Predictive Models of Dairy Cow Thermal State: A Review from a Technological Perspective
Simple Summary
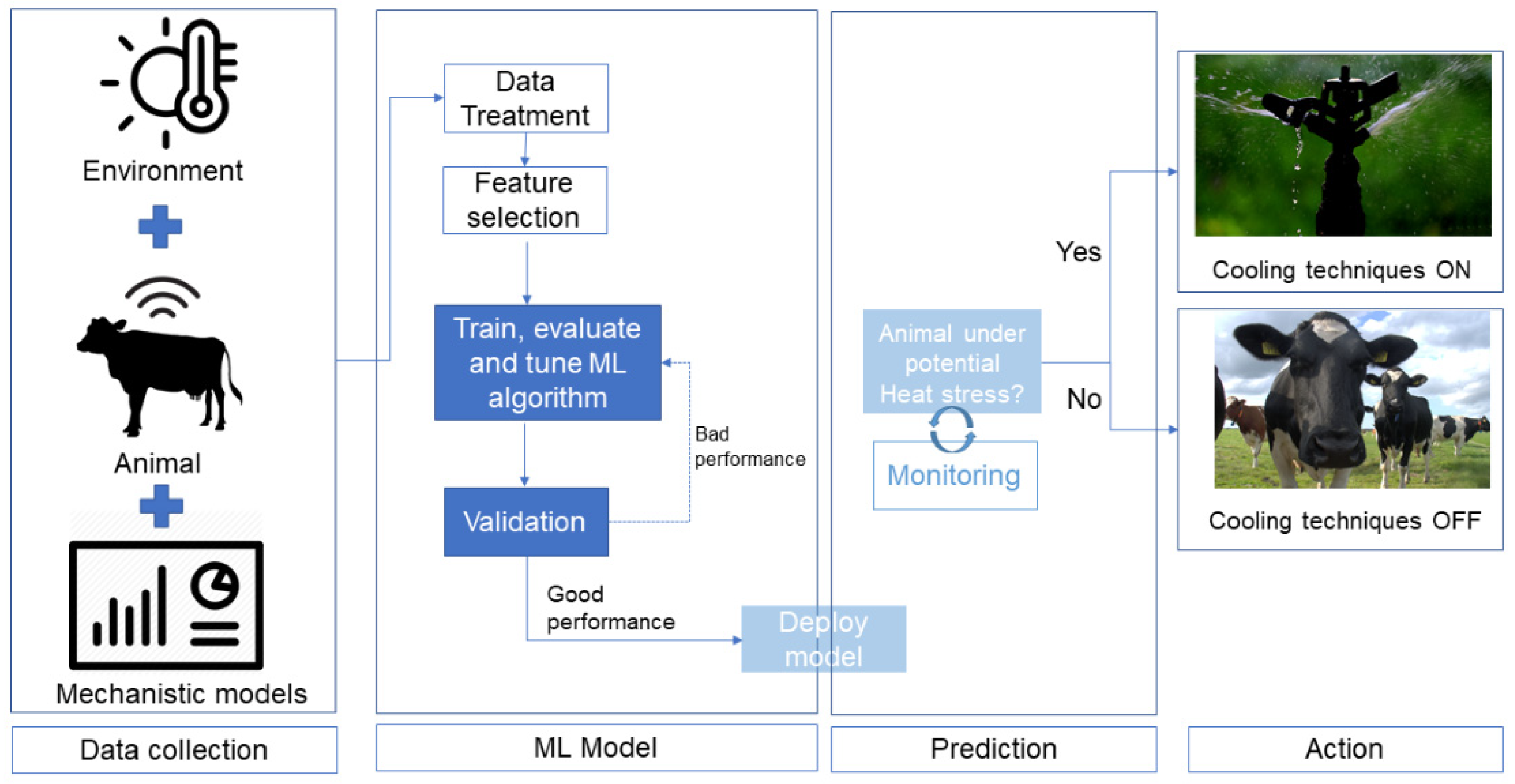
Abstract
1. Introduction
2. Heat Stressed Dairy Cows and the Available Technological Solutions to Detect and Mitigate Heat Load
2.1. Heat Stress Indicators
2.1.1. Physiological Indicators
2.1.2. Behavioural Indicators
2.1.3. Performance-Related Indicators
2.2. Methods to Detect Heat-Stressed Cows
2.2.1. Equipment with Low Data Acquisition Frequency
2.2.2. Equipment with High Data Acquisition Frequency
2.2.3. Observations
2.3. Cooling Technologies
3. Predictive Models
3.1. Bioclimatic Indexes
| Study Goal | Population | Inputs | Outputs | Observations | Ref. |
|---|---|---|---|---|---|
| Temperature-humidity index (THI) developed for bull calves | Four Ayrshire bull calves (9 months old) | Dry-bulb temperature; Wet-bulb temperature. | Rectal temperature | One equation. For five hours, each animal was kept inside a climate chamber. The trials were repeated 3 times per animal. | [80] |
| Correlation between milk production and ambient temperature and humidity | 56 Holstein cows (with several stages of lactation and production levels ranging from 6 to 70 lb per day) | Dry-bulb temperature; Wet-bulb temperature; Normal production level. | Production level | One equation. A good relationship between a THI index and milk production was obtained. A new equation was proposed considering besides air temperature and humidity also the normal production level. | [92] |
| Adjusted THI for cattle, considering wind and solar radiation | Three experiments with a varied number of animals (from 72 to 192) | Air temperature; Relative humidity; Wind velocity; Solar radiation. | Panting score | One equation. Required more than 2000 individual panting score assessments derived from ~12 d of observations. | [7] |
| Linear regression equation to estimate respiration rate of no-shade feedlot cattle | Eight crossbred steers | Dry-bulb temperature; Relative humidity; Wind velocity; Solar radiation. | Respiration rate | Two equations. Responses were studied during eight periods within 4-months. Animals randomly assigned to concrete surfaced pens with shade or no-shade option. | [61] |
| Respiratory heat loss (HER) | Simulation data | Air temperature; Relative humidity; Wind velocity; Animal-related factors (e.g., coat insulation and thickness) | Respiratory heat loss | Lumped model. Outputs of simulations were used to produce estimates of thresholds of maximal respiratory response as a function of ambient conditions for different cows-related factors. | [91] |
| Heat load index (HLI) | Feedlot cattle for seven genotypes (more than 10,000 animals) | Air temperature; Relative humidity; Wind velocity; Solar radiation; Animal-related factors (e.g., genotype, coat color, health status). | Panting score | Two equations. Responses were studied for eight summers. Approximately 162 observations were made per animal (3 times per day for 54 days). | [37] |
| Comprehensive climate index (CCI) for application under a wide range of environmental conditions (hot and cold) | Livestock cattle (number not defined) | Air temperature; Relative humidity; Wind velocity; Solar radiation. | Dry Matter Intake | Multiple non-linear equations. Based on experimental results reported in the literature. Responses were studied for nine summers and six winters. The model performance was compared with wind-chill and heat indexes. | [86] |
3.2. Machine Learning
3.2.1. Fundamentals of Machine Learning (ML)
3.2.2. Prediction of Heat-Stressed Cows
| Study Goal | Population | Inputs | Outputs | Algorithms | Ref. |
|---|---|---|---|---|---|
| Evaluate the heat stress of cattle | 128 heifers | Environmental data: dry bulb temperature, dew point temperature, solar radiation, wind speed. Animal related parameter: temperature of the hair coat color. | Respiration rate. | Regression models; Fuzzy inference systems; ANN. | [106] |
| Predict the physiological response of dairy cows | Holstein dairy cows (experimental + literature data) | Environmental data: dry-bulb air temperature, relative humidity. | Rectal temperature; respiratory rate. | Regression; ANN; Neurofuzzy networks. | [101] |
| Effect of the environmental factors on physiological responses | 19 dairy cows | Environmental data: air temperature; relative humidity, solar radiation, and wind speed. | Respiration rate; Skin temperature; Vaginal temperature. | Penalized linear regression; random forests; Gradient boosted machines; ANN. | [96] |
| Predicting the heat stress for feedlot cattle | 26 Nellore steers | Environmental data: dry and wet bulb temperature. Physiological parameter: temperature of head surface. | Rectal temperature. | Correlations; ANN. | [107] |
| Evaluate the heat stress in naturally ventilated barns for dairy cows | Outdoor conditions: temperature, relative humidity, zonal and meridional wind, sea level pressure, and global radiation; | Conditions inside the husbandries: temperature, relative humidity, and wind components. | Linear regression with and without regularization; random forest; ANN; Support-vector models. | [8] | |
| Definition of dynamic thresholds for heat stress alerts | 126 cows | Environmental data: minimum and mean ambient temperature. Body mass, days in milk, daily milk yields, and milk temperature. | Heat stress thresholds were redefined for the herd taking into consideration the daily milk yield and milk temperature. | Decision tree | [90] |
| Evaluate the milk yield under different thermal conditions | Holstein-Friesian cows | Air temperature around cowsheds. | Milk yield | ANN | [99] |
| Best cow treatment to improve the milk yield | dairy cows in Indonesia | Environmental data: temperature, wind speed, and relative humidity. Physiological parameters: heart rate, body temperature. | Milk yield | ANN | [108] |
| Prediction of the milk yield | 91 dairy cows | Barn environmental data: relative humidity and temperature. Days in milk of the cow. | Daily milk yield | Random forest | [100] |
3.3. Mechanistic Models
4. Future Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dikmen, S.; Hansen, P.J. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a sub-tropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-invasive physiological indicators of heat stress in cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Basiricò, L.; Morera, P.; Nardone, A. Heat stress elicits different responses in peripheral blood mononuclear cells from brown swiss and holstein cows. J. Dairy Sci. 2006, 89, 4606–4612. [Google Scholar] [CrossRef]
- Lacerda, T.F.; Loureiro, B. Selecting thermotolerant animals as a strategy to improve fertility in holstein cows. Glob. J. Anim. Sci. Res. 2014, 3, 119–127. [Google Scholar]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Menz, C.; Pinto, S.; Galán, E.; Janke, D.; Estellés, F.; Müschner-Siemens, T.; Wang, X.; Heinicke, J.; Zhang, G.; et al. Heat stress risk in European dairy cattle husbandry under different climate change scenarios—Uncertainties and potential impacts. Earth Syst. Dyn. 2019, 10, 859–884. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bowman, P.J.; Haile-Mariam, M.; Pryce, J.E.; Hayes, B.J. Genomic selection for tolerance to heat stress in Australian dairy cattle. J. Dairy Sci. 2016, 99, 2849–2862. [Google Scholar] [CrossRef] [PubMed]
- de Haas, Y.; Calus, M.; Veerkamp, R.; Wall, E.; Coffey, M.; Daetwyler, H.; Hayes, B.; Pryce, J. Improved accuracy of genomic prediction for dry matter intake of dairy cattle from combined European and Australian data sets. J. Dairy Sci. 2012, 95, 6103–6112. [Google Scholar] [CrossRef]
- Van Laer, E.; Moons, C.P.H.; Ampe, B.; Sonck, B.; Vandaele, L.; De Campeneere, S.; Tuyttens, F.A.M. Effect of summer conditions and shade on behavioural indicators of thermal discomfort in Holstein dairy and Belgian Blue beef cattle on pasture. Animal 2015, 9, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, B.P.; Abo, Y.; Sakamoto, R.; Furumoto, F.; Yamamoto, S. Diurnal patterns of heat production and heart rate under thermoneutral conditions in Holstein Friesian cows differing in milk production. J. Agric. Sci. 1990, 114, 139–142. [Google Scholar] [CrossRef]
- Peyraud, J.; Macleod, M. Study on Future of EU Livestock: How to Contribute to a Sustainable Agricultural Sector? Final Report. Available online: https://ec.europa.eu/info/food-farming-fisheries/key-policies/common-agricultural-policy/cmef/farmers-and-farming/future-eu-livestock-how-contribute-sustainable-agricultural-sector_en (accessed on 1 December 2020).
- Gunn, K.M.; Holly, M.A.; Veith, T.L.; Buda, A.R.; Prasad, R.; Rotz, C.A.; Soder, K.J.; Stoner, A.M.K. Projected heat stress challenges and abatement opportunities for U.S. milk production. PLoS ONE 2019, 14, e0214665. [Google Scholar] [CrossRef]
- Al-Qaisi, M.; Horst, E.; Kvidera, S.; Mayorga, E.; Timms, L.; Baumgard, L. Technical note: Developing a heat stress model in dairy cows using an electric heat blanket. J. Dairy Sci. 2019, 102, 684–689. [Google Scholar] [CrossRef]
- Curtis, A.; Scharf, B.; Eichen, P.; Spiers, D. Relationships between ambient conditions, thermal status, and feed intake of cattle during summer heat stress with access to shade. J. Therm. Biol. 2017, 63, 104–111. [Google Scholar] [CrossRef]
- Strickland, J.T.; Bucklin, R.A.; Nordstedt, R.A.; Beede, D.K.; Bray, D.R. Sprinkler and Fan Cooling System for Dairy Cows in Hot, Humid Climates. Appl. Eng. Agric. 1989, 5, 231–236. [Google Scholar] [CrossRef]
- Means, S.L.; Bucklin, R.A.; Nordstedt, R.A.; Beede, D.K.; Bray, D.R.; Wilcox, C.J.; Sanchez, W.K. Water Application Rates for a Sprinkler and Fan Dairy Cooling System in Hot, Humid Climates. Appl. Eng. Agric. 1992, 8, 375–379. [Google Scholar] [CrossRef]
- Chen, J.M.; Schütz, K.E.; Tucker, C.B.; Van Os, J. Cooling cows efficiently with sprinklers: Physiological responses to water spray. J. Dairy Sci. 2015, 98, 6925–6938. [Google Scholar] [CrossRef] [PubMed]
- West, J.W. Effects of Heat-Stress on Production in Dairy Cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Heinicke, J.; Hoffmann, G.; Ammon, C.; Amon, B.; Amon, T. Effects of the daily heat load duration exceeding determined heat load thresholds on activity traits of lactating dairy cows. J. Therm. Biol. 2018, 77, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Heinicke, J.; Ibscher, S.; Belik, V.; Amon, T. Cow individual activity response to the accumulation of heat load duration. J. Therm. Biol. 2019, 82, 23–32. [Google Scholar] [CrossRef]
- Nienaber, J.; Hahn, G. Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 2007, 52, 149–157. [Google Scholar] [CrossRef]
- Ferrazza, R.D.A.; Garcia, H.D.M.; Aristizábal, V.H.V.; Nogueira, C.D.S.; Veríssimo, C.J.; Sartori, J.R.; Sartori, R.; Ferreira, J.C.P. Thermoregulatory responses of Holstein cows exposed to experimentally induced heat stress. J. Therm. Biol. 2017, 66, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Brown-Brandl, T.M.; Eigenberg, R.A.; Nienaber, J.A.; Hahn, G.L. Dynamic Response Indicators of Heat Stress in Shaded and Non- shaded Feedlot Cattle, Part 1: Analyses of Indicators. Biosyst. Eng. 2005, 90, 451–462. [Google Scholar] [CrossRef]
- Vizzotto, E.; Fischer, V.; Neto, A.T.; Abreu, A.; Stumpf, M.; Werncke, D.; Schmidt, F.; McManus, C. Access to shade changes behavioral and physiological attributes of dairy cows during the hot season in the subtropics. Animal 2015, 9, 1559–1566. [Google Scholar] [CrossRef]
- Daltro, D.D.S.; Fischer, V.; Alfonzo, E.P.M.; Dalcin, V.C.; Stumpf, M.T.; Kolling, G.J.; Da Silva, M.V.G.B.; McManus, C. Infrared thermography as a method for evaluating the heat tolerance in dairy cows. Rev. Bras. Zootec. 2017, 46, 374–383. [Google Scholar] [CrossRef]
- De Rensis, F.; Lopez-Gatius, F.; García-Ispierto, I.; Morini, G.; Scaramuzzi, R. Causes of declining fertility in dairy cows during the warm season. Theriogenology 2016, 91, 145–153. [Google Scholar] [CrossRef]
- Hoffmann, G.; Herbut, P.; Pinto, S.; Heinicke, J.; Kuhla, B.; Amon, T. Animal-related, non-invasive indicators for determining heat stress in dairy cows. Biosyst. Eng. 2019, 199, 83–96. [Google Scholar] [CrossRef]
- Can, E.; Vieira, A.; Battini, M.; Mattiello, S.; Stilwell, G. Consistency over time of animal-based welfare indicators as a further step for developing a welfare assessment monitoring scheme: The case of the Animal Welfare Indicators protocol for dairy goats. J. Dairy Sci. 2017, 100, 9194–9204. [Google Scholar] [CrossRef]
- Farooq, U.; Samad, H.A.; Shehzad, F.; Qayyum, A. Physiological responses of cattle to heat stress. World Appl. Sci. J. 2010, 8, 38–43. [Google Scholar]
- Vickers, L.; Burfeind, O.; von Keyserlingk, M.; Veira, D.; Weary, D.; Heuwieser, W. Technical note: Comparison of rectal and vaginal temperatures in lactating dairy cows. J. Dairy Sci. 2010, 93, 5246–5251. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Liu, K.; Hao, Z.; Shi, Z.; Li, H. The effects of cow-related factors on rectal temperature, respiration rate, and temper-ature-humidity index thresholds for lactating cows exposed to heat stress. J. Therm. Biol. 2021, 100, 103041. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, K.; Strassburg, P.; Bennett, T.; Oetzel, G.; Cook, N. Thermodynamics of standing and lying behavior in lactating dairy cows in freestall and parlor holding pens during conditions of heat stress. J. Dairy Sci. 2019, 102, 6495–6507. [Google Scholar] [CrossRef]
- Polsky, L.B.; Madureira, A.M.; Filho, E.L.D.; Soriano, S.; Sica, A.F.; Vasconcelos, J.L.; Cerri, R.L. Association between ambient temperature and humidity, vaginal temperature, and automatic activity monitoring on induced estrus in lactating cows. J. Dairy Sci. 2017, 100, 8590–8601. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Lisle, A. A new heat load index for feedlot cattle1. J. Anim. Sci. 2008, 86, 226–234. [Google Scholar] [CrossRef]
- Yadav, B.; Singh, G.; Wankar, A. The use of infrared skin temperature measurements for monitoring heat stress and welfare of crossbred cattle. Indian J. Dairy Sci. 2017, 70, 127–131. [Google Scholar]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Herbut, P.; Angrecka, S. Relationship between THI level and dairy cows’ behaviour during summer period. Ital. J. Anim. Sci. 2017, 17, 226–233. [Google Scholar] [CrossRef]
- Pilatti, J.A.; Vieira, F.M.C.; Rankrape, F.; Vismara, E.S. Diurnal behaviors and herd characteristics of dairy cows housed in a compost-bedded pack barn system under hot and humid conditions. Animal 2019, 13, 399–406. [Google Scholar] [CrossRef]
- Allen, J.; Hall, L.; Collier, R.; Smith, J. Effect of core body temperature, time of day, and climate conditions on behavioral patterns of lactating dairy cows experiencing mild to moderate heat stress. J. Dairy Sci. 2015, 98, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, A. Some factors affecting the behavioural manifestation of oestrus in cattle: A review. Appl. Anim. Behav. Sci. 2000, 70, 1–16. [Google Scholar] [CrossRef]
- Santos, V.; Carvalho, P.; Maia, C.; Carneiro, B.; Valenza, A.; Fricke, P. Fertility of lactating Holstein cows submitted to a Double-Ovsynch protocol and timed artificial insemination versus artificial insemination after synchronization of estrus at a similar day in milk range. J. Dairy Sci. 2017, 100, 8507–8517. [Google Scholar] [CrossRef] [PubMed]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Roth, Z.; Arav, A.; Bor, A.; Zeron, Y.; Braw-Tal, R.; Wolfenson, D. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 2001, 122, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Kovács, L.; Kézér, F.L.; Bakony, M.; Jurkovich, V.; Szenci, O. Lying down frequency as a discomfort index in heat stressed Holstein bull calves. Sci. Rep. 2018, 8, 15065. [Google Scholar] [CrossRef]
- Ammer, S.; Lambertz, C.; Gauly, M. Is reticular temperature a useful indicator of heat stress in dairy cattle? J. Dairy Sci. 2016, 99, 10067–10076. [Google Scholar] [CrossRef]
- National Research Council. Effect of Environment on Nutrient Requirements of Domestic Animals; National Academies Press: Washington, DC, USA, 1981; p. 78. ISBN 0-309-53374-0. [Google Scholar] [CrossRef]
- Bernabucci, U.; Basiricò, L.; Morera, P.; DiPasquale, D.; Vitali, A.; Cappelli, F.P.; Calamari, L. Effect of summer season on milk protein fractions in Holstein cows. J. Dairy Sci. 2015, 98, 1815–1827. [Google Scholar] [CrossRef]
- Hill, D.; Wall, E. Dairy cattle in a temperate climate: The effects of weather on milk yield and composition depend on management. Animal 2015, 9, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ezernieks, V.; Wang, J.; Arachchillage, N.W.; Garner, J.B.; Wales, W.J.; Cocks, B.G.; Rochfort, S. Heat Stress in Dairy Cattle Alters Lipid Composition of Milk. Sci. Rep. 2017, 7, 961. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001; p. 138. ISBN 978-0-309-06997-7. [Google Scholar] [CrossRef]
- Van Laer, E.; Tuyttens, F.; Ampe, B.; Sonck, B.; Moons, C.P.H.; Vandaele, L. Effect of summer conditions and shade on the production and metabolism of Holstein dairy cows on pasture in temperate climate. Animal 2015, 9, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Banhazi, T.; Perano, K.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. A review of measuring, assessing and mitigating heat stress in dairy cattle. Biosyst. Eng. 2020, 199, 4–26. [Google Scholar] [CrossRef]
- Rashamol, V.P.; Sejian, V.; Pragna, P.; Lees, A.M.; Bagath, M.; Krishnan, G.; Gaughan, J.B. Prediction models, assessment methodologies and biotechnological tools to quantify heat stress response in ruminant livestock. Int. J. Biometeorol. 2019, 63, 1265–1281. [Google Scholar] [CrossRef]
- Müschner-Siemens, T.; Hoffmann, G.; Ammon, C.; Amon, T. Daily rumination time of lactating dairy cows under heat stress conditions. J. Therm. Biol. 2020, 88, 102484. [Google Scholar] [CrossRef]
- Chung, H.; Li, J.; Kim, Y.; Van Os, J.M.; Brounts, S.H.; Choi, C.Y. Using implantable biosensors and wearable scanners to monitor dairy cattle’s core body temperature in re-al-time. Comput. Electron. Agric. 2020, 174, 105453. [Google Scholar] [CrossRef]
- Eigenberg, R.A.; Nienaber, J.A.; Hahn, G.L. Dynamic response indicators of heat stress in shaded and non-shadedfeedlot cattle, Part 2: Predictive Relationships. Biosyst. Eng. 2005, 91, 111–118. [Google Scholar] [CrossRef]
- Sellier, N.; Guettier, E.; Staub, C. A Review of Methods to Measure Animal Body Temperature in Precision Farming. Am. J. Agric. Sci. Technol. 2014, 2, 74–99. [Google Scholar] [CrossRef]
- Rungruang, S.; Collier, J.; Rhoads, R.; Baumgard, L.; De Veth, M.; Collier, R. A dose-response evaluation of rumen-protected niacin in thermoneutral or heat-stressed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5023–5034. [Google Scholar] [CrossRef]
- Kunc, P.; Knizkova, I. The use of infrared thermography in livestock production and veterinary field. In Infrared Thermog-Raphy: Recent Advances and Future Trends; Bentham Science Publisher: Sharjah, United Arab Emirates, 2012; pp. 85–101. [Google Scholar]
- Unruh, E.M.; Theurer, M.E.; White, B.J.; Larson, R.L.; Drouillard, J.S.; Schrag, N. Evaluation of infrared thermography as a diagnostic tool to predict heat stress events in feedlot cattle. Am. J. Veter-Res. 2017, 78, 771–777. [Google Scholar] [CrossRef]
- Koltes, J.E.; Koltes, D.A.; Mote, B.E.; Tucker, J.; Hubbell, D.S. Automated collection of heat stress data in livestock: New technologies and opportunities. Transl. Anim. Sci. 2018, 2, 319–323. [Google Scholar] [CrossRef]
- Cantor, M.C.; Costa, J.H.C.; Bewley, J.M. Impact of Observed and Controlled Water Intake on Reticulorumen Temperature in Lactating Dairy Cattle. Animals 2018, 8, 194. [Google Scholar] [CrossRef]
- Eslamizad, M.; Lamp, O.; Derno, M.; Kuhla, B. The control of short-term feed intake by metabolic oxidation in late-pregnant and early lactating dairy cows exposed to high ambient temperatures. Physiol. Behav. 2015, 145, 64–70. [Google Scholar] [CrossRef]
- Borchers, M.R.; Chang, Y.M.; Proudfoot, K.L.; Wadsworth, B.A.; Stone, A.E.; Bewley, J.M. Machine-learning-based calving prediction from activity, lying, and ruminating behaviors in dairy cattle. J. Dairy Sci. 2017, 100, 5664–5674. [Google Scholar] [CrossRef] [PubMed]
- Eigenberg, R.A.; Hahn, G.L.; Nienaber, J.A.; Brown-Brandl, T.M.; Spiers, D.E. Development of a new respiration rate monitor for cattle. Trans. ASAE 2000, 43, 723–728. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Mader, T.L. Body temperature and respiratory dynamics in un-shaded beef cattle. Int. J. Biometeorol. 2014, 58, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Sullivan, M.L.; Hahn, G.L. Assessing the heat tolerance of 17 beef cattle genotypes. Int. J. Biometeorol. 2010, 54, 617–627. [Google Scholar] [CrossRef]
- Min, L.; Li, D.; Tong, X.; Nan, X.; Ding, D.; Xu, B.; Wang, G. Nutritional strategies for alleviating the detrimental effects of heat stress in dairy cows: A review. Int. J. Biometeorol. 2019, 63, 1283–1302. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D. Heat Stress Interaction with Shade and Cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Drwencke, A.M.; Tresoldi, G.; Stevens, M.M.; Narayanan, V.; Carrazco, A.V.; Mitloehner, F.M.; Pistochini, T.; Tucker, C.B. Innovative cooling strategies: Dairy cow responses and water and energy use. J. Dairy Sci. 2020, 103, 5440–5454. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Banhazi, T.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. Modelling of heat stress in a robotic dairy farm. Part 1: Thermal comfort indices as the indicators of production loss. Biosyst. Eng. 2019, 199, 27–42. [Google Scholar] [CrossRef]
- Wijffels, G.; Sullivan, M.; Gaughan, J. Methods to quantify heat stress in ruminants: Current status and future prospects. Methods 2020, 186, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Hoffmann, G.; Ammon, C.; Amon, T. Critical THI thresholds based on the physiological parameters of lactating dairy cows. J. Therm. Biol. 2020, 88, 102523. [Google Scholar] [CrossRef]
- Thom, E.C. The Discomfort Index. Weatherwise 1959, 12, 57–61. [Google Scholar] [CrossRef]
- Bianca, W. Relative Importance of Dry- and Wet-Bulb Temperatures in Causing Heat Stress in Cattle. Nature 1962, 195, 251–252. [Google Scholar] [CrossRef]
- Johnson, H.D. Environmental temperature and lactation (with special reference to cattle). Int. J. Biometeorol. 1965, 9, 103–116. [Google Scholar] [CrossRef]
- Gorniak, T.; Meyer, U.; Südekum, K.-H.; Dänicke, S. Impact of mild heat stress on dry matter intake, milk yield and milk composition in mid-lactation Holstein dairy cows in a temperate climate. Arch. Anim. Nutr. 2014, 68, 358–369. [Google Scholar] [CrossRef]
- Hammami, H.; Bormann, J.; M’Hamdi, N.; Montaldo, H.; Gengler, N. Evaluation of heat stress effects on production traits and somatic cell score of Holsteins in a temperate environment. J. Dairy Sci. 2013, 96, 1844–1855. [Google Scholar] [CrossRef]
- Lees, J.C.; Lees, A.; Gaughan, J. Developing a heat load index for lactating dairy cows. Anim. Prod. Sci. 2018, 58, 1387. [Google Scholar] [CrossRef]
- Wang, X.; Gao, H.; Gebremedhin, K.G.; Bjerg, B.; Van Os, J.; Tucker, C.; Zhang, G. A predictive model of equivalent temperature index for dairy cattle (ETIC). J. Therm. Biol. 2018, 76, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.L.; Johnson, L.J.; Gaughan, J. A comprehensive index for assessing environmental stress in animals1. J. Anim. Sci. 2010, 88, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Banhazi, T.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. Modelling of heat stress in a robotic dairy farm. Part 4: Time constant and cumulative effects of heat stress. Biosyst. Eng. 2020, 199, 73–82. [Google Scholar] [CrossRef]
- Yan, G.; Li, H.; Zhao, W.; Shi, Z. Evaluation of thermal indices based on their relationships with some physiological responses of housed lactating cows under heat stress. Int. J. Biometeorol. 2020, 64, 2077–2091. [Google Scholar] [CrossRef]
- DeVoe, K.R.; Hoff, S.J.; Ramirez, B.C.; Baumgard, L.H. Climate Dependent Heat Stress Mitigation Modeling for Dairy Cattle Housing. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, WA, USA, 16–19 July 2017. [Google Scholar]
- Ji, B.; Banhazi, T.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. Modelling of heat stress in a robotic dairy farm. Part 2: Identifying the specific thresholds with production factors. Biosyst. Eng. 2019, 199, 43–57. [Google Scholar] [CrossRef]
- Berman, A. Estimates of heat stress relief needs for Holstein dairy cows1. J. Anim. Sci. 2005, 83, 1377–1384. [Google Scholar] [CrossRef]
- Berry, I.L.; Shanklin, M.D.; Johnson, A.H.D. Dairy Shelter Design Based on Milk Production Decline as Affected by Temperature and Humidity. Trans. ASAE 1964, 7, 0329–0331. [Google Scholar] [CrossRef]
- Hudson, C.; Kaler, J.; Down, P. Use of big data in cattle practice. Practice 2018, 40, 396–410. [Google Scholar] [CrossRef]
- Frank, M.; Drikakis, D.; Charissis, V. Machine-learning methods for computational science and engineering. Computation 2020, 8, 15. [Google Scholar] [CrossRef]
- Kotsiantis, S.B.; Zaharakis, I.D.; Pintelas, P.E. Machine learning: A review of classification and combining techniques. Artif. Intell. Rev. 2006, 26, 159–190. [Google Scholar] [CrossRef]
- Gorczyca, M.T.; Gebremedhin, K.G. Ranking of environmental heat stressors for dairy cows using machine learning algo-rithms. Comput. Electron. Agric. 2020, 168, 105124. [Google Scholar] [CrossRef]
- Anifowose, F.A.; Labadin, J.; Abdulraheem, A. Ensemble machine learning: An untapped modeling paradigm for petro-leum reservoir characterization. J. Pet. Sci. Eng. 2017, 151, 480–487. [Google Scholar] [CrossRef]
- Krollner, B.; Vanstone, B.; Finnie, G. Financial Time Series Forecasting with Machine Learning Techniques: A Survey; Computational and machine learning; European Symposium on Artificialneural Networks: Bruges, Belgium, 2010; pp. 25–30. [Google Scholar]
- Boniecki, P.; Lipiński, M.; Koszela, K.; Przybył, J. Neural prediction of cows milk yield according to environment tem-perature. Afr. J. Biotechnol. 2013, 12, 4707–4712. [Google Scholar] [CrossRef]
- Bovo, M.; Agrusti, M.; Benni, S.; Torreggiani, D.; Tassinari, P. Random Forest Modelling of Milk Yield of Dairy Cows under Heat Stress Conditions. Animals 2021, 11, 1305. [Google Scholar] [CrossRef]
- Hernández-Julio, Y.F.; Yanagi, T., Jr.; Pires, M.D.F.; Lopes, M.A.; De Lima, R.R. Models for Prediction of Physiological Responses of Holstein Dairy Cows. Appl. Artif. Intell. 2014, 28, 766–792. [Google Scholar] [CrossRef]
- Jain, V.K. Machine Learning; Khanna Publishing House: Delhi, India, 1990; pp. 1–108. [Google Scholar]
- Ruczinski, I.; Kooperberg, C.; Leblanc, M. Logic Regression. J. Comput. Graph. Stat. 2003, 12, 475–511. [Google Scholar] [CrossRef]
- Maimon, O.; Rokach, L. Data Mining and Knowledge Discovery Handbook; Springer: New York, NY, USA, 2005; pp. 149–164. [Google Scholar] [CrossRef]
- Gehrke, J.; Ramakrishnan, R.; Ganti, V. Rainforest—A framework for fast decision tree construction of large datasets. Data Min. Knowl. Discov. 2000, 4, 127–162. [Google Scholar] [CrossRef]
- Brown-Brandl, T.; Jones, D.; Woldt, W. Evaluating modelling techniques for cattle heat stress prediction. Biosyst. Eng. 2005, 91, 513–524. [Google Scholar] [CrossRef]
- de Sousa, R.V.; Rodrigues, A.V.D.S.; de Abreu, M.G.; Tabile, R.A.; Martello, L.S. Predictive model based on artificial neural network for assessing beef cattle thermal stress using weather and physiological variables. Comput. Electron. Agric. 2018, 144, 37–43. [Google Scholar] [CrossRef]
- Sugiono, S.; Soenoko, R.; Andriani, D.P. Analysis the relationship of physiological, environmental, and cow milk productivity using AI. In Proceedings of the 2016 International Conference on Data and Software Engineering (ICoDSE), Denpasar, Indonesia, 26–27 May 2016. [Google Scholar]
- Turnpenny, J.; Wathes, C.; Clark, J.; McArthur, A. Thermal balance of livestock: 2. Applications of a parsimonious model. Agric. For. Meteorol. 2000, 101, 29–52. [Google Scholar] [CrossRef]
- Chen, E.; Narayanan, V.; Pistochini, T.; Rasouli, E. Transient simultaneous heat and mass transfer model to estimate drying time in a wetted fur of a cow. Biosyst. Eng. 2020, 195, 116–135. [Google Scholar] [CrossRef]
- Gebremedhin, K.G.; Wu, B.; Perano, K. Modeling conductive cooling for thermally stressed dairy cows. J. Therm. Biol. 2016, 56, 91–99. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A. Thermal interaction between animal and microclimate: A comprehensive model. J. Theor. Biol. 1987, 126, 203–238. [Google Scholar] [CrossRef]
- Thompson, V.A.; Sainz, R.D.; Strathe, A.B.; Rumsey, T.R.; Fadel, J.G. The evaluation of a dynamic, mechanistic, thermal balance model for Bos indicus and Bos taurus. J. Agric. Sci. 2013, 152, 483–496. [Google Scholar] [CrossRef]
- McGovern, R.; Bruce, J. AP—Animal Production Technology: A Model of the Thermal Balance for Cattle in Hot Conditions. J. Agric. Eng. Res. 2000, 77, 81–92. [Google Scholar] [CrossRef]
- Gebremedhin, K.G.; Wu, B. Modeling heat loss from the udder of a dairy cow. J. Therm. Biol. 2016, 59, 34–38. [Google Scholar] [CrossRef]
- Maia, A.S.C.; Da Silva, R.G.; Loureiro, C.M.B. Latent heat loss of Holstein cows in a tropical environment: A prediction model. Rev. Bras. Zootec. 2008, 37, 1837–1843. [Google Scholar] [CrossRef][Green Version]
- Gebremedhin, K.G.; Wu, B. A model of evaporative cooling of wet skin surface and fur layer. J. Therm. Biol. 2001, 26, 537–545. [Google Scholar] [CrossRef]
- Gebremedhin, K.; Wu, B. Characterization of flow field in a ventilated space and simulation of heat exchange between cows and their environment. J. Therm. Biol. 2003, 28, 301–319. [Google Scholar] [CrossRef]
- Malaquias, A.F.; Neves, S.; Campos, J.M. The impact of water on firefighter protective clothing thermal performance and steam burn occurrence in firefighters. Fire Saf. J. 2022, 127, 103506. [Google Scholar] [CrossRef]
- Das, K.; Mishra, S.C. Study of thermal behavior of a biological tissue: An equivalence of Pennes bioheat equation and Wulff continuum model. J. Therm. Biol. 2014, 45, 103–109. [Google Scholar] [CrossRef]
- Herzog, A.; Winckler, C.; Hörtenhuber, S.; Zollitsch, W. Environmental impacts of implementing basket fans for heat abatement in dairy farms. Animal 2021, 15, 100274. [Google Scholar] [CrossRef]
- Stolwijk, J.A.J. A Mathematical Model of Physiological Temperature Regulation in Man; Report number: NASA-CR-1855; NASA: Washington, DC, USA, 1971.

| Model Description | Main Mathematical Assumptions | Inputs | Outputs | Observations | Ref. |
|---|---|---|---|---|---|
| Thermal balance for cattle in hot conditions. | Three node model: core, skin, and coat. Main transfer phenomena at the cow surface (skin + hair): heat transfer by convection and radiation, and mass transfer by convection. Main thermoregulation mechanisms: panting and sweating. | Environmental conditions: temperature, humidity, air velocity, solar radiation. Animal-related parameters: e.g., weight, metabolic heat, body-specific heat, coat reflection coefficient and thickness. | Core, skin, and coat temperature. Sensible and heat loss from respiration. Stored heat. Latent heat loss from the skin. | Only qualitative behavior of the numerical results was assessed. | [114] |
| Thermal balance for Holstein cows in hot conditions. | Based on [118]. Additionally, the author adjusted several animal-related parameters. | Based on [118]. | Based on [118]. | Improvement of the accuracy of total skin and respiratory heat loss prediction. | [91] |
| Thermal balance of livestock. | Three node model: core, skin, and coat. Main transfer phenomena at the cow surface: convection, evaporation, radiation, and solar radiation gain (for animals outdoors). Furthermore, considers the rain effect. Physiological responses: vasomotor action, sweating and panting. | Environmental conditions: temperature, humidity, air velocity, precipitation, direct and diffuse solar radiation. Animal-related parameters: e.g., tissues thermal resistance. | Skin and coat temperature Sensible and latent heat loss. | Simplification of the physical and physiological mechanisms to simulate long data sets for climate change impact analysis. | [109] |
| Simulation of udder heat loss. | One dimensional approach (heat transfer through skin; from core to ambient). Main phenomena at the skin surface: convection and sweat evaporation. Main phenomena through the skin: conduction, convection (heating by infused blood flow), and metabolic heat production. | Environmental conditions: air temperature and velocity. Animal-related factors: e.g., tissue density and specific heat, metabolic heat production. | Udder skin temperature. Evaporative heat loss from the udder skin. Convective heat loss from the udder skin. | The approach can be used to study the heat loss of other body zones and the performance of cooling technologies (e.g., fans). | [115] |
| Heat and mass transfer model to estimate drying time of a wetted fur. | One dimensional approach. Simulation domain: hair coat. Main phenomena: heat conduction, diffusion of water vapor, and evaporation. | Environmental conditions: air temperature, humidity and velocity. Animal-related factors: e.g., tissue thermal resistance, fur thermal conductivity, coat thickness. | Skin temperature. Total heat flux at the skin and coat surfaces. Water mass fraction. | It can be used to study the efficiency of water sprays coupled with fan-induced air flow. | [110] |
| Heat loss from cattle randomly distributed along a ventilated barn. | Domain: ventilated space occupied by 10 cows. Fluid flow fields characterized through 3-dimensional simulation. Cows thermal balance calculated through a coupled heat and mass transfer model (based on [114,121]). | Environmental conditions: air temperature, humidity, and air velocity. Animal-related factors: e.g., tissue thermal resistance, fur thermal conductivity, coat thickness, and animal position inside the barn. | Fluid field around each cow. Skin temperature. Total heat loss for each cow convective and radiant heat losses, sensible and latent heat components). | The approach can be used to obtain realistic convective heat and mass transfer coefficients, assuming different cows’ dimensions and spatial distribution. | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, S.F.; Silva, M.C.F.; Miranda, J.M.; Stilwell, G.; Cortez, P.P. Predictive Models of Dairy Cow Thermal State: A Review from a Technological Perspective. Vet. Sci. 2022, 9, 416. https://doi.org/10.3390/vetsci9080416
Neves SF, Silva MCF, Miranda JM, Stilwell G, Cortez PP. Predictive Models of Dairy Cow Thermal State: A Review from a Technological Perspective. Veterinary Sciences. 2022; 9(8):416. https://doi.org/10.3390/vetsci9080416
Chicago/Turabian StyleNeves, Soraia F., Mónica C. F. Silva, João M. Miranda, George Stilwell, and Paulo P. Cortez. 2022. "Predictive Models of Dairy Cow Thermal State: A Review from a Technological Perspective" Veterinary Sciences 9, no. 8: 416. https://doi.org/10.3390/vetsci9080416
APA StyleNeves, S. F., Silva, M. C. F., Miranda, J. M., Stilwell, G., & Cortez, P. P. (2022). Predictive Models of Dairy Cow Thermal State: A Review from a Technological Perspective. Veterinary Sciences, 9(8), 416. https://doi.org/10.3390/vetsci9080416










