Disseminated Nocardiosis Caused by Nocardia farcinica in Two Puppy Siblings
Abstract
Simple Summary
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical History and Presentation
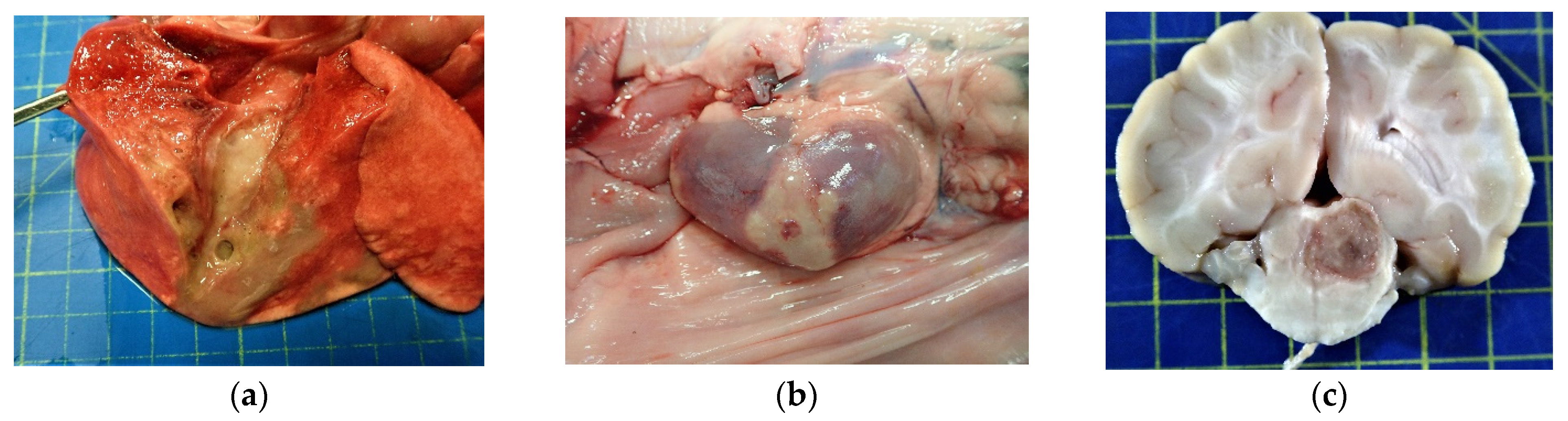
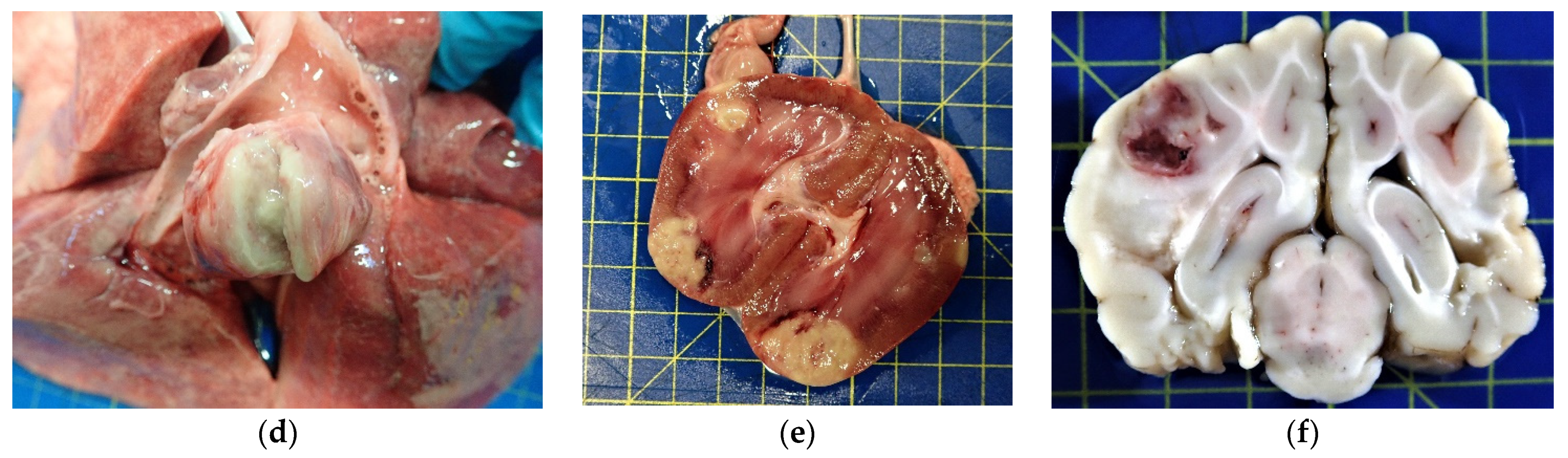
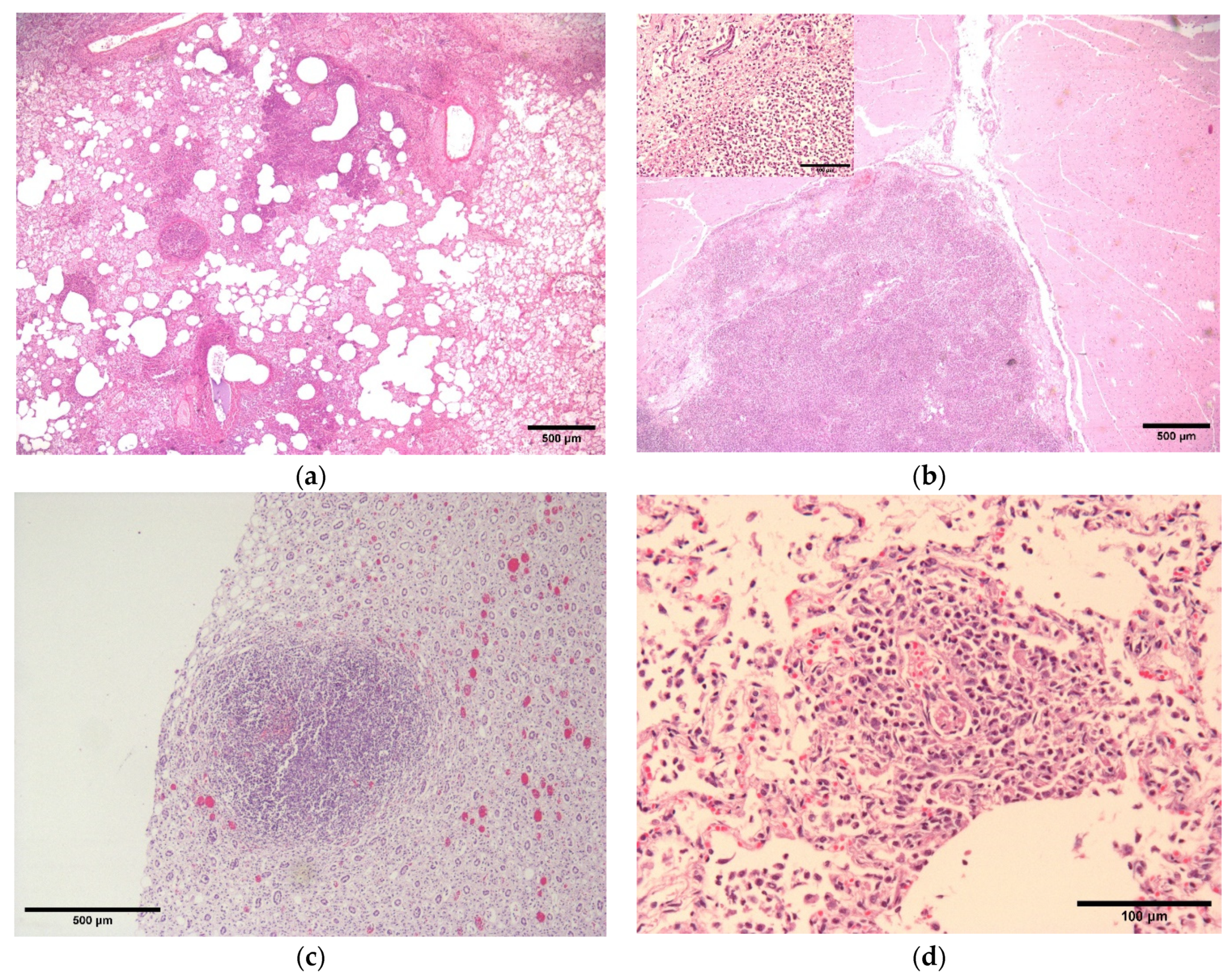
2.2. Pathology and Immunohistochemistry
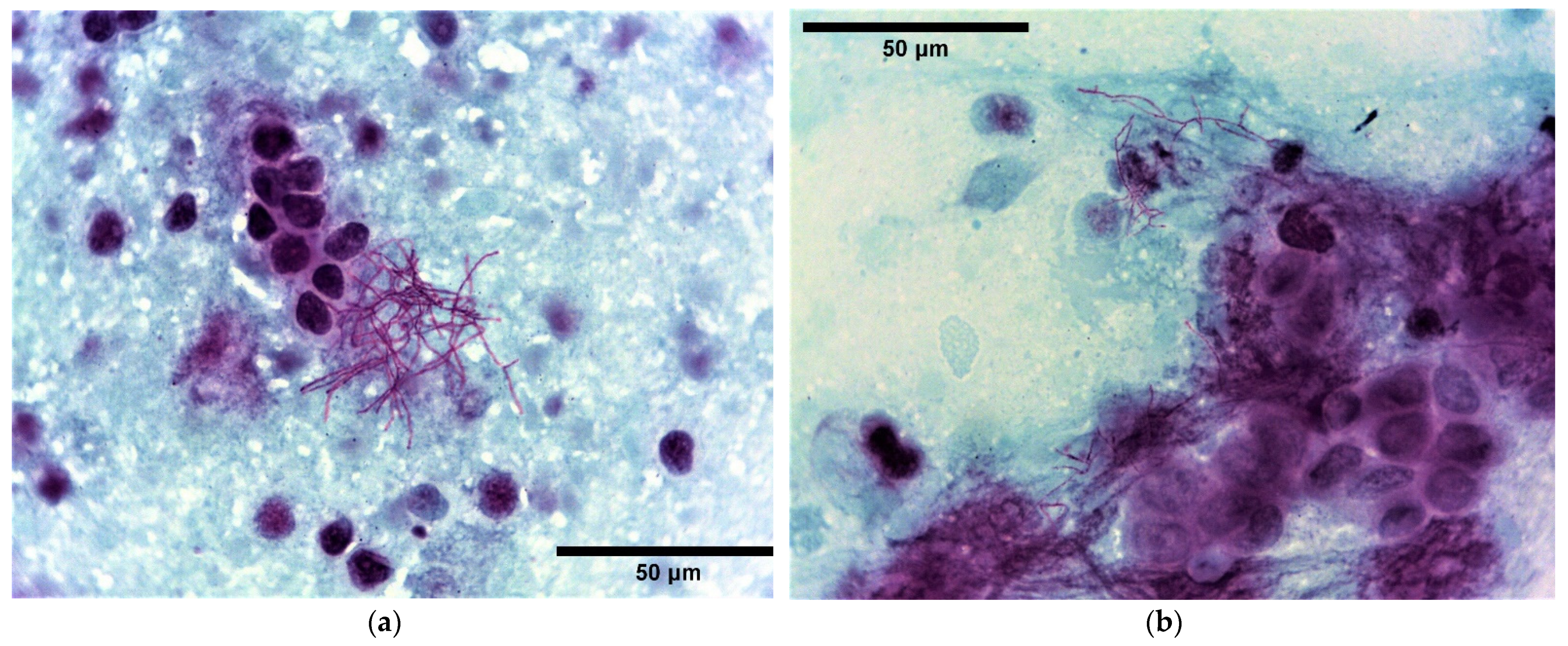
2.3. Bacterial Culture, AST and 16S rRNA Sequencing
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahendra, P.; Dave, P. Nocardiosis: An Emerging Infectious Actinomycetic Disease of Humans and Animals. J. Microbiol. Microb. Technol. 2016, 1, 4. [Google Scholar]
- Fatahi-Bafghi, M. Nocardiosis from 1888 to 2017. Microb. Pathog. 2018, 114, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Augustine, D.; Panikar, D.; Nandakumar, A.; Dinesh, K.R.; Karim, S.; Philip, R. Nocardia Farcinica Brain Abscess: Epidemiology, Pathophysiology, and Literature Review. Surg. Infect. 2014, 15, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kirpensteijn, J.; Fingland, R.B. Cutaneous Actinomycosis and Nocardiosis in Dogs: 48 Cases (1980–1990). J. Am. Vet. Med. Assoc. 1992, 201, 917–920. [Google Scholar]
- Sykes, J.E. Actinomycosis and Nocardiosis. In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012. [Google Scholar]
- Whitney, J.; Barrs, V.R. Actinomycosis, Nocardiosis, and Mycobacterial Infections. In Clinical Small Animal Internal Medicine; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 977–984. ISBN 978-1-119-50123-7. [Google Scholar]
- Marino, D.J.; Jaggy, A. Nocardiosis—A Literature Review with Selected Case Reports in Two Digs. J. Vet. Intern. Med. 1993, 7, 4–11. [Google Scholar] [CrossRef]
- Conville, P.S.; Witebsky, F.G. Organisms Designated as Nocardia Asteroides Drug Pattern Type VI Are Members of the Species Nocardia Cyriacigeorgica. J. Clin. Microbiol. 2007, 45, 2257–2259. [Google Scholar] [CrossRef]
- Roth, A.; Andrees, S.; Kroppenstedt, R.M.; Harmsen, D.; Mauch, H. Phylogeny of the Genus Nocardia Based on Reassessed 16S RRNA Gene Sequences Reveals Underspeciation and Division of Strains Classified as Nocardia Asteroides into Three Established Species and Two Unnamed Taxons. J. Clin. Microbiol. 2003, 41, 851–856. [Google Scholar] [CrossRef]
- Anagnostou, T.; Arvanitis, M.; Kourkoumpetis, T.K.; Desalermos, A.; Carneiro, H.A.; Mylonakis, E. Nocardiosis of the Central Nervous System: Experience from a General Hospital and Review of 84 Cases from the Literature. Medicine 2014, 93, 19–32. [Google Scholar] [CrossRef]
- Budzik, J.M.; Hosseini, M.; Mackinnon, A.C., Jr.; Taxy, J.B. Disseminated Nocardia Farcinica: Literature Review and Fatal Outcome in an Immunocompetent Patient. Surg. Infect. 2012, 13, 163–170. [Google Scholar] [CrossRef]
- Eroksuz, Y.; Gursoy, N.C.; Karapinar, T.; Karabulut, B.; Incili, C.A.; Yerlikaya, Z.; Toraman, Z.A.; Timurkan, M.O.; Eroksuz, H. Systemic Nocardiosis in a Dog Caused by Nocardia Cyriacigeorgica. BMC Vet. Res. 2016, 13, 30. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, M.G.; Salerno, T.; de Mattos-Guaraldi, A.L.; Camello, T.C.F.; Langoni, H.; Siqueira, A.K.; Paes, A.C.; Fernandes, M.C.; Lara, G.H.B. Nocardiosis: An Overview and Additional Report of 28 Cases in Cattle and Dogs. Rev. Inst. Med. Trop. São Paulo 2008, 50, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Ribeiro, A.I.; da Cruz Burema, M.; de Souza Borges, A.P.; de Melo Bruno, V.C.; Brandini Nespoli, P.E.; Colodel, E.M.; Furlan Gouvea, F.H.; Dutra, V.; Nakazato, L.; Ribeiro, M.G. Pyogranulomatous Pleuropneumonia Caused by Nocardia Asiatica in a Dog Coinfected with Canine Morbillivirus (Canine Distemper Virus). Vet. Med. Sci. 2020, 6, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Uhde, A.-K.; Kilwinski, J.; Peters, M.; Verspohl, J.; Feßler, A.T.; Schwarz, S.; Wohlsein, P. Fatal Nocardiosis in a Dog Caused by Multiresistant Nocardia Veterana. Vet. Microbiol. 2016, 183, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, Y.; Pandey, P.; Singh, U.B. Granulomatous Hepatitis by Nocardia Species: An Unusual Case. Int. J. Infect. Dis. 2019, 81, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Kennis, B.; Sandey, M.; White, A. Successful Medical Management of Cutaneous Nocardia Species Infection in a Dog Receiving Ciclosporin (Atopica). Vet. Rec. Case Rep. 2017, 5, e000493. [Google Scholar] [CrossRef]
- MacNeill, A.L.; Steeil, J.C.; Dossin, O.; Hoien-Dalen, P.S.; Maddox, C.W. Disseminated Nocardiosis Caused by Nocardia Abscessus in a Dog. Vet. Clin. Pathol. 2010, 39, 381–385. [Google Scholar] [CrossRef]
- Siak, M.K.; Burrows, A.K. Cutaneous Nocardiosis in Two Dogs Receiving Ciclosporin Therapy for the Management of Canine Atopic Dermatitis. Vet. Dermatol. 2013, 24, 453-e103. [Google Scholar] [CrossRef]
- Nocard, E. Note Sur La Maladie Des Boeufs de La Guadeloupe Connue Sous Le Nom de Farcin. Ann. L’Inst. Pasteur. 1888, 2, 293–302. [Google Scholar]
- Angeles, R.M.; LaSala, R.P.; Fanning, C.V. Disseminated Subcutaneous Nocardiosis Caused by Nocardia Farcinica Diagnosed by FNA Biopsy and 16S Ribosomal Gene Sequencing. Diagn. Cytopathol. 2008, 36, 266–269. [Google Scholar] [CrossRef]
- Corsini Campioli, C.; Castillo Almeida, N.E.; O’Horo, J.C.; Challener, D.; Go, J.R.; DeSimone, D.C.; Sohail, M.R. Clinical Presentation, Management, and Outcomes of Patients With Brain Abscess Due to Nocardia Species. Open Forum Infect. Dis. 2021, 8, ofab067. [Google Scholar] [CrossRef]
- Beaman, B.L.; Sugar, A.M. Nocardia in Naturally Acquired and Experimental Infections in Animals. Epidemiol. Infect. 1983, 91, 393–419. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard, 2nd ed.; CLSI Document M24-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; ISBN 1-56238-746-4.
- Randall, L.P.; Lemma, F.; Koylass, M.; Rogers, J.; Ayling, R.D.; Worth, D.; Klita, M.; Steventon, A.; Line, K.; Wragg, P.; et al. Evaluation of MALDI-ToF as a Method for the Identification of Bacteria in the Veterinary Diagnostic Laboratory. Res. Vet. Sci. 2015, 101, 42–49. [Google Scholar] [CrossRef]
- Desjardins, P.; Conklin, D. NanoDrop Microvolume Quantitation of Nucleic Acids. JoVE (J. Vis. Exp.) 2010, 45, e2565. [Google Scholar] [CrossRef]
- Beaman, B.L.; Beaman, L. Nocardia Species: Host-Parasite Relationships. Clin. Microbiol. Rev. 1994, 7, 213–264. [Google Scholar] [CrossRef] [PubMed]
- Duggal, S.D.; Chugh, T.D. Nocardiosis: A Neglected Disease. MPP 2020, 29, 514–523. [Google Scholar] [CrossRef]
- Williams, E.; Jenney, A.W.; Spelman, D.W. Nocardia Bacteremia: A Single-Center Retrospective Review and a Systematic Review of the Literature. Int. J. Infect. Dis. 2020, 92, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Yaemsiri, S.; Sykes, J.E. Successful Treatment of Disseminated Nocardiosis Caused by Nocardia Veterana in a Dog. J. Vet. Intern. Med. 2018, 32, 418–422. [Google Scholar] [CrossRef]
- Martínez-Barricarte, R. Isolated Nocardiosis, an Unrecognized Primary Immunodeficiency? Front. Immunol. 2020, 11, 590239. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.E.; Sturgis, C.D.; Procop, G.W.; Rhoads, D.D. The Cytopathology of Actinomyces, Nocardia, and Their Mimickers. Diagn. Cytopathol. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Kapadia, M.; Rolston, K.V.; Han, X.Y. Invasive Streptomyces Infections: Six Cases and Literature Review. Am. J. Clin. Pathol. 2007, 127, 619–624. [Google Scholar] [CrossRef]
- Girard, V.; Mailler, S.; Polsinelli, S.; Jacob, D.; Saccomani, M.C.; Celliere, B.; Monnin, V.; van Belkum, A.; Hagen, F.; Meis, J.F. Routine Identification of Nocardia Species by MALDI-TOF Mass Spectrometry. Diagn. Microbiol. Infect. Dis. 2017, 87, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-S.; So, H.; Joo, B.; Park, J.; Jeong, I.-S.; Lee, G.-J.; Park, J. A Canine Case of Nocardia Africana Infection Detected by Matrix-Assisted Laser Desorption Ionization—Time-of-Flight Mass Spectrometry. Vet. Sci. 2022, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Ruiz, A.; Iglesias, C.; Quiroga, L.; Cercenado, E.; Martín-Rabadán, P.; Bouza, E.; Rodríguez-Sánchez, B. Identification of Nocardia Species from Clinical Isolates Using MALDI-TOF Mass Spectrometry. Clin. Microbiol. Infect. 2018, 24, 1342.e5–1342.e8. [Google Scholar] [CrossRef] [PubMed]
- Conville, P.S.; Brown-Elliott, B.A.; Smith, T.; Zelazny, A.M. The Complexities of Nocardia Taxonomy and Identification. J. Clin. Microbiol. 2018, 56, e01419-17. [Google Scholar] [CrossRef]
- Minero, M.V.; Marín, M.; Cercenado, E.; Rabadán, P.M.; Bouza, E.; Muñoz, P. Nocardiosis at the Turn of the Century. Medicine 2009, 88, 250–261. [Google Scholar] [CrossRef]
- Welsh, O.; Vera-Cabrera, L.; Salinas-Carmona, M.C. Current Treatment for Nocardia Infections. Expert Opin. Pharmacother. 2013, 14, 2387–2398. [Google Scholar] [CrossRef]
- Hitti, W.; Wolff, M. Two Cases of Multidrug-Resistant Nocardia Farcinica Infection in Immunosuppressed Patients and Implications for Empiric Therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 142–144. [Google Scholar] [CrossRef]
- Senard, O.; Blanot, S.; Jouvion, G.; Rodriguez-Nava, V.; Lortholary, O.; Join-Lambert, O.; Toubiana, J. Fulminant Nocardiosis Due to a Multidrug-Resistant Isolate in a 12-Year-Old Immunocompetent Child. Pediatrics 2018, 141, e20163131. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.P.; Dias, R.F.F.; da Silva, R.A.; de Melo, R.P.B.; de Araujo, C.A.S.C.; Nogueira, J.F.; da Costa, M.M.; Junior, J.W.P.; Mota, R.A. Bovine Mastitis Caused by Multidrug-Resistant Nocardia Farcinica. Acta Sci. Vet. 2020, 48 (Suppl. 1), 520. [Google Scholar] [CrossRef]
- Strzok, E.; Siepker, C.; Armwood, A.; Howerth, E.; Smith, J.; Banovic, F. Successful Treatment of Cutaneous Curvularia Geniculata, Nocardia Niigatensis, and Viral Papillomatosis in a Dog during the Therapeutic Management of Immune-Mediated Hemolytic Anemia. Front. Vet. Sci. 2019, 6, 249. [Google Scholar] [CrossRef]
- Mund, G.M.; Häußler, T.C.; Aurich, S.H.; Ewers, C.; Thiel, C. Successful Treatment of Peritoneal Nocardiosis Caused by Nocardia Paucivorans in a Dog. N. Z. Vet. J. 2021, 69, 355–360. [Google Scholar] [CrossRef] [PubMed]
| Tissue Type (Including Abscess) | MZN-Positive | Nocardia Culture Positive | |
|---|---|---|---|
| Dog 1 | Lung | Yes | Yes |
| Liver | No | No | |
| Kidney | Yes | Yes | |
| Spleen | No | No | |
| Bone Marrow | No | No | |
| Small Intestine | No | No | |
| Bronchial lymph node | Yes | Yes | |
| R Popliteal lymph node | No | No | |
| Dog 2 | Lung | No | No |
| Liver | No | No | |
| Kidney | Yes | Yes | |
| Spleen | No | No | |
| Bone Marrow | No | No | |
| Small Intestine | No | No | |
| L Scapular abscess fluid | Yes | Yes | |
| L Axillary lymph node | Yes | Yes |
| Antibiotic | Broth Microdilution |
|---|---|
| Ampicillin | >8 (R) |
| Amoxicillin-clavulanic acid | 8 (R) |
| Cefovecin | >8 (R) |
| Cefpodoxime | >8 (R) |
| Imipenem | >4 (R) |
| Amikacin | <16 (S) |
| Gentamicin | >16 (R) |
| Doxycycline | >0.5 (R) |
| Tetracycline | >1 (R) |
| Minocycline | 2 (R) |
| Marbofloxacin | 4 (R) |
| Pradofloxacin | ≤0.25 (S) |
| Trimethoprim-sulfamethoxazole | ≤2 (S) |
| Nitrofurantoin | >64 (R) |
| Rifampicin | >2 (R) |
| Vancomycin | >16 (R) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zendri, F.; Richards-Rios, P.; Maciuca, I.; Ricci, E.; Timofte, D. Disseminated Nocardiosis Caused by Nocardia farcinica in Two Puppy Siblings. Vet. Sci. 2023, 10, 28. https://doi.org/10.3390/vetsci10010028
Zendri F, Richards-Rios P, Maciuca I, Ricci E, Timofte D. Disseminated Nocardiosis Caused by Nocardia farcinica in Two Puppy Siblings. Veterinary Sciences. 2023; 10(1):28. https://doi.org/10.3390/vetsci10010028
Chicago/Turabian StyleZendri, Flavia, Peter Richards-Rios, Iuliana Maciuca, Emanuele Ricci, and Dorina Timofte. 2023. "Disseminated Nocardiosis Caused by Nocardia farcinica in Two Puppy Siblings" Veterinary Sciences 10, no. 1: 28. https://doi.org/10.3390/vetsci10010028
APA StyleZendri, F., Richards-Rios, P., Maciuca, I., Ricci, E., & Timofte, D. (2023). Disseminated Nocardiosis Caused by Nocardia farcinica in Two Puppy Siblings. Veterinary Sciences, 10(1), 28. https://doi.org/10.3390/vetsci10010028





