Sphingolipidomics of Bovine Pink Eye: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Lipidomics
2.3. Statistical Analysis
3. Results
3.1. Clinical Samples
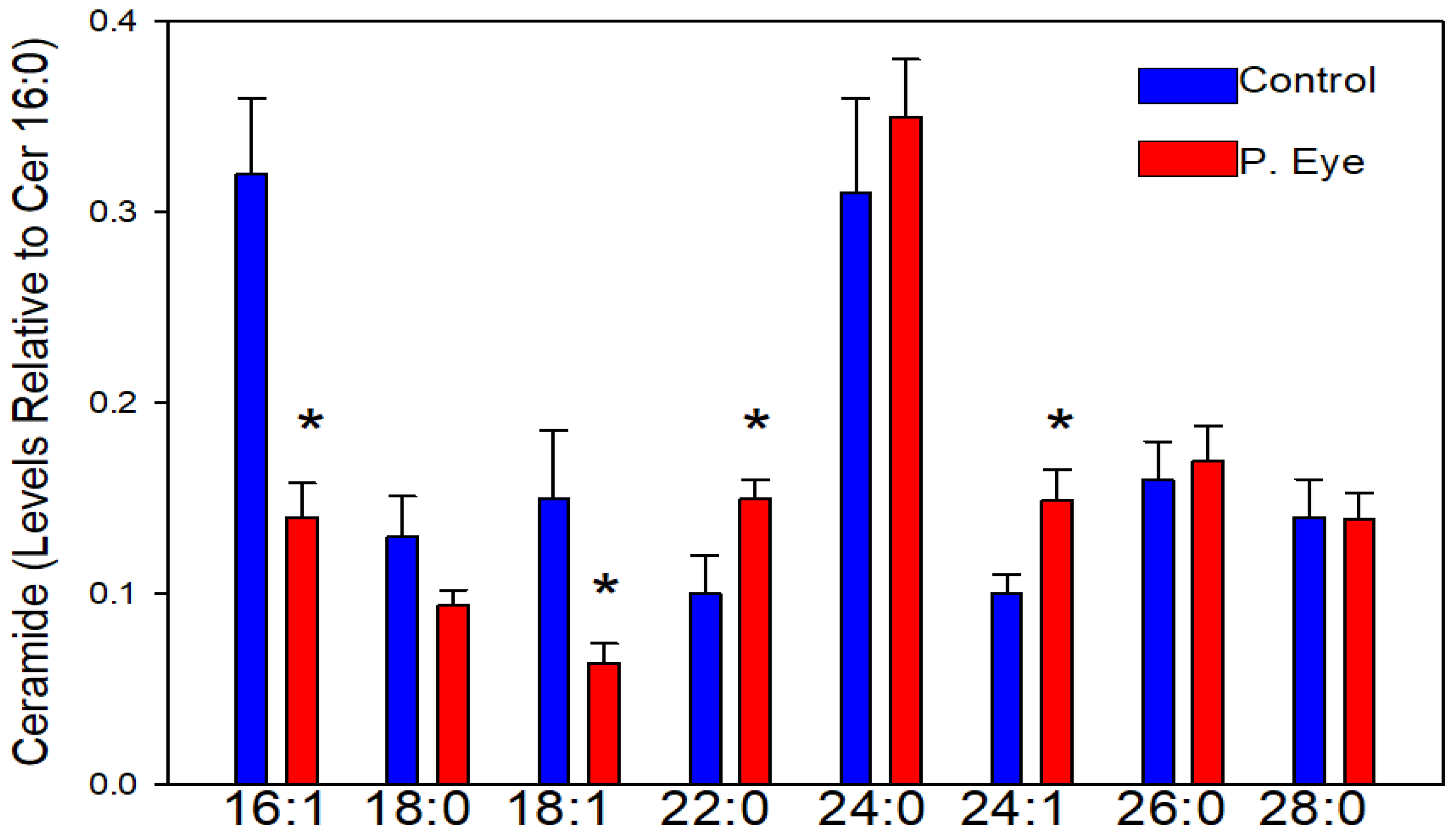
3.2. Ceramides
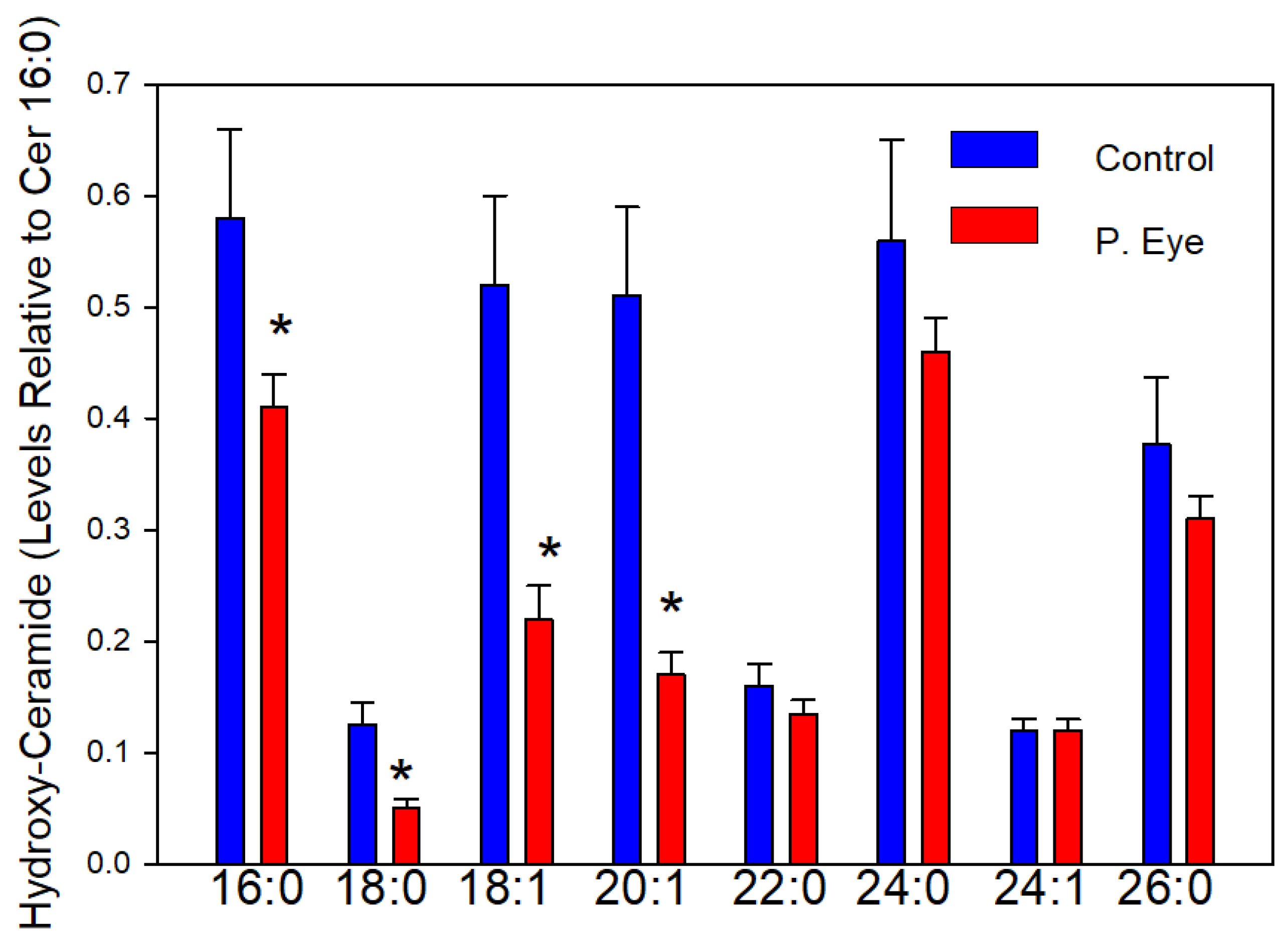
3.3. Hydroxy-Ceramides
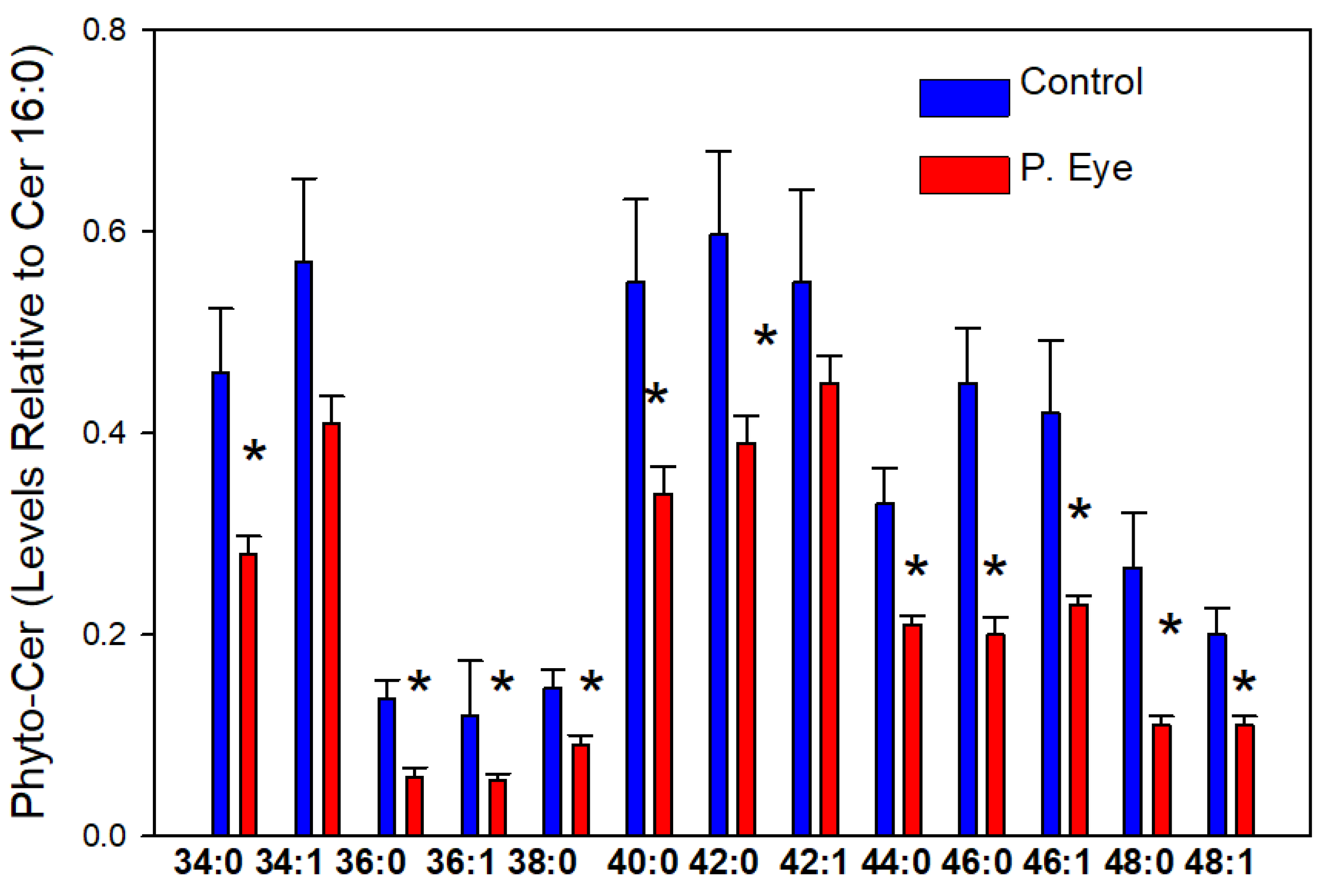
3.4. Phytoceramides (Cer X-O3)
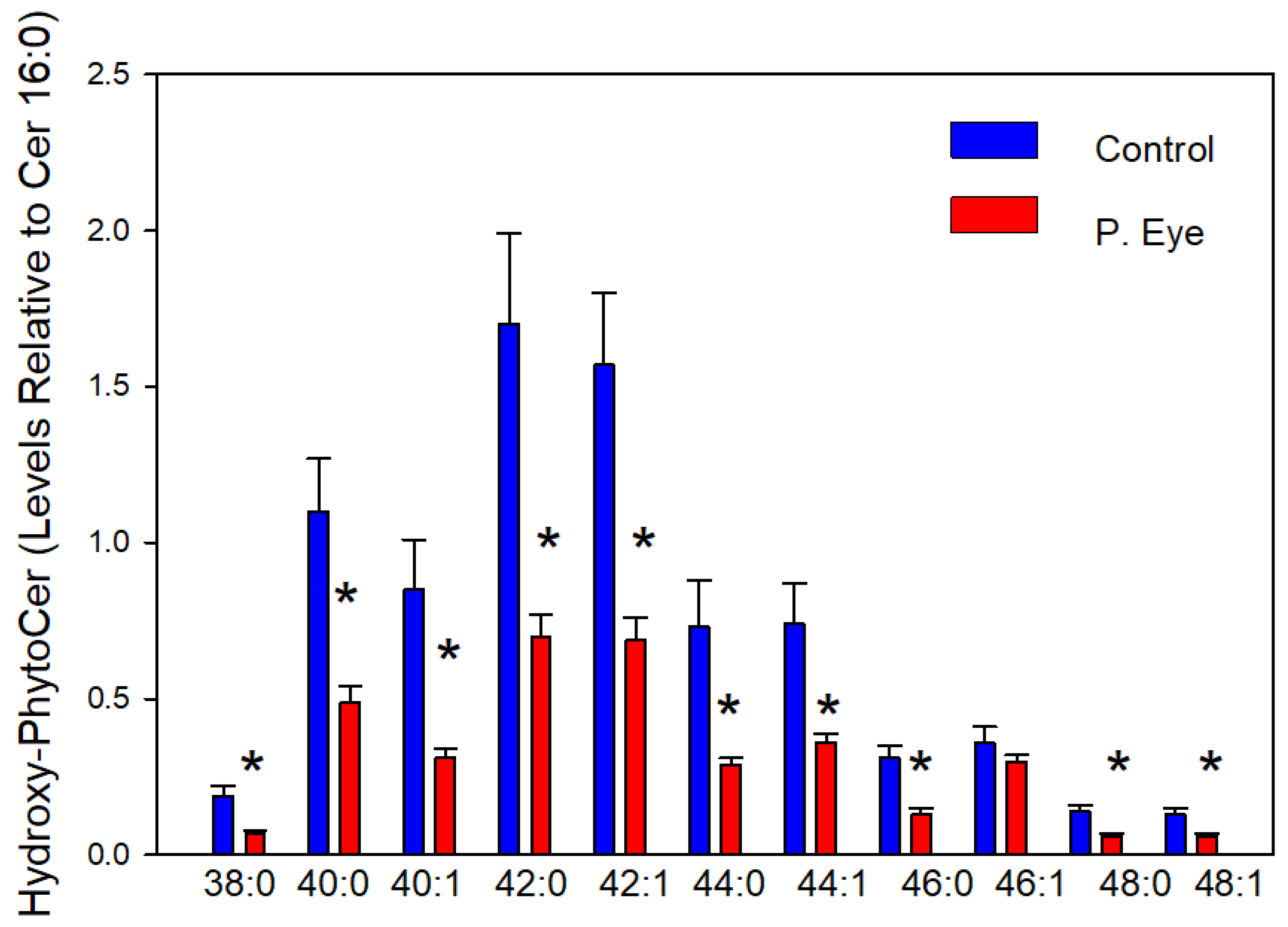
3.5. Hydroxy-Phytoceramides (Cer X-O4)
3.6. Hydroxy-Phytoceramides (Cer X-O5)
3.7. Dihdroceramides
3.8. Biomarkers of Ocular Infection
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 97, 108115. [Google Scholar] [CrossRef]
- Cwiklik, L. Tear film lipid layer: A molecular level view. Biochim. Biophys. Acta 2016, 1858, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.K.; Catanese, S.; Emond, P.; Corcia, P.; Blasco, H.; Pisella, P.J. Metabolomics and lipidomics approaches in human tears: A systematic review. Surv. Ophthalmol. 2022, 67, 1229–1243. [Google Scholar] [CrossRef]
- Wood, P.L.; Donohue, M.N.; Cebak, J.E.; Beckmann, T.G.; Treece, M.; Johnson, J.W.; Miller, L.M.J. Tear film amphiphilic and anti-inflammatory lipids in bovine pink eye. Metabolites 2018, 8, 81. [Google Scholar] [CrossRef]
- Chen, J.; Green-Church, K.B.; Nichols, K.K. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6220–6231. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Fatty acyl esters of hydroxy fatty acid (FAHFA) lipid families. Metabolites 2020, 10, 512. [Google Scholar] [CrossRef]
- Wood, P.L.; Woltjer, R.L. Electrospray Ionization High Resolution Mass Spectrometry of the Chloride Adducts of Steroids, Mono- and Oligo-Saccharides, Xyloglucans, Ceramides, Gangliosides, and Phenols. In Springer Protocols, Neuromethods: Metabolomics; Wood, P.L., Ed.; Human Press: New York, NY, USA, 2021; Volume 159, pp. 69–76. ISBN1 978-1-0716-0863-0. ISBN2 eBook 978-1-0716-0864-7. [Google Scholar]
- Wood, P.L.; Hauther, K.A.; Scarborough, J.H.; Craney, D.J.; Dudzik, B.; Cebak, J.E.; Woltjer, R.L. Human brain lipidomics: Utilities of chloride adducts in flow injection analysis. Life 2021, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Klatt, S.; Brammananth, R.; O’Callaghan, S.; Kouremenos, K.A.; Tull, D.; Crellin, P.K.; Coppel, R.L.; McConville, M.J. Identification of novel lipid modifications and intermembrane dynamics in Corynebacterium glutamicum using high-resolution mass spectrometry. J. Lipid Res. 2018, 59, 1190–1204. [Google Scholar] [CrossRef]
- Shine, W.E.; McCulley, J.P. Polar lipids in human meibomian gland secretions. Curr. Eye Res. 2003, 26, 89–94. [Google Scholar] [CrossRef]
- Galor, A.; Sanchez, V.; Jensen, A.; Burton, M.; Maus, K.; Stephenson, D.; Chalfant, C.; Mandal, N. Meibum sphingolipid composition is altered in individuals with meibomian gland dysfunction-a side by side comparison of Meibum and Tear Sphingolipids. Ocul. Surf. 2022, 23, 87–95. [Google Scholar] [CrossRef]
- Paranjpe, V.; Tan, J.; Nguyen, J.; Lee, J.; Allegood, J.; Galor, A.; Mandal, N. Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul. Surf. 2019, 17, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, H.; Xie, H.T.; Xu, K.K.; Shi, B.J.; Huang, Y.K. Changes in meibum lipid composition with ocular demodex infestation. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Olżyńska, A.; Cwiklik, L. Behavior of sphingomyelin and ceramide in a tear film lipid layer model. Ann. Anat. 2017, 210, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.; Vogel, H.J.; Prenner, E.J. The effect of repeated lateral compression and expansions mimicking blinking on selected tear film polar lipid monofilms. Biochim. Biophys. Acta Biomembr. 2017, 1859, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Marquês, J.T.; Marinho, H.S.; de Almeida, R.F.M. Sphingolipid hydroxylation in mammals, yeast and plants—An integrated view. Prog. Lipid Res. 2018, 71, 18–42. [Google Scholar] [CrossRef]
- Mizutani, Y.; Kihara, A.; Igarashi, Y. Identification of the human sphingolipid C4-hydroxylase, hDES2, and its up-regulation during keratinocyte differentiation. FEBS Lett. 2004, 563, 93–97. [Google Scholar] [CrossRef]
- Enomoto, A.; Omae, F.; Miyazaki, M.; Kozutsumi, Y.; Yubisui, T.; Suzuki, A. Dihydroceramide: Sphinganine C-4-hydroxylation requires Des2 hydroxylase and the membrane form of cytochrome b5. Biochem. J. 2006, 397, 289–295. [Google Scholar] [CrossRef][Green Version]
- Mizutani, Y.; Kihara, A.; Chiba, H.; Tojo, H.; Igarashi, Y. 2-hydroxy-ceramide synthesis by ceramide synthase family: Enzymatic basis for the preference of FA chain length. J. Lipid Res. 2008, 49, 2356–2364. [Google Scholar] [CrossRef]
- Alderson, N.L.; Rembiesa, B.M.; Walla, M.D.; Bielawska, A.; Bielawski, J.; Hama, H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 2004, 279, 48562–48568. [Google Scholar] [CrossRef]
- Hama, H. Fatty acid 2-hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 405–414. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Alderson, N.L.; Monje, P.V.; Wood, P.M.; Hama, H. FA2H is responsible for the formation of 2-hydroxy galactolipids in peripheral nervous system myelin. J. Lipid Res. 2008, 49, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Downing, D.T. The omega-hydroxyceramides of pig epidermis are attached to corneocytes solely through omega-hydroxyl groups. J. Lipid Res. 2001, 42, 1105–1110. [Google Scholar] [CrossRef]
- Wertz, P.W. Lipids and the permeability and antimicrobial barriers of the skin. J. Lipids 2018, 2018, 5954034. [Google Scholar] [CrossRef]
- Mudgil, P. Antimicrobial tear lipids in the ocular surface defense. Front. Cell. Infect. Microbiol. 2022, 12, 866900. [Google Scholar] [CrossRef]


| Lipid | Exact Mass | [M+Cl]− | Level Relative to Cer d18:1/16:0 | ppm |
|---|---|---|---|---|
| hTMCM 28:2 | 760.4973 | 795.4671 | 0.0051 ± 0.0015 | 0.44 |
| hTMCN 33:3 | 828.5599 | 863.5297 | 0.010 ± 0.0015 | 1.5 |
| hTMCM 34:3 | 842.5755 | 877.5454 | 0.0070 ± 0.0023 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, P.L.; Miller, L.M.J. Sphingolipidomics of Bovine Pink Eye: A Pilot Study. Vet. Sci. 2022, 9, 388. https://doi.org/10.3390/vetsci9080388
Wood PL, Miller LMJ. Sphingolipidomics of Bovine Pink Eye: A Pilot Study. Veterinary Sciences. 2022; 9(8):388. https://doi.org/10.3390/vetsci9080388
Chicago/Turabian StyleWood, Paul L., and Lynda M. J. Miller. 2022. "Sphingolipidomics of Bovine Pink Eye: A Pilot Study" Veterinary Sciences 9, no. 8: 388. https://doi.org/10.3390/vetsci9080388
APA StyleWood, P. L., & Miller, L. M. J. (2022). Sphingolipidomics of Bovine Pink Eye: A Pilot Study. Veterinary Sciences, 9(8), 388. https://doi.org/10.3390/vetsci9080388





