quantitative PCR assay for detection of goose parvovirus. Chin. J. Vet. Sci. 2018, 38, 1100–1104. [Google Scholar] [CrossRef]
- Ting, C.H.; Lin, C.Y.; Huang, Y.C.; Liu, S.S.; Peng, S.Y.; Wang, C.W.; Wu, H.Y. Correlation between goose circovirus and goose parvovirus with gosling feather loss disease and goose broke feather disease in southern Taiwan. J. Vet. Sci. 2021, 22, e1. [Google Scholar] [CrossRef] [PubMed]
- Kardoğan, Ö.; Müştak, H.K.; Müştak, İ.B. The first detection and characterization of goose parvovirus (GPV) in Turkey. Trop. Anim. Health Prod. 2020, 53, 36. [Google Scholar] [CrossRef]
- Sirivan, P.; Obayashi, M.; Nakamura, M.; Tantaswasdi, U.; Takehara, K. Detection of goose and Muscovy duck parvoviruses using polymerase chain reaction-restriction enzyme fragment length polymorphism analysis. Avian Dis. 1998, 42, 133–139. [Google Scholar] [CrossRef]
- Takehara, K.; Nishio, T.; Hayashi, Y.; Kanda, J.; Sasaki, M.; Abe, N.; Hiraizumi, M.; Saito, S.; Yamada, T.; Haritani, M.; et al. An outbreak of goose parvovirus infection in Japan. J. Vet. Med. Sci. 1995, 57, 777–779. [Google Scholar] [CrossRef]
- Ning, K.; Wang, M.; Qu, S.; Lv, J.; Yang, L.; Zhang, D. Pathogenicity of Pekin duck- and goose-origin parvoviruses in Pekin ducklings. Vet. Microbiol. 2017, 210, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.J.; Bu, R.E.; Han, X.J.; Ma, B.; Wang, J.W. Preparation and application of immunosera against GPV. Chin. Vet. Sci. Technol. 2004, 12, 65–68. [Google Scholar] [CrossRef]
- Wan, C.; Liu, R.; Chen, C.; Cheng, L.; Shi, S.; Fu, G.; Chen, H.; Fu, Q.; Huang, Y. Novel goose parvovirus in domestic Linwu sheldrakes with short beak and dwarfism syndrome, China. Transbound. Emerg. Dis. 2019, 66, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Chen, S.; Cheng, X.; Lin, F.; Wang, S.; Zhu, X.; Yu, B.; Huang, M.; Wang, J.; Wu, N.; et al. The newly emerging duck-origin goose parvovirus in China exhibits a wide range of pathogenicity to main domesticated waterfowl. Vet. Microbiol. 2017, 203, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Kisary, J. Cross-neutralization tests on parvoviruses isolated from goslings. Avian Pathol. 1974, 3, 293–296. [Google Scholar] [CrossRef]
- She, Y.J.; Hu, S.J.; Huang, W.; Zhou, X.F.; Zhou, G.R. Detection of Goose Fever Virus Antigen and Antibody by Agar Immunodiffusion Test. Anim. Hus. Vet. Med. China 1987, 5, 202–204. [Google Scholar]
- Lin, S.; Wang, S.; Cheng, X.; Xiao, S.; Chen, X.; Chen, S.; Chen, S.; Yu, F. Development of a duplex SYBR Green I-based quantitative real-time PCR assay for the rapid differentiation of goose and Muscovy duck parvoviruses. Virol. J. 2019, 16, 6. [Google Scholar] [CrossRef]
- Wan, C.; Chen, C.; Cheng, L.; Liu, R.; Shi, S.; Fu, G.; Chen, H.; Fu, Q.; Huang, Y. Specific detection and differentiation of classic goose parvovirus and novel goose parvovirus by TaqMan real-time PCR assay, coupled with host specificity. BMC Vet. Res. 2019, 15, 389. [Google Scholar] [CrossRef]
- Yang, J.; Yang, R.; Cheng, A.; Wang, M.; Fu, L.; Yang, S.; Zhang, S.; Yang, L.; Xu, Z. A simple and rapid method for detection of Goose Parvovirus in the field by loop-mediated isothermal amplification. Virol. J. 2010, 7, 14. [Google Scholar] [CrossRef]
- Huang, C.; Cheng, A.; Wang, M.; Liu, F.; Han, X.; Wang, G.; Zhou, W.; Wen, M.; Jia, R.; Guo, Y. Development and application of PCR to detect goose parvovirus. Vet. Sci. China 2004, 34, 54–60. [Google Scholar] [CrossRef]
- Limn, C.K.; Yamada, T.; Nakamura, M.; Takehara, K. Detection of Goose parvovirus genome by polymerase chain reaction: Distribution of Goose parvovirus in Muscovy ducklings. Virus Res. 1996, 42, 167–172. [Google Scholar] [CrossRef]
- Hu, Q.L.; Chen, S.Y.; Lin, T.L.; Cheng, Y.Q.; Li, Y.Y. Rapid Differnetiation of Muscovy Duck and Goose Parvoviruses by Polymerase Chain Reaction. Chin. J. Vet. Sci. 2001, 6, 47–50. [Google Scholar]
- Wang, J.; Cheng, Y.; Zhang, M.; Zhao, H.; Lin, P.; Yi, L.; Tong, M.; Cheng, S. Development of a nanoparticle-assisted PCR (nanoPCR) assay for detection of mink enteritis virus (MEV) and genetic characterization of the NS1 gene in four Chinese MEV strains. BMC Vet. Res. 2015, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Chen, Z.; Liu, G.; Jiang, S.; Li, C. A multiplex nanoparticle-assisted polymerase chain reaction assay for detecting three canine epidemic viruses using a dual priming oligonucleotide system. J. Virol. Methods 2021, 298, 114290. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, L.; Chen, H.; Niu, X.; Dou, Y.; Yang, J.; Wang, Z.; Tang, Y.; Diao, Y. Development of Colloidal Gold-Based Immunochromatographic Assay for Rapid Detection of Goose Parvovirus. Front. Microbiol. 2018, 9, 953. [Google Scholar] [CrossRef]
- Watson, D.E.; Li, B. TaqMan applications in genetic and molecular toxicology. Int. J. Toxicol. 2005, 24, 139–145. [Google Scholar] [CrossRef] [Green Version]







| Primers | Primer Sequences (5′–3′) | Product Size (bp) |
|---|---|---|
| GPV-VP3-F1 | CCTGGACCAGAGAGTTAGGGCCTAT | 386 |
| GPV-VP3-R1 | TCTGCCAAACCATTCCTGGTAAAGC | |
| GPV-VP3-F2 | CTCGAGATGGCAGAGGGAGGAG | 1605 |
| GPV-VP3-R2 | CGGTCGACTTACAGATTTTGAGTTAG | |
| GPV-VP3-F3 | CAACCATTGGGGAATCAGAC | 121 |
| GPV-VP3-R3 | TTGAATTGTTGACGTGAGATTGT | |
| GPV-P | FAM-TCTGATCCTGCGTTGTGACTTCTTTG-BHQ1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Gao, X.; Fu, J.; Xue, H.; Song, Y.; Zhu, K. Development and Evaluation of NanoPCR for the Detection of Goose Parvovirus. Vet. Sci. 2022, 9, 460. https://doi.org/10.3390/vetsci9090460
Ma H, Gao X, Fu J, Xue H, Song Y, Zhu K. Development and Evaluation of NanoPCR for the Detection of Goose Parvovirus. Veterinary Sciences. 2022; 9(9):460. https://doi.org/10.3390/vetsci9090460
Chicago/Turabian StyleMa, Haoyuan, Xu Gao, Jingfeng Fu, Haowen Xue, Yanhao Song, and Kunru Zhu. 2022. "Development and Evaluation of NanoPCR for the Detection of Goose Parvovirus" Veterinary Sciences 9, no. 9: 460. https://doi.org/10.3390/vetsci9090460
APA StyleMa, H., Gao, X., Fu, J., Xue, H., Song, Y., & Zhu, K. (2022). Development and Evaluation of NanoPCR for the Detection of Goose Parvovirus. Veterinary Sciences, 9(9), 460. https://doi.org/10.3390/vetsci9090460






