Simple summary
One of the limitations of vaccine development against E. canis infection is the indefinite knowledge of the protective immunity in the host. In this study, recombinant protein GP19 was produced as a vaccine prototype, rGP19, for inducing protective immune responses in a mouse model against E. canis. Antibody responses against E. canis were evaluated and revealed that the immunized mice with rGP19 showed higher antibody levels than in adjuvant-immunized and naive mice, both pre- and post-challenging with E. canis. DNA from blood, liver, and spleen were extracted to determine ehrlichial loads. The rGP19-immunized mice showed significantly lower ehrlichial loads in blood, liver, and spleen DNA compared with adjuvant-immunized mice. This study also detected IFN-γ-producing CD4+ T cells in the rGP19-immunized mice and then were later infected with E. canis on day 14 of the post-infection period using flow cytometry. Additionally, Cytokine mRNA expression was investigated and revealed up-regulation of IFNG and IL1 mRNA expression in rGP19-immunized mice. The present study provides evidence of rGP19 that can eliminate E. canis by manipulating both humoral and cell-mediated immune responses in the laboratory animal model.
Abstract
The intracellular bacterium Ehrlichia canis is the causative pathogen of canine monocytic ehrlichiosis (CME) in dogs. Despite its veterinary and medical importance, there is currently no available vaccine against this pathogen. In this study, the recombinant GP19 (rGP19) was produced and used as a recombinant vaccine prototype in a mouse model against experimental E. canis infection. The efficacy of the rGP19 vaccine prototype in the part of stimulating B and T cell responses and conferring protection in mice later challenged with E. canis pathogen were evaluated. The rGP19-specific antibody response was evaluated by ELISA after E. canis challenge exposure (on days 0, 7, and 14 post-challenge), and demonstrated significantly higher mean antibody levels in rGP19-immunized mice compared with adjuvant-immunized and naive mice. Significantly lower ehrlichial loads in blood, liver, and spleen DNA samples were detected in the immunized mice with rGP19 by qPCR. The up-regulation of IFNG and IL1 mRNA expression were observed in mice immunized with rGP19. In addition, this study detected IFN-γ-producing memory CD4+ T cells in the rGP19-immunized mice and later infected with E. canis on day 14 post-infection period using flow cytometry. The present study provided a piece of evidence that rGP19 may eliminate E. canis by manipulating Th1 and B cell roles and demonstrated a promising strategy in vaccine development against E. canis infection in the definitive host for further study.
1. Introduction
Canine monocytic ehrlichiosis (CME) is one of the major infectious diseases for dogs, globally. CME is a rickettsial disease which leads to a multi-systemic disease in dogs, caused by Ehrlichia canis. In addition, there were shreds of evidence of E. canis infection reported in wild mice and humans [1,2]. Despite its potential veterinary and medical importance, there is currently no commercial vaccine against E. canis. Previous studies have shown partial clinical protection of inactivated and attenuated vaccine candidates after being challenged with the virulent strain [3,4]. One of the disadvantages of primitive inactivated and live-attenuated vaccines is the undesirable effects associated with some of the antigenic proteins, this has led to the need for the development of modern vaccines [5,6]. In Ehrlichia spp., recombinant P29 protein was developed and substantiated an ability to protect against Ehrlichia muris in mice [6]; however, there is no available information on this type of vaccine against E. canis infection.
Ehrlichia spp. have been characterized by their reactivity with antibodies from infected hosts, several antigens have been identified [6,7]. One of the noticeably major immunoreactive proteins of E. canis, 19-kDa glycoprotein (GP19) is detected with dog sera during the acute phase of canine monocytic ehrlichiosis. The GP19 was highly conserved among the geographically distributed E. canis strains [7,8,9,10]. GP19 of E. canis was found to have orthologs with variable-length PCR target proteins (VLPT) of Ehrlichia chaffeensis, but without the tandem repeats (TRs) that were present in E. chaffeensis VLPT [11]. Furthermore, E. canis GP19 protein consists of a serine/threonine/glutamate (STE)-rich patch at the amino-terminal that contains a major species-specific antibody epitope recognized by the host [11]. Although this protein seems to play an essential role in host-microbe interaction, the information on an application using GP19 as a vaccine candidate against E. canis infection is not available.
In the present study, recombinant protein GP19 (rGP19) was produced and purified to use as a vaccine prototype against E. canis infection in mice. The efficacy of the rGP19 vaccine candidate was investigated in the protection and immune response after mice were challenged with E. canis pathogen at the in vivo level. There was no report that E. canis causes disease in mice; however, E. canis infection in wild mouse and cultivation in BALB/c mouse macrophage cells were reported [1,12]. A mouse model was used in this study for immunization and challenge infection at the laboratory level, indicating the possibility of this vaccine being used in animals, especially dogs which are the definitive host, or for further industrial production. The ehrlichial loads were determined after E. canis challenge exposure using real-time PCR. Likewise, the cytokine gene expression in mice derived from GP19 peptide-based vaccine was explored. In addition, the frequencies of E. canis rGP19-specific IFN-γ-producing CD4+ and CD8+ of mice immunized with or without rGP19 were investigated as well using flow cytometry.
2. Materials and Methods
2.1. E. canis Organism Cultivation and Stocks
E. canis BF W053712 X + 5 (PTA-5811™, ATCC®, Manassas, VA, USA) was cultured in DH82 canine macrophage cells at 37 °C and 5% CO2 as previously described [13]. DH82 cells with intracellular bacteria, E. canis, were propagated until 80% of infected cells were reached. A number of the 1 × 106 infected DH82 cells were collected and stored at −80 °C until they were used. For infection, ehrlichial stocks were used for experimental inoculation of the mice by the intraperitoneal route (i.p.) as described previously [6].
2.2. Mice
The mice used in this study were 6- to 8-week-old female BALB/c 40 mice, which were obtained from the National Laboratory Animal Center at Mahidol University, Thailand. All mice were acclimated for 1 week before starting the experiment. The experiment was carried out under the animal biosafety condition at 21 ± 1 °C with 50 ± 10% relative humidity and a 12 h light and dark cycle. The mice were provided laboratory grade of food and water, ad libitum. The experimental procedures for the mouse model were approved by the Institutional Biosafety Committee (IBC), Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand (IBC Approved no. A-0763001). All experiments using animals were performed at Laboratory Animal Center (Office of Research Administration, Chiang Mai University) with the approval of Animal Use Protocol (AUP) for Permission of Animal Care and Use (code: 2563/MC-0008).
2.3. Expression of Recombinant E. canis GP19 Proteins
Ehrlichia open reading frame (ORF) gp19-cm03 (GenBank accession no. MF771088) was directionally cloned into pET30a (Genscript, Piscataway, NJ, USA). The Sequence analysis was performed to verify the appropriate frame of the insert. The plasmids were transformed into BL21 Star™ (DE3) (Invitrogen™, Thermo Fisher Scientific, Waltham, MA, USA) for producing the recombinant protein (rGP19). Briefly, the competent cells with a recombinant GP19 pET30a plasmid, were cultured in Luria–Bertani (LB) broth containing the kanamycin at 37 °C for 12–16 h and shaken at 200 rpm until the cell count reached the optical density (OD) at a wavelength of 600 nm reached 0.5–0.6. Then, Isopropyl-β-D-thiogalactopyranoside (IPTG) solution was added to the cells to a final concentration 0.5 mM and continually shaken at 125 rpm at 16 °C for another 16 h. Cell pellets were then collected by centrifugation at 4000× g for 20 min. Only the sedimented cells were collected for protein extraction by lysis solution at a ratio of 5 mL per 1 g of coagulant cells, shaken at 75 rpm at room temperature for 60 min, and centrifuged at 10,000× g for 20 min at 4 °C. The solution was collected for protein extraction.
For the vaccine prototype, rGP19 was purified by Ni-NTA affinity chromatography using HisTrap HP columns (GE Healthcare Life Sciences, Piscataway, NJ, USA). The protein size was then isolated by electrophoresis (NATIVEN®; ATTO Gentaur, Tokyo, Japan). SDS-PAGE and Western immunoblotting were performed to verify the rGP19 molecular mass and the HRP binding attribute. The protein concentration was measured by the BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) as described in the instruction manual, before processing of immunogen formulation and immunization in the further step.
2.4. Vaccine Immunizations and E. canis Challenge
BALB/c mice were used in this study for animal immunization and challenge infection. A total of 40 BALB/c mice, 6 to 8 weeks old were categorized into eight groups based on the immunogen formulations (n = 5 in each group). Groups 1 to 6, mice were immunized with rGP19 (0 µg, 50 µg and 100 µg, respectively) in Montanide adjuvant (Montanide™ ISA, SEPPIC, Paris, France), while groups 7 to 8 are non-immunized groups (control groups). Mice were intraperitoneally (i.p.) immunized, followed by a booster at 2 weeks from primary immunization. Except for groups 7 and 8, mice were challenged with E. canis (1 × 104 E. canis) that were grown in DH82 cells. The challenge was performed after the booster immunization for 4 weeks by i.p. route and then observed daily. Five mice in each group were sacrificed on days 7 and 14 post-challenge (Table 1). Whole blood samples, sera, liver, and spleen were collected for further procedure. All procedures using mice including challenge exposure, and blood and organ collection were performed in the laminar flow biological cabinet of Biosafety level 2.

Table 1.
Immunization and Ehrlichia canis challenge experiments in mice.
2.5. Measurement of rGP19 Antibody Elicits in Mice
Sera of all mice groups were collected pre- and post-E. canis challenging to determine antibody titers. An in-house indirect ELISA was performed, a concentration of 10 µg/ml rGP19 coated the immunoplates. The plates were incubated with the individual mice sera with the dilution of 1:1000. HRP conjugated rabbit anti-Mouse IgG (InvitrogenTM, Thermo Fisher Scientific, Waltham, MA, USA) was used as the secondary antibody at a dilution of 1:5000. Uncoated wells were used as blanks. The data were expressed as means with standard errors measured at the optical density (OD) of 450 nm.
2.6. Assessment of Ehrlichial Load in Organs by Real-Time PCR
Blood, liver, and spleen samples of the mice were obtained to extract the genomic DNA following the manufacturer’s instructions. Quantitative estimation of the ehrlichial load was performed using the quantitative PCR (qPCR) E. canis-16S rRNA, as performed in a previous study [14]. A total of 100 ng of DNA was used for each reaction. The qPCR reaction was performed using a SensiFAST SYBR Lo-ROX Kit (Bioline, London, UK) according to the manufacturer, and the reaction was the procedure under CFX96 Touch™ Real-Time PCR (Bio-Rad Laboratories, Hercules, CA, USA). A negative result for ehrlichial DNA was identified if the critical threshold values (Ct) of the qPCR reaction exceed 40 cycles. The expression analysis of the ehrlichial load was normalized relative to the total DNA.
2.7. Relevance of rGP19 on Cytokine mRNA Expression by qPCR
For investigation of the effects of rGP19 recombinant protein vaccine on cytokine expression, cytokine genes related to monocytes, macrophages, T cells, and B cells were quantitatively analyzed with qPCR as well. RNA in whole blood samples of each mouse was isolated and then used in cDNA synthesis for quantitative analysis of the mRNA transcriptions of gene coding that involved cytokine, including interferon-gamma (IFNG), interleukin 2 (IL-1), interleukin 4 (IL-4), interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF), with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. The expression levels (fold-difference) were reported using the 2−ΔΔCt method [15]. Specific information regarding the oligonucleotide sequences of primers used in this part of the study is presented in Table 2.

Table 2.
PCR and oligonucleotide sequences of primers targeting cytokine genes used in this experiment.
2.8. Preparation of Mice Splenocyte and Flow Cytometry Analysis
The frequencies of E. canis GP19-specific IFN-γ-producing T cells in mice spleens were investigated using flow cytometry modified from previous studies [16,17]. A number of 2 × 106 cells of the splenocytes from individual mouse were isolated and then cultured in a 12-well culture plate in cell culture media, containing RPMI 1640 media (GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% inactivated fetal bovine serum (FBS; GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) and 1% antibiotic-antimycotic (GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) in the presence of three stimuli, including E. canis GP19 antigen (2 µg/mL), cultured media (negative control), or 1x cell stimulation cocktail (eBioscience™, San Diego, CA, USA) containing phorbol 12-myristate 13-acetate (PMA) and ionomycin (positive control). After 18 h of in vitro stimulation, the cells were harvested and then followed by 4 h of incubation with brefeldin A (BD GolgiPlug; BD Biosciences, San Diego, CA, USA). Fc receptors were blocked by adding 20 µL per test of anti-Fc II/III receptor monoclonal antibodies (MAbs) (BD Biosciences) to the sample and then incubate on ice for 20 min. Without washing, the splenocytes then were labeled with fluorescent-conjugated antibodies (eBioscience™, San Diego, CA, USA) for mice, including CD3 (APC; clone 17A2) for 0.5 μg/test, CD4 (FITC; clone RM4–5) for 0.25 μg/test, and CD8 (PerCP Cyn5.5; clone 53–6.7) for 0.25 μg/test of surface molecules. The cells were incubated on ice and protected from light for 60 min, and then washed two times with a flow cytometry staining buffer (cold PBS + 1% FBS) and centrifuged. The cells were resuspended in serum/protein-free PBS and labeled with a viability dye (LIVE/DEAD™ Fixable Dead Cell Stain Kits; Thermo Fisher Scientific, Waltham, MA, USA) on ice and protected from light for 30 min.
For intracellular IFN-γ straining, the splenocytes were fixed and stained with IFN-γ MAbs (PE; clone XMG1.2) for 0.25 μg/test. The sample acquisition (50,000 events) was performed on IFN-γ-containing CD4+ and CD8+ cells using a CyAnTM ADP Flow Cytometer (Beckman Coulter, Brea, CA, USA). The frequencies of antigen-specific IFN-γ-producing T cells in the spleens of the mice were investigated after the background staining of unstimulated cells was subtracted.
2.9. Data Analysis
The data in this study were analyzed for outliers and the normality test was performed prior to performing statistical analysis. The statistical analysis was performed using one-way ANOVA or the multiple t-test using GraphPad Prism 8.2.0 (GraphPad Software, Inc., San Diego, CA, USA). Multiple comparisons were analyzed using the Holm-Sidak method. Results of statistical analyses were considered significant in all experiments when p < 0.05. Results were reported as the mean and standard error (SE) or median value.
3. Results
3.1. Recombinant GP19 Protein (rGP19) Production
The purified rGP19 were examined and analyzed by Western blot. The rGP19 displayed the characteristic of reactive protein bands at a target molecular mass approximately 20–25 kDa (Figure 1; the original figure was provided as Figure S1 of supplementary materials). The result provided evidence of successful expression of rGP19 and the specificity of the HRP-conjugated antibody to the synthetic rGP19.
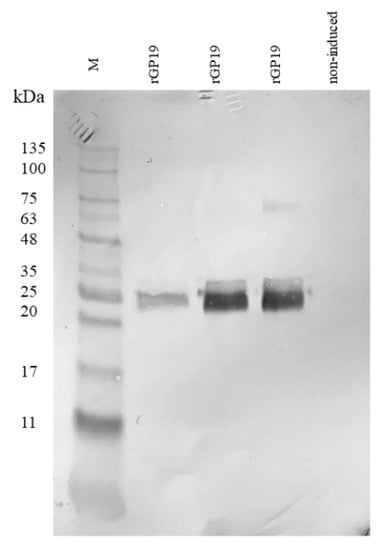
Figure 1.
Recombinant protein of Ehrlichia canis (rGP19) expression was affirmed with the HRP conjugated IgG antibody against rGP19 in Western blot analysis. Lane M: protein marker; rGP19: recombinant protein GP19 induced with IPTG; non-induced: non-induced rGP19 with IPTG.
3.2. rGP19 Vaccine Prototypes Induce a Strong Antibody Response in Mice
Analysis of specific anti-rGP19 responses by ELISA on days 0, 7, and 14 after E. canis challenging demonstrated that the mean antibody levels in immunized mice (50 µg and 100 µg of rGP19) were statistically significantly higher (p < 0.01) than those immunized with only adjuvant and naïve mice, pre- and post-challenge with E. canis (Figure 2).
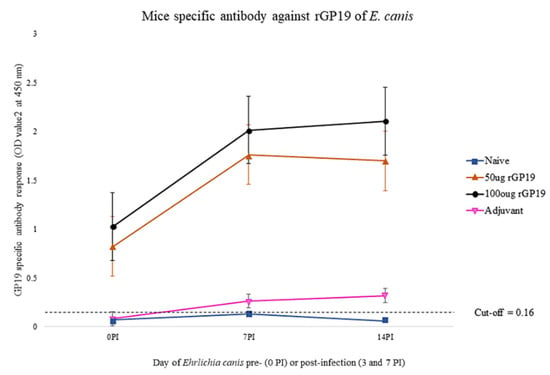
Figure 2.
The association between antibody responses induced by the rGP19 vaccine and the development of antigen-specific antibody responses, pre- and post-E. canis infection in mice. rGP19 vaccine prototypes provide the protection against the pathogen measured by an ELISA using a purified rGP19 as the antigen on day 0 (pre-Ehrlichia canis challenging), and days 7 and 14 (post-E. canis challenging).
With the results, antibody response induced by the rGP19 vaccine prototypes was associated with the development of antigen-specific antibody responses.
3.3. rGP19 Vaccine Prototypes Provide the Protection against the Pathogen
There was no evidence of bacterial loads in the naïve mice groups (groups 7 and 8). The protection against E. canis was evaluated with E. canis challenging and determined the bacterial copy number by real-time PCR at different days post-challenge. The results showed lower (p < 0.01) E. canis load in blood, liver, and spleen on days 7 and 14 post-challenge in the immunized mice (both 50 µg and 100 µg) compared with the adjuvant-immunized mice (Figure 3).
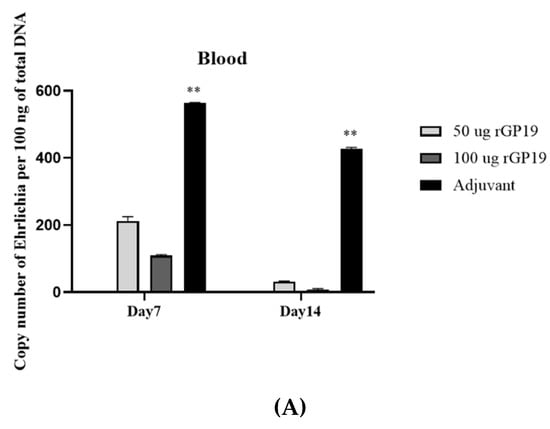
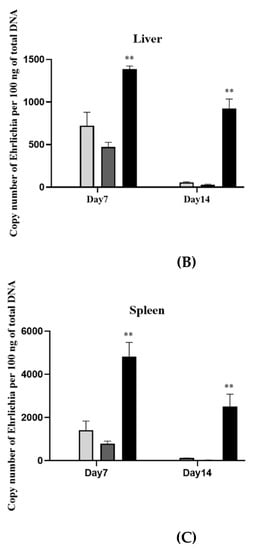
Figure 3.
Immunization with rGP19 provided protection against Ehrlichia canis infection in mice. The protection against E. canis infection in the immunized mice with rGP19 was determined by the ehrlichial load in the blood (A), liver (B), and spleen (C) samples measured by quantitative PCR (qPCR) and showed lower ehrlichial loads on days 7 and 14 post-challenge (** p < 0.01) compared with mice immunized with adjuvant.
3.4. Relevance of rGP19 on Cytokine-Relative Gene Expression by qPCR
Cytokine gene expression levels in naive mice and mice challenged with E. canis were investigated, including IFNG, IL1a, IL4, IL-6, and TNF genes. On day 7 post-infection period, cytokine gene expression levels showed overall no different expression among the mice group, except for IFNG. The immunized mice with 100 µg of rGP19 and adjuvant showed a higher expression level of IFNG than other groups (p < 0.05).
On day 14 post-infection period, the immunized mice with rGP19 (both 50 µg and 100 µg combined with Montanide adjuvant) and later infected with E. canis showed significantly up-regulated expression of IFNG (p < 0.01), IL1a (p < 0.01), and IL4 (p < 0.05) compared with naive mice on day 14 post-infection, as shown in Figure 4.
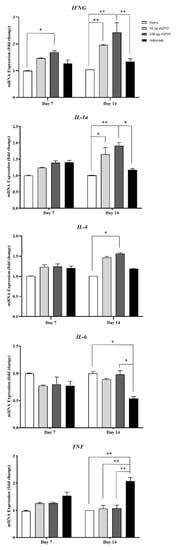
Figure 4.
Analyses of cytokine mRNA expression in mice using qPCR. Bar graphs represent relative IFNG, IL-1a, IL-4, IL-6, and TNF mRNA expression levels after normalization to the GAPDH gene in naive and immunized mice with later Ehrlichia canis challenge group (50 µg rGP19, 100 µg rGP19, and adjuvant). Data are represented as mean ± SE (n = 5 each treatment), one-way ANOVA, * p < 0.05, ** p < 0.01.
The results revealed that the immunized mice with Montanide adjuvant and later infected with E. canis had significantly higher TNF gene expression (p < 0.01) than other mice groups 14 days after infection. In contrast, IL6 gene expression of adjuvant immunized, and later infected mice showed down-regulation compared with other mice groups.
3.5. rGP19 Manipulated Specific Memory CD4+ Th1 Responses during E. canis Infection
The immunized and non-immunized mice displayed no difference in the frequencies of IFN-γ-producing CD8+ T cells in splenocytes after being stimulated with the E. canis rGP19 antigen. In the part of CD4+ T cells, there is no difference among mice splenocytes that were stimulated with PMA, cell media, or E. canis rGP19 antigen on day 7 post-infection (Figure 5A).
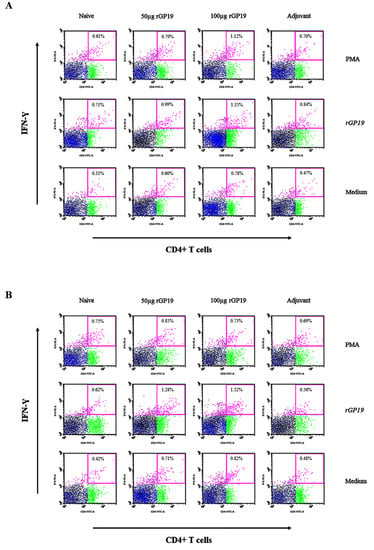
Figure 5.
Ehrlichia canis GP19-specific IFN-γ-producing CD4+ T cells develop during E. canis infection in mouse splenocyte determined by flow cytometry. Naive and immunized mice infected with E. canis over a period of 7 days (A) and 14 days (B) post-infection. The data plot represents the populations of lymphocytes (black dot), CD3+ T lymphocytes (blue dot), CD4+ T lymphocytes (green dot), and IFN-γ molecules in CD4+ T lymphocytes (pink dot).
However, there are significantly different (p = 0.011) splenocytes on day 14 post-infection when stimulated E. canis antigen on day 14 post-infection. Compared with uninfected na-ive mice, the immunized mice (with 50 µg and 100 µg of rGP19) had significantly higher frequencies of IFN-γ-producing CD4+ T cells in splenocytes (Figure 5B and Figure 6).
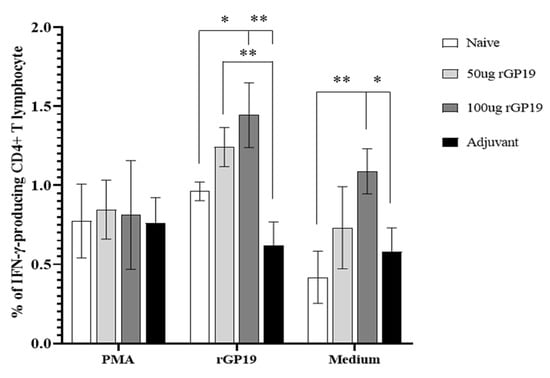
Figure 6.
Mean of the frequencies of IFN-γ-producing CD4+ T lymphocytes in the splenocytes of mice on days 14 post-Ehrlichia canis infection when stimulated with different treatments. Data are represented as mean ± SE, one-way ANOVA, * p < 0.05, ** p < 0.01.
4. Discussion
GP19 of E. canis was found to have orthologs with variable-length PCR target proteins (VLPT) of E. chaffeensis, but it lacked the tandem repeats (TRs) that were present in E. chaffeensis VLPT [11]. Moreover, the GP19 protein exhibited O-linked glycosylation sites that were stated in the STE-rich patch. The amino-terminus of the STE-rich patch consisted of an epitope recognized by the host [11]. Although this protein seems to play an important role in host-microbe interaction, the information of the application using GP19 as a vaccine candidate against E. canis infection is not available.
In this present study, the ability of recombinant GP19 protein vaccine in protecting mice against E. canis infection was analyzed. The rGP19 at the molecular mass of approximately 20–25 kDa was used as a prototype vaccine against E. canis infection in mice. The protection against E. canis infection after challenge exposure for 7 days and 14 days was explored and revealed that the mean antibody levels in immunized mice (50 µg and 100 µg of rGP19) were statistically significantly higher than immunized with only adjuvant and naive mice, pre- and post-challenge with E. canis. Interestingly, the elevated antibodies in rGP19-immunized mice were along with the lower ehrlichial load in blood, liver, and spleen on days 7 and 14 post-challenge compared with the adjuvant-immunized mice detected by qPCR. The results of the protection role by reduction in E. canis in the rGP19-immunized mice in this study showed similarity to the previous studies of the protection role of P28 against Ehrlichia in mice, E. cheffeensis, and E. muris [16,18,19,20]. A previous study demonstrated the spontaneous clearance of E. cheffeensis infection in the BALB/c mice that were immunized with the rP28 [18]. Moreover, the results in this study supported the possibility of humoral immunity role in the part of the protection in mice challenged with E. canis in the rGP19-immunized mice.
The activation of phagocytes and cell-mediated immune response plays major roles against Ehrlichia with various soluble cell products [21,22]. There was the possibility of rGP19 provoked CD4+ memory T cell responses due to the detection of the antigen-specific IFN-γ-producing memory CD4+ T cells in the rGP19-immunized mice later infected with E. canis on day 14 post-infection period. The results at the cellular level in this study provided evidence of rGP19 that can eliminate E. canis by manipulating Th1 and B cell roles, similar to the previous studies that determined the effect of structural-based peptide and recombinant P28 against E. muris [16,17].
Cytokines are cell-signaling molecules that are synthesized by different immune cells. Specific receptors on the target cells are bonded with the cytokines, and then trigger signal-transduction pathways that are involved in innate and adaptive immunity to defend against pathogens or disease conditions [23,24]. Accordingly, cytokines are imperative for the phenomenon of immunity, including their role in developing and regulating [25]. The results in the part of in vivo of this study provide a perspective on IFNG genes that involved macrophage-Th1 cells, according to in vitro study. The IFNG expression of immunized mice with 100 µg of rGP19 displayed significantly up-regulated levels since day 7 post-challenge of E. canis. The result confirmed the possibility that IFNG expression in the rGP19-immunized mice infected with E. canis can be related to the elimination of the microorganism, E. canis.
IL-4 is one of the cytokines involved with the Th2 response. The inactivated vaccine of Ehrlichia ruminantium provided the elicit of CD4+ and CD8+ to produce IFN-γ; however, there was an absence of IL-4 in a previous study [26]. The results from the previous study indicated the type 1 response that was influenced by the inactivated vaccine [26]. Contrastingly, the IL4 gene expression of the 100 µg of rGP19-immunized mice later infected with E. canis in vivo in this study displayed a positive relationship with IFN on day 14 post-infection period. The animal model showed the possibility that rGP19 might stimulate both Th1 and Th2 responses. Thus, the investigation of the cell-mediated immune response including type 1 and 2 cytokines in immunization with the GP19 peptide and protein in animal models is further needed for the full understanding of the protective ability.
TNF-α is a pro-inflammatory cytokine produced predominantly by macrophages [27,28]. The TNF gene in mouse blood samples showed up-regulated expression in the E. canis-infected mice with the absence of rGP19 immunization on day 14 of the post-infection period. In addition, IL6 gene encoding of IL-6 is one of the pro-inflammatory and anti-inflammatory myokines mediated through its inhibitory effects on TNF-alpha activation, which showed a negative relationship with TNF expression. Importantly, IL-1 which is expressed in various immune cells, including macrophages, monocytes, dendritic cells, B cells, NK cells, and epithelial cells, promotes adaptive immunity [29,30]. In this study, rGP19 non-immunized and immunized mice with/without E. canis challenge were investigated in the IL1a encoding IL-1α. The rGP19-immunized mice (50 µg and 100 µg) later infected with E. canis showed up-regulation of IL1a compared with naive mice on day 14 post-infection period, according to the T cell responses examined by flow cytometry. However, the relationship between cytokine genes and adaptive immune response needs further study for the rational development of vaccines.
Our analyses of the cytokine networks suggest that there are supplementary cytokines involved in the E. canis infection responses with the cooperation of macrophages and lymphocytes (T and B cells). The possible linkages of cytokine expressions resulting from macrophages and cell-mediated immune response against intracellular microorganisms including E. canis are shown in Figure 7.
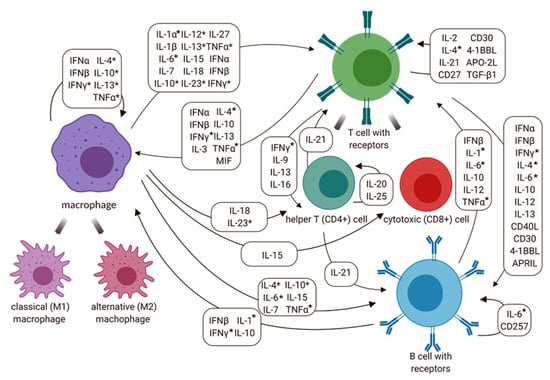
Figure 7.
Cytokine network involved macrophage, T cell (CD4+ and CD8+) and B cell activation in the immune response against Ehrlichia canis. Abbreviations: 4-1BBL: 4-1BB ligand; APRIL: a proliferation-inducing ligand; CD: cluster of differentiation; IFN: interferon; IL-: interleukin; MIF: macrophage migration inhibitory factor; TNF: tumor necrosis factor; TGF: transforming growth factor. Additionally, * represents the cytokine expression at the molecular level in this study and previous study [13].
5. Conclusions
The limitation of vaccine development against E. canis infection is that the knowledge of the protective immunity of the host is still unknown. In this study, recombinant protein GP19 was produced as a vaccine prototype, rGP19, for inducing protective immune responses in a mouse model against E. canis. Antibody responses against E. canis were evaluated and revealed that the antibody levels in rGP19-immunized mice were significantly higher than adjuvant-immunized and naive mice, pre- and post-E. canis challenging. DNA from blood, liver, and spleen were extracted to determine ehrlichial loads. The rGP19-immunized mice showed significantly lower ehrlichial loads in blood, liver, and spleen DNA compared with adjuvant-immunized mice. This study also provided the detection of IFN-γ-producing memory CD4+ T cells in the rGP19-immunized mice later infected with E. canis on day 14 post-infection period using flow cytometry. Additionally, Cytokine expression was investigated and revealed up-regulation of IFNG and IL1 mRNA expression in rGP19-immunized mice. The present studies provided evidence of rGP19 that can eliminate E. canis by manipulating both humoral and phagocyte cell-mediated immune responses in a laboratory animal model.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci9080386/s1, Figure S1: Recombinant protein of Ehrlichia canis (rGP19) expression affirmed with Western blot analysis.
Author Contributions
Conceptualization, B.N. and N.S.; methodology, B.N., S.T. (Saruda Tiwanathagorn) and N.S.; software, B.N.; validation, S.T.(Sahatchai Tangtrongsup), S.T. (Saruda Tiwananthagorn) and N.S.; formal analysis, B.N. and N.S.; investigation, B.N., A.M., A.R., P.K., P.C. and K.S.; resources, B.N. and N.S.; data curation, B.N. and N.S.; writing—original draft preparation, B.N.; writing—review and editing, N.S.; visualization, B.N.; supervision, S.T. (Sahatchai Tangtronsup), S.T. (Saruda Tiwananthagorn) and N.S.; project administration, N.S.; funding acquisition, B.N. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Research and Researchers for Industries (RRi), Thailand Research Fund (TRF), grant no. PHD58I0063. This work was also supported by the National Research Council of Thailand, grant no. FF65/089, and partial funded by the Center of Excellence in Veterinary Bioscience, Chiang Mai University (grant number 08/2565).
Institutional Review Board Statement
The study was conducted according to the guidelines and approved by the Chiang Mai University-Animal Care and Use Committee (CMU-ACUC) approval number 2563/MC-0008 and Chiang Mai University Institutional Biosafety Committee (CMU-IBC) approval number CMUIBC A-0763001.
Informed Consent Statement
Informed consent was obtained from the animals’ owner.
Data Availability Statement
All data in this study are included in the manuscript.
Acknowledgments
The authors would like to acknowledge the staff of the Laboratory Animal Center, Office of Research Administration, Chiang Mai University, for facilitating the experiment and taking care of the laboratory animals according to animal welfare principles.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kawahara, M.; Suto, C.; Rikihisa, Y.; Yamamoto, S.; Tsuboi, Y. Characterization of ehrlichial organisms isolated from a wild mouse. J. Clin. Microbiol. 1993, 31, 89–96. [Google Scholar] [CrossRef]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mahan, S.; Kelly, P.J.; Mahan, S.M. A preliminary study to evaluate the immune responses induced by immunization of dogs with inactivated Ehrlichia canis organisms. Onderstepoort J. Vet. Res. 2005, 72, 119–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rudoler, N.; Baneth, G.; Eyal, O.; van Straten, M.; Harrus, S. Evaluation of an attenuated strain of Ehrlichia canis as a vaccine for canine monocytic ehrlichiosis. Vaccine 2012, 31, 226–233. [Google Scholar] [CrossRef]
- Shams, H. Recent developments in veterinary vaccinology. Vet. J. 2005, 170, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Thirumalapura, N.R.; Crocquet-Valdes, P.A.; Saito, T.B.; Thomas, S.; McBride, J.W.; Walker, D.H. Recombinant Ehrlichia P29 protein induces a protective immune response in a mouse model of ehrlichiosis. Vaccine 2013, 31, 5960–5967. [Google Scholar] [CrossRef][Green Version]
- McBride, J.W.; Corstvet, R.E.; Gaunt, S.D.; Boudreaux, C.; Guedry, T.; Walker, D.H. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect. Immun. 2003, 71, 2516–2524. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, T.; Keysary, A.; Baneth, G.; Miyashiro, S.; Strenger, C.; Waner, T.; McBride, J.W. Genetic and antigenic diversities of major immunoreactive proteins in globally distributed Ehrlichia canis strains. Clin. Vaccine Immunol. 2008, 15, 1080–1088. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Lee, C.C.; Tsang, C.L.; Chung, Y.T. Detection and characterization of four novel genotypes of Ehrlichia canis from dogs. Vet. Microbiol. 2010, 146, 70–75. [Google Scholar] [CrossRef]
- Nambooppha, B.; Rittipornlertrak, A.; Tattiyapong, M.; Tangtrongsup, S.; Tiwananthagorn, S.; Chung, Y.T.; Sthitmatee, N. Two different genogroups of Ehrlichia canis from dogs in Thailand using immunodominant protein genes. Infect. Genet. Evol. 2018, 63, 116–125. [Google Scholar] [CrossRef]
- McBride, J.W.; Doyle, C.K.; Zhang, X.; Cardenas, A.M.; Popov, V.L.; Nethery, K.A.; Woods, M.E. Identification of a glycosylated Ehrlichia canis 19-kilodalton major immunoreactive protein with a species-specific serine-rich glycopeptide epitope. Infect. Immun. 2007, 75, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Keysary, A.; Waner, T.; Strenger, C.; Harrus, S. Cultivation of Ehrlichia canis in a continuous BALB/C mouse macrophage cell culture line. J. Vet. Diagn. Investig. 2001, 13, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Nambooppha, B.; Rittipornlertrak, A.; Muenthaisong, A.; Koonyosying, P.; Tangtrongsup, S.; Tiwananthagorn, S.; Chung, Y.T.; Sthitmatee, N. Effect of GP19 peptide hyperimmune antiserum on activated macrophage during Ehrlichia canis infection in canine macrophage-like cells. Animals 2021, 11, 2310. [Google Scholar] [CrossRef]
- Peleg, O.; Baneth, G.; Eyal, O.; Inbar, J.; Harrus, S. Multiplex real-time qPCR for the detection of Ehrlichia canis and Babesia canis vogeli. Vet. Parasitol. 2010, 173, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Crocquet-Valdes, P.A.; Thirumalapura, N.R.; Ismail, N.; Yu, X.; Saito, T.B.; Stevenson, H.L.; Pietzsch, C.A.; Thomas, S.; Walker, D.H. Immunization with Ehrlichia P28 outer membrane proteins confers protection in a mouse model of ehrlichiosis. Clin. Vaccine Immunol. 2011, 18, 2018–2025. [Google Scholar] [CrossRef]
- Thomas, S.; Thirumalapura, N.R.; Crocquet-Valdes, P.A.; Luxon, B.A.; Walker, D.H. Structure-based vaccines provide protection in a mouse model of ehrlichiosis. PLoS ONE 2011, 6, e27981. [Google Scholar] [CrossRef]
- Ohashi, N.; Zhi, N.; Zhang, Y.; Rikihisa, Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 1998, 66, 132–139. [Google Scholar] [CrossRef]
- Li, J.S.; Yager, E.; Reilly, M.; Freeman, C.; Reddy, G.R.; Reilly, A.A.; Chu, F.K.; Winslow, G.M. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 2001, 166, 1855–1862. [Google Scholar] [CrossRef]
- Nandi, B.; Hogle, K.; Vitko, N.; Winslow, G.M. CD4 T-cell epitopes associated with protective immunity induced following vaccination of mice with an ehrlichial variable outer membrane protein. Infect. Immun. 2007, 75, 5453–5459. [Google Scholar] [CrossRef]
- Barnewall, R.E.; Rikihisa, Y. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron-transferrin. Infect. Immun. 1994, 62, 4804–4810. [Google Scholar] [CrossRef]
- Tajima, T.; Wada, M. Inhibitory effect of interferon gamma on frequency of Ehrlichia canis-infected cells in vitro. Vet. Immunol. Immunopathol. 2013, 156, 200–204. [Google Scholar] [CrossRef]
- Bach, E.A.; Aguet, M.; Schreiber, R.D. The IFN gamma receptor: A paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 1997, 15, 563–591. [Google Scholar] [CrossRef]
- Dhama, K.; Mahendran, M.; Chauhan, R.S.; Tomar, S. Cytokines: Their functional roles and prospective in veterinary practice—A review. Vet. Immunol. Immunopathol. 2008, 10, 79–89. [Google Scholar]
- Ferreira, V.L.; Borba, H.H.L.; Bonetti, A.F.; Leonart, L.P.; Pontarolo, R. Cytokines and Interferons: Types and Functions. Autoantibodies Cytokines 2018, 13, 65–87. [Google Scholar]
- Esteves, I.; Vachiéry, N.; Martinez, D.; Totté, P. Analysis of Ehrlichia ruminantium–specific T1/T2 responses during vaccination with a protective killed vaccine and challenge of goats. Parasite Immunol. 2004, 26, 95–103. [Google Scholar] [CrossRef]
- Riches, D.W.H.; Chan, E.D.; Winston, B.W. Chapter 7—TNF-α-induced regulation and signalling in macrophages. Immunobiology 1996, 195, 477–490. [Google Scholar] [CrossRef]
- Varma, T.K.; Toliver-Kinsky, T.E.; Lin, C.Y.; Koutrouvelis, A.P.; Nichols, J.E.; Sherwood, E.R. Cellular mechanisms that cause suppressed gamma interferon secretion in endotoxin-tolerant mice. Infect. Immun. 2001, 69, 5249–5263. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nature. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eeckhout, B.; Tavernier, J.; Gerlo, S. Interleukin-1 as innate mediator of T cell Immunity. Front. Immunol. 2011, 11, 621931. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).