Tolerability of Atovaquone—Proguanil Application in Common Buzzard Nestlings
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Malarone® Treatment
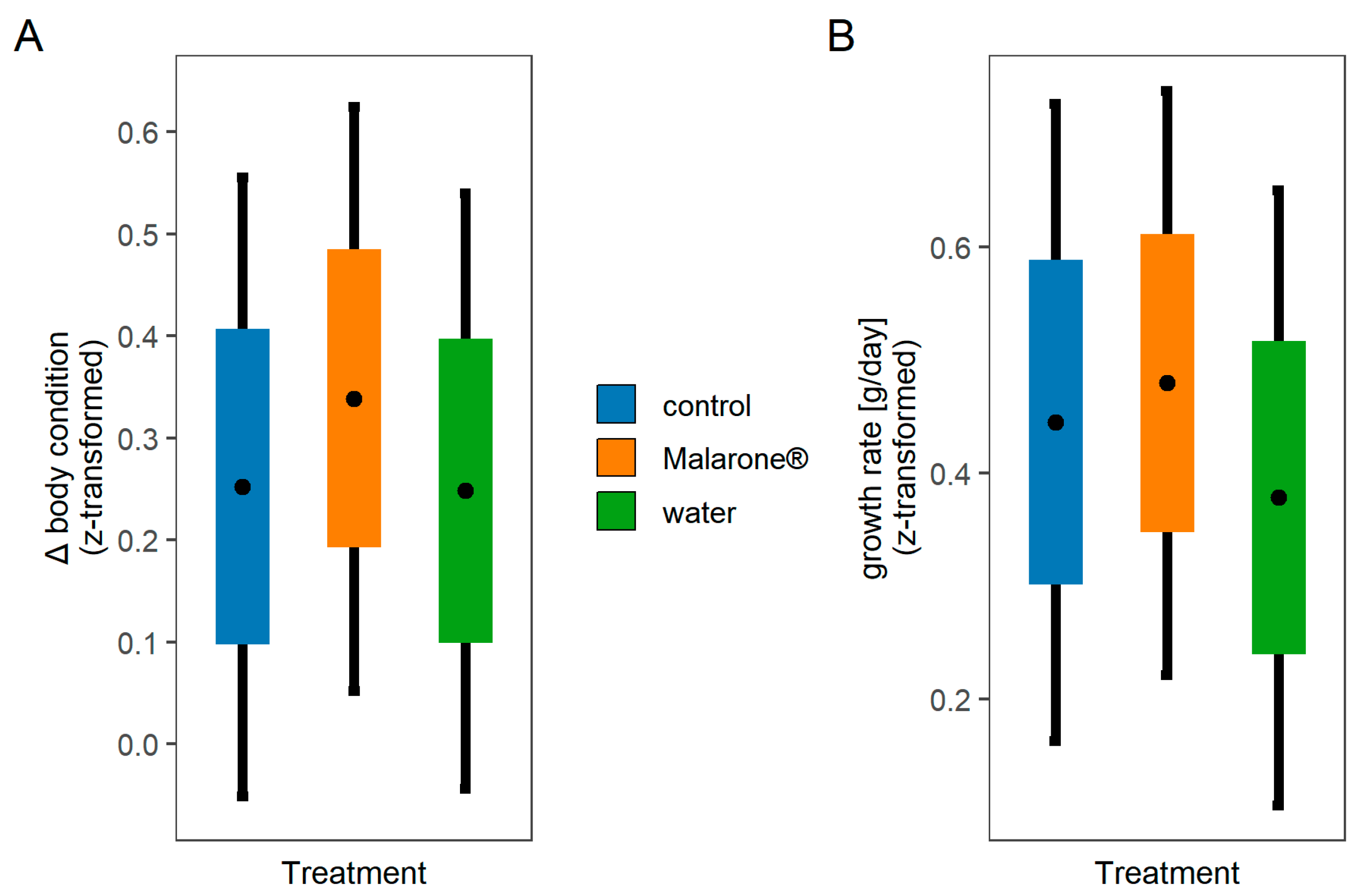
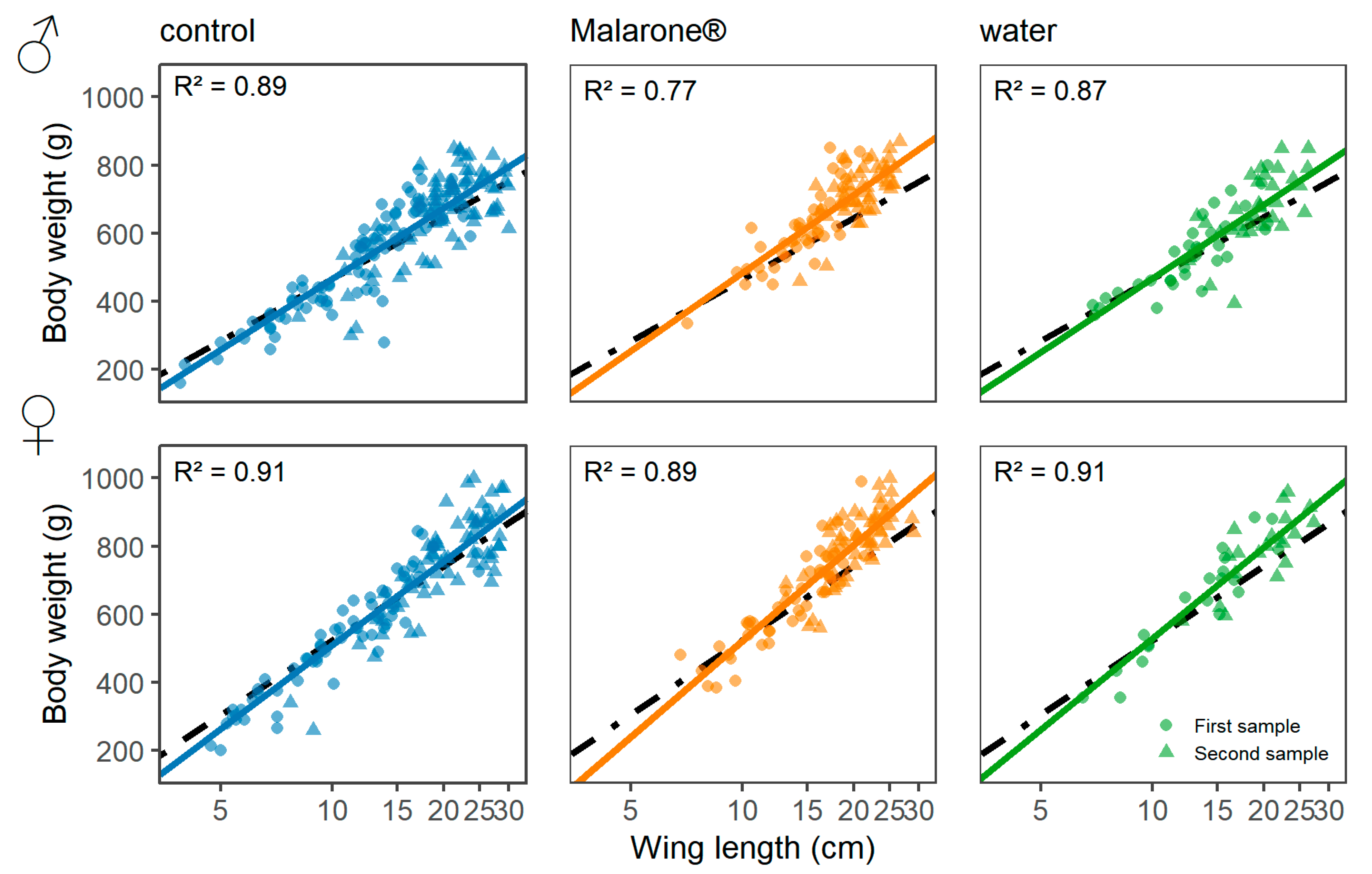
2.3. Body Condition and Growth Rate
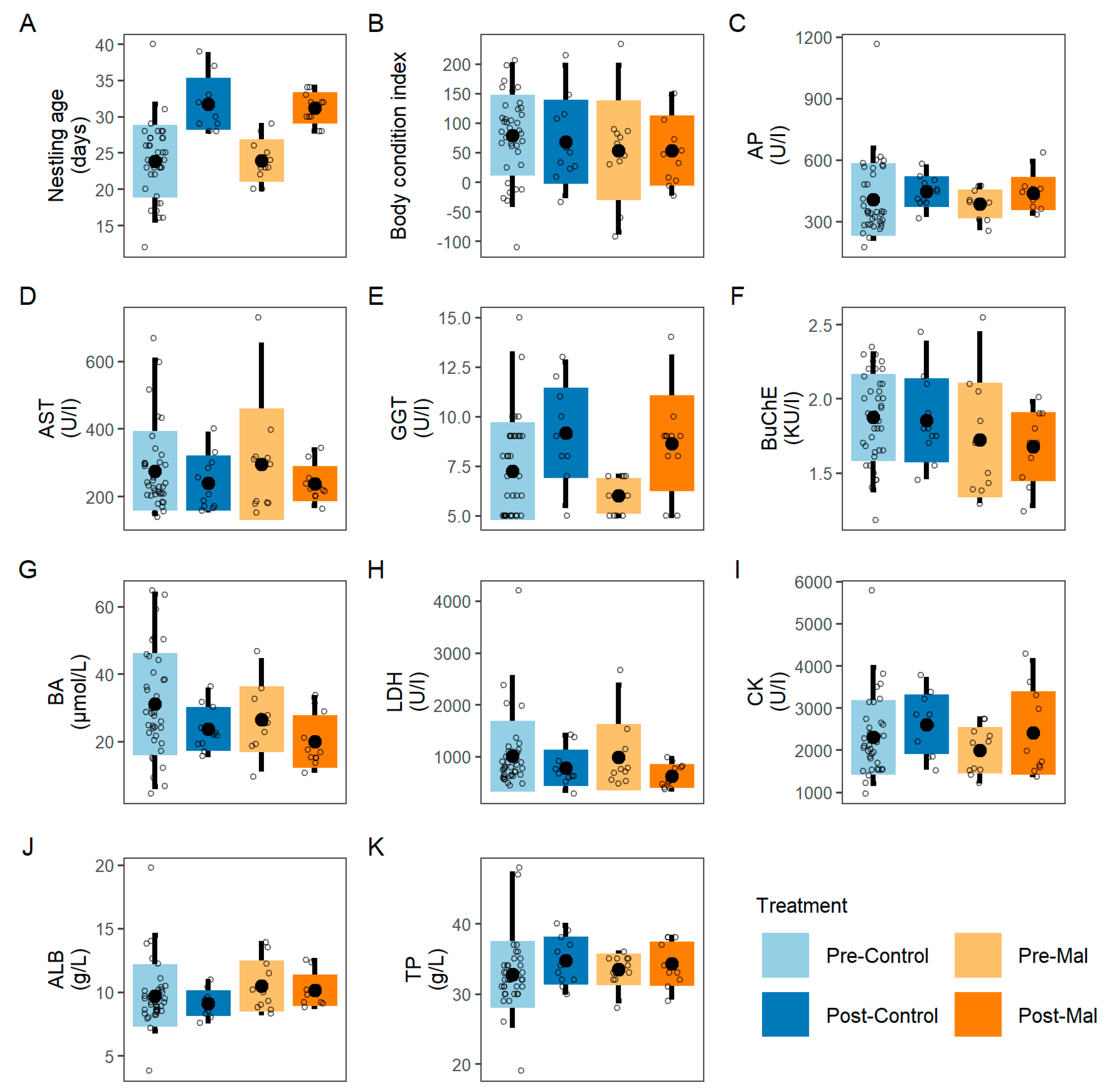
2.4. Blood Chemistry
2.5. Statistical analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davey, D.G. The use of avian malaria for the discovery of drugs effective in the treatment and prevention of human malaria: II.—Drugs for causal prophylaxis and radical cure or the chemotherapy of exo-erythrocytic forms. Ann. Trop. Med. Parasitol. 1946, 40, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Palinauskas, V.; la Puente, J.M.-D.; Hernández-Soto, S.R.; Marzal, A. Experimental parasitology and ecoimmunology: Concepts and opportunities in avian haemosporidian studies. In Avian Malaria and Related Parasites in the Tropics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 527–558. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Valkiūnas, G.; Guimarães, L.D.O.; Monteiro, E.; Guida, F.J.V.; Simões, R.; Rodrigues, P.T.; Luna, E.J.D.A.; Kirchgatter, K. Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malar. J. 2017, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bush, S.E.; Clayton, D.H. Anti-parasite behaviour of birds. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170196. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, D.A.; Atkinson, C.T.; Samuel, M.D. Ecology and conservation biology of avian malaria. Ann. New York Acad. Sci. 2012, 1249, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.W. Exotic Animal Formulary, 5th ed.; Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
- Remple, J.D. Intracellular Hematozoa of Raptors: A Review and Update. J. Avian Med. Surg. 2004, 18, 75–88. [Google Scholar] [CrossRef]
- Lee, C.C.; Kinter, L.D.; Heiffer, M.H. Subacute toxicity of primaquine in dogs, monkeys, and rats. Bull. World Health Organ. 1981, 59, 439–448. [Google Scholar]
- AlKadi, H.O. Antimalarial Drug Toxicity: A Review. Chemotherapy 2007, 53, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Avni-Magen, N.; Pe’Er, O.; Berkowitz, A.; Ofri, R. Treatment with chloroquine is retinotoxic in captive African penguins (Speniscus demersus). Attenuation and recovery of electroretinographic responses. Veter-Ophthalmol. 2021, 24, 336–345. [Google Scholar] [CrossRef]
- Wünschmann, A.; Armien, A.; Wallace, R.; Wictor, M.; Oglesbee, M. Neuronal storage disease in a group of captive humboldt penguins (Spheniscus humboldti). Veter-Pathol. 2006, 43, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Toutain, P.-L.; Ferran, A.; Bousquet-Melou, A. Species differences in pharmacokinetics and pharmacodynamics. In Comparative and Veterinary Pharmacology; Cunningham, F., Elliott, J., Peter Lees, P., Eds.; Springer: Cham, Switzerland, 2010; pp. 19–48. [Google Scholar] [CrossRef]
- Styka, A.N.; Savitz, D.A. Assessment of Long-Term Health Effects of Antimalarial Drugs When Used for Prophylaxis; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- McKeage, K.; Scott, L.J. Atovaquone/proguanil-a review of its use for the prophylaxis of Plasmodium falciparum malaria. Drugs 2003, 63, 597–623. [Google Scholar] [CrossRef]
- Knowles, S.C.L.; Palinauskas, V.; Sheldon, B.C. Chronic malaria infections increase family inequalities and reduce parental fitness: Experimental evidence from a wild bird population. J. Evol. Biol. 2010, 23, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kwak, D.; Kim, K.-T. The first clinical cases of Haemoproteus infection in a snowy owl (Bubo scandiacus) and a goshawk (Accipiter gentilis) at a zoo in the Republic of Korea. J. Veter-Med. Sci. 2018, 80, 1255–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palinauskas, V.; Valkiūnas, G.; Križanauskienė, A.; Bensch, S.; Bolshakov, C.V. Plasmodium relictum (lineage P-SGS1): Further observation of effects on experimentally infected passeriform birds, with remarks on treatment with Malarone™. Exp. Parasitol. 2009, 123, 134–139. [Google Scholar] [CrossRef]
- Chakarov, N.; Boerner, M.; Krüger, O. Fitness in common buzzards at the cross-point of opposite melanin-parasite interactions. Funct. Ecol. 2008, 22, 1062–1069. [Google Scholar] [CrossRef]
- Chakarov, N.; Pauli, M.; Krüger, O. Immune responses link parasite genetic diversity, prevalence and plumage morphs in common buzzards. Evol. Ecol. 2016, 31, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Bijlmsa, R. Sex determination of nestling Common Buzzards Buteo buteo. Limosa 1999, 72, 1–10. [Google Scholar]
- Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Wiegmann, A.; Springer, A.; Rinaud, T.; Ottensmann, M.; Legler, M.; Krüger, O.; Fehr, M.; Chakarov, N.; Strube, C. The prevalence of Leucocytozoon spp. in nestlings of three wild raptor species including implications on haematological and blood chemistry values. Int. J. Parasitol. Parasites Wildl. 2021, 16, 236–243. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar] [CrossRef]
- Guzman, D.S.-M. Advances in Avian Clinical Therapeutics. J. Exot. Pet Med. 2014, 23, 6–20. [Google Scholar] [CrossRef]
- Hyatt, M.W.; Georoff, T.A.; Nollens, H.H.; Wells, R.L.; Clauss, T.M.; Ialeggio, D.M.; Harms, C.; Wack, A.N. Voriconazole toxicity in multiple penguin species. J. Zoo Wildl. Med. 2015, 46, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Van Der Mast, H.; Dorrestein, G.M.; Westerhof, J. A Fatal Treatment of Sinusitus in an African Grey. J. Assoc. Avian Veter. 1990, 4, 189. [Google Scholar] [CrossRef]
- Bird, J.E.; Miller, K.W.; Larson, A.A.; Duke, G.E. Pharmacokinetics of gentamicin in birds of prey. Am. J. Veter.-Res. 1983, 44, 1245–1247. [Google Scholar]
- Flammer, K.; Clark, C.H.; Drewes, L.A.; Wilson, R.C.; Fiorello-Barrett, J. Adverse effects of gentamicin in scarlet macaws and galahs. Am. J. Veter.-Res. 1990, 51, 404–407. [Google Scholar]
- Jimenez-Lopez, O.; Ponder, J.; Nault, A.; Bueno, I. Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) and their Effect on Old World Vultures: A Scoping Review. J. Raptor Res. 2021, 55, 297–310. [Google Scholar] [CrossRef]
- Swan, G.E.; Cuthbert, R.; Quevedo, M.; Green, R.E.; Pain, D.J.; Bartels, P.; Cunningham, A.A.; Duncan, N.; Meharg, A.A.; Oaks, J.L.; et al. Toxicity of diclofenac to Gyps vultures. Biol. Lett. 2006, 2, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorrestein, G.M.; Miert, A.V. Pharmacotherapeutic aspects of medication of birds. J. Veter.-Pharmacol. Ther. 1988, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- FDA, Package Insert for Malarone® (Atovaquone and Proguanil Hydrochloride) Tablets and Malarone® (Atovaquone and Proguanil Hydrochloride) Pediatric Tablets. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021078s023lbl.pdf (accessed on 22 February 2019).
- Beernaert, L.A.; Baert, K.; Marin, P.; Chiers, K.; De Backer, P.; Pasmans, F.; Martel, A. Designing voriconazole treatment for racing pigeons: Balancing between hepatic enzyme auto induction and toxicity. Med. Mycol. 2009, 47, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grilo, M.; Vanstreels, R.E.T.; Wallace, R.; García-Párraga, D.; Braga, E.; Chitty, J.; Catão-Dias, J.L.; de Carvalho, L.M. Malaria in penguins–current perceptions. Avian Pathol. 2016, 45, 393–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaud, T.; Krüger, O.; Ottensmann, M.; Chakarov, N. Apparent Physiological Costs of Blood Parasites in the Early-Life of a Vertebrate Host-Only during Acute Infections. 2022. Available online: https://ecoevorxiv.org/4tcqu/ (accessed on 29 July 2022).
- Souza, M.J.; Martin-Jimenez, T.; Jones, M.P.; Cox, S.K. Pharmacokinetics of intravenous and oral tramadol in the bald eagle (Haliaeetus leucocephalus). J. Avian Med. Surg. 2009, 23, 247–252. [Google Scholar] [CrossRef]
- Dorrestein, G.M.; Van Gogh, H.; Rinzema, J.D. Pharmacokinetic aspects of penicillins, aminoglycosides and chloramphenicol in birds compared to mammals. A review. Veter.-Q. 1984, 6, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Baert, K.; De Backer, P. Comparative pharmacokinetics of three non-steroidal anti-inflammatory drugs in five bird species. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2002, 134, 25–33. [Google Scholar] [CrossRef]
- Carag, J.H.; Sander, S.J.; Kottyan, J.; Phillips, J.; Brubaker, J.; Cruz-Espindola, C.; Boothe, D.; Bronson, E. Pharmacokinetics of primaquine phosphate after a single oral administration to african penguins (spheniscus demersus). J. Zoo Wildl. Med. 2021, 52, 75–80. [Google Scholar] [CrossRef]
- Boggild, A.K.; Parise, M.E.; Lewis, L.S.; Kain, K.C. Atovaquone-proguanil: Report from the cdc expert meeting on malaria chemoprophylaxis (II). Am. J. Trop. Med. Hyg. 2007, 76, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Adawaren, E.O.; Du Plessis, M.; Suleman, E.; Kindler, D.; Oosthuizen, A.O.; Mukandiwa, L.; Naidoo, V. The complete mitochondrial genome of Gyps coprotheres (Aves, Accipitridae, Accipitriformes): Phylogenetic analysis of mitogenome among raptors. PeerJ 2020, 8, e10034. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.P.; Kawai, Y.K.; Ikenaka, Y.; Kawata, M.; Ikushiro, S.-I.; Sakaki, T.; Ishizuka, M. Avian Cytochrome P450 (CYP) 1–3 Family Genes: Isoforms, Evolutionary Relationships, and mRNA Expression in Chicken Liver. PLoS ONE 2013, 8, e75689. [Google Scholar] [CrossRef] [Green Version]
- Orosz, S.E. Overview of aspergillosis: Pathogenesis and treatment options. Semin. Avian Exot. Pet. Med. 2000, 9, 59–65. [Google Scholar] [CrossRef]
- Looareesuwan, S.; Hutchinson, D.B.; Chulay, J.D.; Canfield, C.J. Malarone (atovaquone and proguanil hydrochloride): A review of its clinical development for treatment of malaria. Malarone Clinical Trials Study Group. Am. J. Trop. Med. Hyg. 1999, 60, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.B. Avian malaria: Clinical and chemical pathology of Plasmodium gallinaceum in the domesticated fowl Gallus gallus. Avian Pathol. 2005, 34, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.D.E.; Pires, J.R.; Nascimento, C.L.; Dutra, G.H.P.; Torres-Filho, R.A.; Toma, H.K.; Brener, B.; Almosny, N.R. Analysis of hematologic and serum chemistry values of Spheniscus magellanicus with molecular detection of avian malarial parasites (Plasmodium spp.). Pesq. Vet. Bras. 2014, 34, 1236–1242. [Google Scholar] [CrossRef] [Green Version]
| Predictors | Δ Body Condition | Growth Rate | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimates | CI | t-Value | p | Estimates | CI | t-Value | p | |
| Intercept | 0.41 | −0.53–1.35 | 0.86 | 0.391 | 2.83 | 2.02–3.64 | 6.91 | <0.001 |
| Mean Age (days) * | −0.01 | −0.04–0.02 | −0.42 | 0.676 | −0.09 | −0.12–−0.07 | −6.91 | <0.001 |
| Resampling interval (days) | 0.00 | −0.07–0.08 | 0.13 | 0.895 | −0.02 | −0.08–0.04 | −0.68 | 0.498 |
| Sex (Males) | −0.40 | −0.61–−0.18 | −3.67 | <0.001 | ||||
| Treatment (Control) | Reference | Reference | ||||||
| Treatment (Water) | −0.04 | −0.39–0.32 | −0.20 | 0.841 | −0.09 | −0.41–0.23 | −0.55 | 0.580 |
| Treatment (Malarone®) | 0.05 | −0.27–0.37 | 0.29 | 0.774 | 0.01 | −0.28–0.29 | 0.04 | 0.970 |
| Year (2020) | Reference | Reference | ||||||
| Year (2016) | −1.46 | −3.50–0.58 | −1.41 | 0.159 | −1.02 | −2.81–0.76 | −1.13 | 0.260 |
| Year (2018) | −0.38 | −0.75–−0.02 | −2.08 | 0.039 | −0.28 | −0.59–0.03 | −1.78 | 0.077 |
| Year (2019) | −0.56 | −0.95–−0.17 | −2.83 | 0.005 | −0.44 | −0.76–−0.11 | −2.62 | 0.010 |
| Random Effect | ||||||||
| σ2 | 0.65 | 0.62 | ||||||
| ICC | 0.38 | 0.23 | ||||||
| N | 109 Broods | 109 Broods | ||||||
| Observations | 261 | 261 | ||||||
| Marginal R2/Conditional R2 | 0.058/0.416 | 0.267/0.439 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiegmann, A.; Rinaud, T.; Ottensmann, M.; Krüger, O.; Springer, A.; Legler, M.; Fehr, M.; Strube, C.; Chakarov, N. Tolerability of Atovaquone—Proguanil Application in Common Buzzard Nestlings. Vet. Sci. 2022, 9, 397. https://doi.org/10.3390/vetsci9080397
Wiegmann A, Rinaud T, Ottensmann M, Krüger O, Springer A, Legler M, Fehr M, Strube C, Chakarov N. Tolerability of Atovaquone—Proguanil Application in Common Buzzard Nestlings. Veterinary Sciences. 2022; 9(8):397. https://doi.org/10.3390/vetsci9080397
Chicago/Turabian StyleWiegmann, Anja, Tony Rinaud, Meinolf Ottensmann, Oliver Krüger, Andrea Springer, Marko Legler, Michael Fehr, Christina Strube, and Nayden Chakarov. 2022. "Tolerability of Atovaquone—Proguanil Application in Common Buzzard Nestlings" Veterinary Sciences 9, no. 8: 397. https://doi.org/10.3390/vetsci9080397
APA StyleWiegmann, A., Rinaud, T., Ottensmann, M., Krüger, O., Springer, A., Legler, M., Fehr, M., Strube, C., & Chakarov, N. (2022). Tolerability of Atovaquone—Proguanil Application in Common Buzzard Nestlings. Veterinary Sciences, 9(8), 397. https://doi.org/10.3390/vetsci9080397








