Flow Cytometric Analysis of Leukocyte Populations in the Lung Tissue of Dromedary Camels
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Materials
2.3. Animals and Clinical Examination
2.4. Blood Sampling and Cell Separation
2.5. Monoclonal Antibodies
2.6. Cell Labeling and Flow Cytometry
2.7. Analysis of Phagocytosis Activity
2.8. Measurement of Reactive Oxygen Species Production
2.9. Statistical Analyses
3. Results
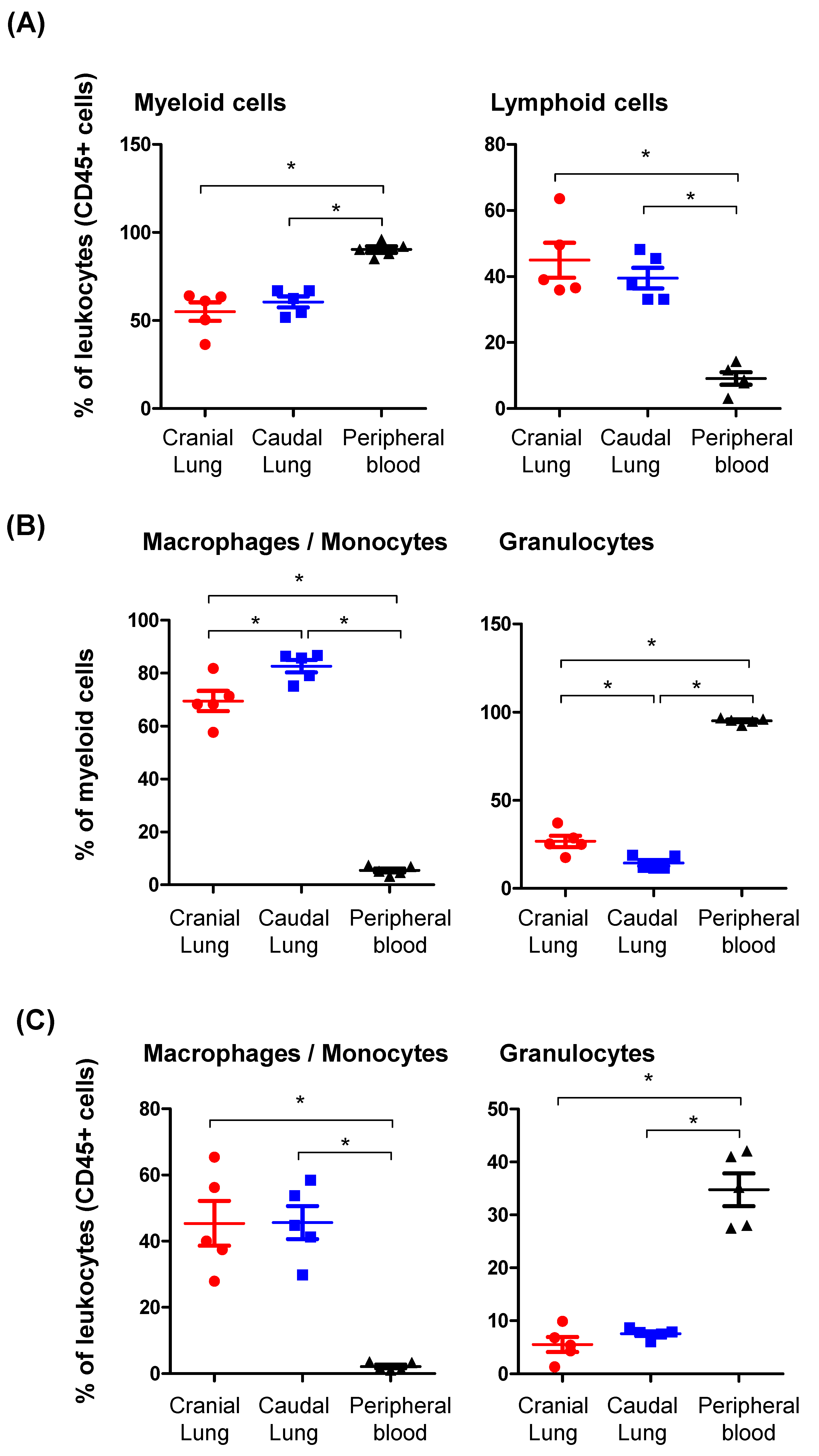
3.1. The Identification of Main Leukocyte Populations in the Lung Tissue of Camels
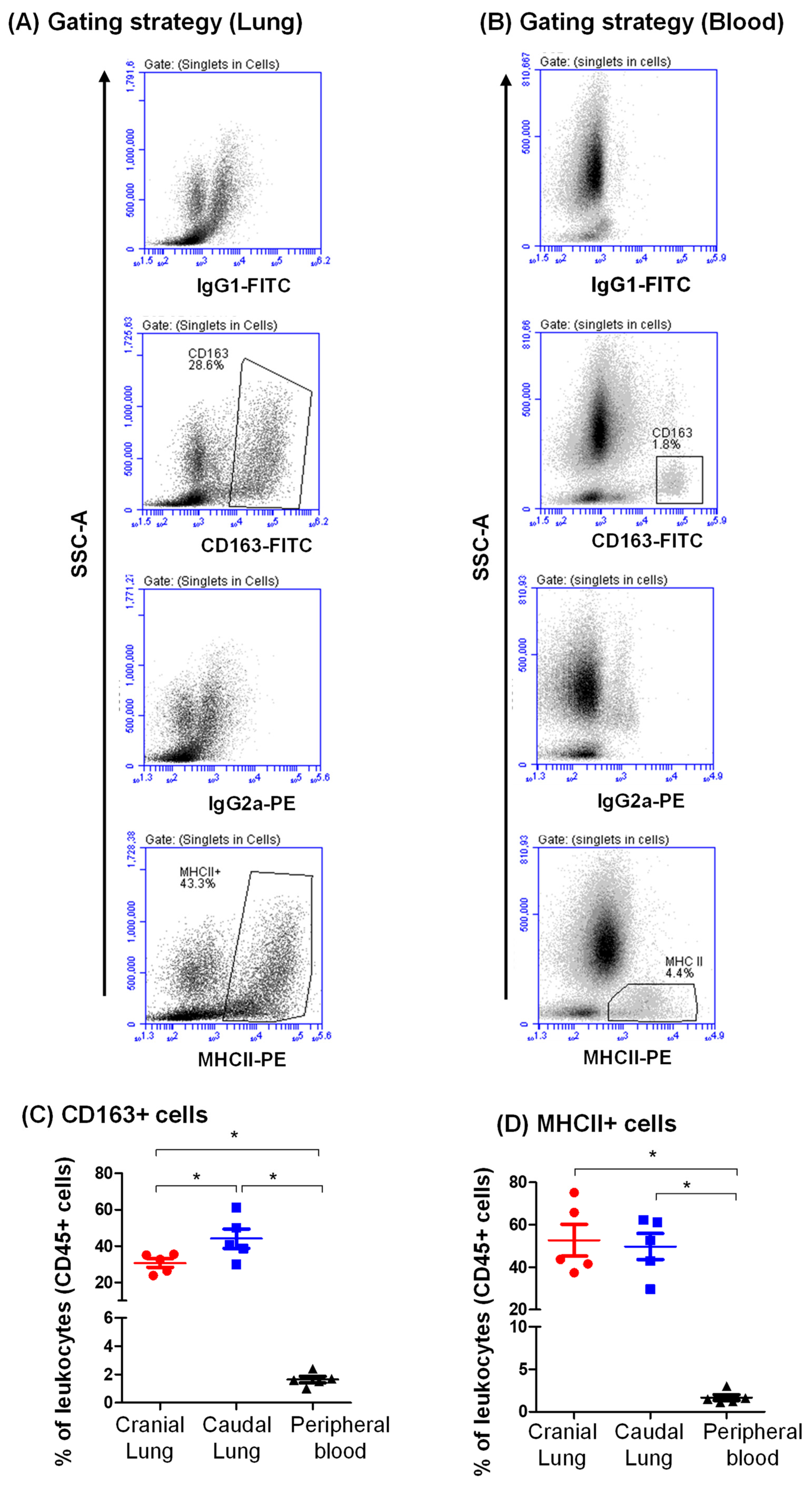
3.2. Expression Pattern of CD163 and MHCII on Camel Lung Leukocytes
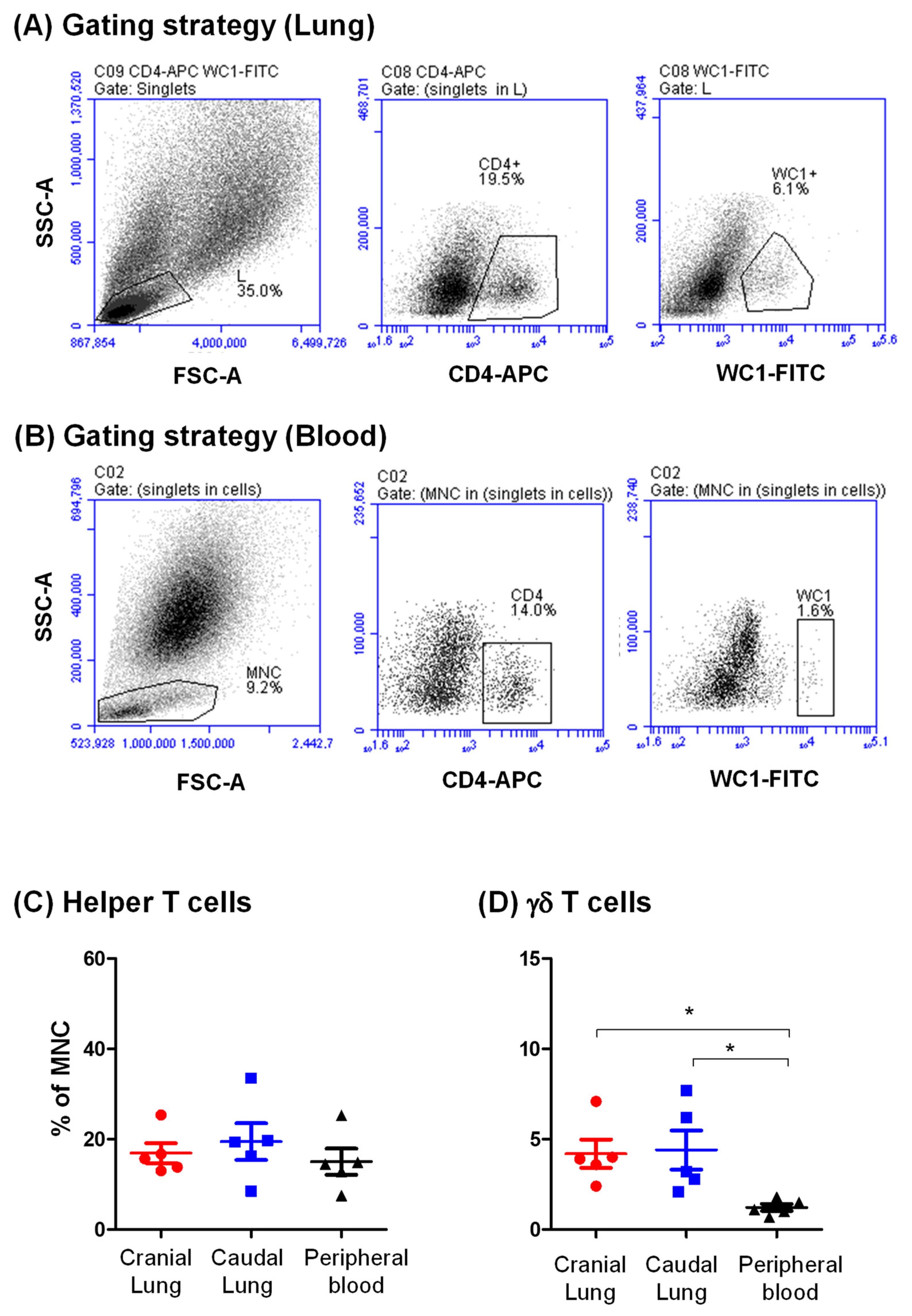
3.3. Identification of CD4-Positive Helper T Cells and Gd T Cells in the Camel Lung
3.4. Cell Adhesion Molecules Expression on Lung Leukocytes
3.5. The Antibacterial Activity of Lung Phagocytes
4. Discussion
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braciale, T.J.; Sun, J.; Kim, T.S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 2012, 12, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Hotten, D.F.; Malakhau, Y.; Volker, E.; Ghio, A.J.; Noble, P.W.; Kraft, M.; Hollingsworth, J.W.; Gunn, M.D.; Tighe, R.M. Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am. J. Respir. Cell Mol. Biol. 2016, 54, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Tirouvanziam, R.; Laval, J.; Greene, C.M.; Habiel, D.; Sharma, L.; Yildirim, A.O.; Dela Cruz, C.S.; Hogaboam, C.M. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J. Innate Immun. 2018, 10, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Buscher, M. Flow Cytometry Instrumentation—An Overview. Curr. Protoc. Cytom. 2019, 87, e52. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; Lhermitte, L.; Bottcher, S.; Almeida, J.; van der Velden, V.H.; Flores-Montero, J.; Rawstron, A.; Asnafi, V.; Lecrevisse, Q.; Lucio, P.; et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012, 26, 1908–1975. [Google Scholar] [CrossRef]
- Sykora, M.M.; Reschke, M. Immunophenotyping of Tissue Samples Using Multicolor Flow Cytometry. Methods Mol. Biol. 2019, 1953, 253–268. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef]
- Ma, W.; Cui, W.; Lin, Q. Improved immnunophenotyping of lymphocytes in bronchoalveolar lavage fluid (BALF) by flow cytometry. Clin. Chim. Acta 2001, 313, 133–138. [Google Scholar] [CrossRef]
- Alhafiz, G.A.; Alghatam, F.H.; Almohammed, H.; Hussen, J. Milk Immune Cell Composition in Dromedary Camels With Subclinical Mastitis. Front. Vet. Sci. 2022, 9, 885523. [Google Scholar] [CrossRef]
- Tan, S.Y.; Krasnow, M.A. Developmental origin of lung macrophage diversity. Development 2016, 143, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Janssen, W.J.; McPhillips, K.A.; Dickinson, M.G.; Linderman, D.J.; Morimoto, K.; Xiao, Y.Q.; Oldham, K.M.; Vandivier, R.W.; Henson, P.M.; Gardai, S.J. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am. J. Respir. Crit. Care Med. 2008, 178, 158–167. [Google Scholar] [CrossRef]
- Kong, X.N.; Yan, H.X.; Chen, L.; Dong, L.W.; Yang, W.; Liu, Q.; Yu, L.X.; Huang, D.D.; Liu, S.Q.; Liu, H.; et al. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. J. Exp. Med. 2007, 204, 2719–2731. [Google Scholar] [CrossRef]
- Kitchens, R.L. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem. Immunol. 2000, 74, 61–82. [Google Scholar] [CrossRef]
- Etzerodt, A.; Berg, R.M.; Plovsing, R.R.; Andersen, M.N.; Bebien, M.; Habbeddine, M.; Lawrence, T.; Moller, H.J.; Moestrup, S.K. Soluble ectodomain CD163 and extracellular vesicle-associated CD163 are two differently regulated forms of ‘soluble CD163′ in plasma. Sci. Rep. 2017, 7, 40286. [Google Scholar] [CrossRef]
- Abualrous, E.T.; Sticht, J.; Freund, C. Major histocompatibility complex (MHC) class I and class II proteins: Impact of polymorphism on antigen presentation. Curr. Opin. Immunol. 2021, 70, 95–104. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Alvaro-Benito, M.; Stolzenberg, S.; Noe, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sugimoto, C.; Arainga, M.; Alvarez, X.; Didier, E.S.; Kuroda, M.J. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: Implications for understanding lung disease in humans. J. Immunol. 2014, 192, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kolar, Q.K.; Waddell, L.A.; Raper, A.; Rocchi, M.S.; Shaw, D.J.; Corbishley, A.; Hope, J.C. Anatomical distribution of respiratory tract leukocyte cell subsets in neonatal calves. Vet. Immunol. Immunopathol. 2020, 227, 110090. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Davies, M.; Villarreal-Ramos, B.; Hope, J. Differential distribution of WC1(+) gammadelta TCR(+) T lymphocyte subsets within lymphoid tissues of the head and respiratory tract and effects of intranasal M. bovis BCG vaccination. Vet. Immunol. Immunopathol. 2010, 136, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Bordet, E.; Maisonnasse, P.; Renson, P.; Bouguyon, E.; Crisci, E.; Tiret, M.; Descamps, D.; Bernelin-Cottet, C.; Urien, C.; Lefevre, F.; et al. Porcine Alveolar Macrophage-like cells are pro-inflammatory Pulmonary Intravascular Macrophages that produce large titers of Porcine Reproductive and Respiratory Syndrome Virus. Sci. Rep. 2018, 8, 10172. [Google Scholar] [CrossRef]
- Lee, Y.; Kiupel, M.; Soboll Hussey, G. Characterization of respiratory dendritic cells from equine lung tissues. BMC Vet. Res. 2017, 13, 313. [Google Scholar] [CrossRef]
- Rabiger, F.V.; Rothe, K.; von Buttlar, H.; Bismarck, D.; Buttner, M.; Moore, P.F.; Eschke, M.; Alber, G. Distinct Features of Canine Non-conventional CD4(-)CD8alpha(-) Double-Negative TCRalphabeta(+) vs. TCRgammadelta(+) T Cells. Front. Immunol. 2019, 10, 2748. [Google Scholar] [CrossRef]
- Aljasim, T.A.; Almasoud, A.; Aljami, H.A.; Alenazi, M.W.; Alsagaby, S.A.; Alsaleh, A.N.; Alharbi, N.K. High Rate of Circulating MERS-CoV in Dromedary Camels at Slaughterhouses in Riyadh, 2019. Viruses 2020, 12, 1215. [Google Scholar] [CrossRef]
- Mosaad, A.A.; Elbagory, A.R.; Khalid, A.M.; Waters, W.; Tibary, A.; Hamilton, M.J.; Davis, W.C. Identification of monoclonal antibody reagents for use in the study of the immune response to infectious agents in camel and water buffalo. J. Camel Pract. Res. 2006, 13, 91–101. [Google Scholar]
- Al-Mubarak, A.I.A. Differential expression of the coronavirus (Mers-cov) Receptor, Dipeptidyl Peptidase 4, on normal and stimulated leukocytes of dromedary camels. J. Camel Pract. Res. 2018, 25, 249. [Google Scholar] [CrossRef]
- Hussen, J.; Shawaf, T.; Al-herz, A.I.; Alturaifi, H.R.; Al khamees, M.; Alluwaimi, A.M. Expression Patterns of Cell Adhesion Molecules on CD4+ T Cells and WC1+ T Cells in the Peripheral Blood of Dromedary Camels. Pak. Vet. J. 2018, 38, 231–236. [Google Scholar] [CrossRef]
- Hussen, J.; Shawaf, T.; Al-herz, A.I.; Alturaifi, H.R.; Alluwaimi, A.M. Reactivity of commercially available monoclonal antibodies to human CD antigens with peripheral blood leucocytes of dromedary camels (Camelus dromedarius). Open Vet. J. 2017, 7, 150–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hussen, J.; Shawaf, T.; Al-Mubarak, A.I.A.; Al Humam, N.A.; Almathen, F.; Schuberth, H.J. Dromedary camel CD14(high) MHCII(high) monocytes display inflammatory properties and are reduced in newborn camel calves. BMC Vet. Res. 2020, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Miyazawa, M.; Zibiki, Y.; Kamikakimoto, R.; Hobo, S. Flow cytometric analysis of bronchoalveolar lavage fluid immune dynamics in calves. J. Vet. Med. Sci. 2022, 84, 548–557. [Google Scholar] [CrossRef]
- Hussen, J.; Al-Sukruwah, M.A. The Impact of the Animal Housing System on Immune Cell Composition and Function in the Blood of Dromedary Camels. Animals 2022, 12, 317. [Google Scholar] [CrossRef]
- He, W.; Zhang, W.; Cheng, C.; Li, J.; Wu, X.; Li, M.; Chen, Z.; Wang, W. The distributive and structural characteristics of bronchus-associated lymphoid tissue (BALT) in Bactrian camels (Camelus bactrianus). PeerJ 2019, 7, e6571. [Google Scholar] [CrossRef]
- He, W.H.; Zhang, W.D.; Cheng, C.C.; Lu, J.; Liu, L.; Chen, Z.H.; Wang, W.H. Expression characteristics of polymeric immunoglobulin receptor in Bactrian camel (Camelus bactrianus) lungs. PLoS ONE 2022, 17, e0264815. [Google Scholar] [CrossRef]
- Elhussieny, O.; Zidan, M. Temporospatial characterization of the bronchus associated lymphoid tissue (BALT) of the one humped camel (Camelus dromedarius). Trop. Anim. Health Prod. 2021, 53, 265. [Google Scholar] [CrossRef] [PubMed]
- Hussen, J.; Schuberth, H.J. Heterogeneity of Bovine Peripheral Blood Monocytes. Front. Immunol. 2017, 8, 1875. [Google Scholar] [CrossRef]
- Hussen, J.; Schuberth, H.J. Recent Advances in Camel Immunology. Front. Immunol. 2020, 11, 614150. [Google Scholar] [CrossRef]
- Hu, J.M.; Liu, K.; Liu, J.H.; Jiang, X.L.; Wang, X.L.; Chen, Y.Z.; Li, S.G.; Zou, H.; Pang, L.J.; Liu, C.X.; et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 21526–21538. [Google Scholar] [CrossRef] [PubMed]
- Vidyarthi, A.; Khan, N.; Agnihotri, T.; Negi, S.; Das, D.K.; Aqdas, M.; Chatterjee, D.; Colegio, O.R.; Tewari, M.K.; Agrewala, J.N. TLR-3 Stimulation Skews M2 Macrophages to M1 Through IFN-alphabeta Signaling and Restricts Tumor Progression. Front. Immunol. 2018, 9, 1650. [Google Scholar] [CrossRef]
- Buxade, M.; Huerga Encabo, H.; Riera-Borrull, M.; Quintana-Gallardo, L.; Lopez-Cotarelo, P.; Tellechea, M.; Martinez-Martinez, S.; Redondo, J.M.; Martin-Caballero, J.; Flores, J.M.; et al. Macrophage-specific MHCII expression is regulated by a remote Ciita enhancer controlled by NFAT5. J. Exp. Med. 2018, 215, 2901–2918. [Google Scholar] [CrossRef] [PubMed]
- ten Broeke, T.; Wubbolts, R.; Stoorvogel, W. MHC class II antigen presentation by dendritic cells regulated through endosomal sorting. Cold Spring Harb. Perspect. Biol. 2013, 5, a016873. [Google Scholar] [CrossRef] [PubMed]
- Katikaneni, D.S.; Jin, L. B cell MHC class II signaling: A story of life and death. Hum. Immunol. 2019, 80, 37–43. [Google Scholar] [CrossRef]
- Hussen, J.; Duvel, A.; Sandra, O.; Smith, D.; Sheldon, I.M.; Zieger, P.; Schuberth, H.J. Phenotypic and functional heterogeneity of bovine blood monocytes. PLoS ONE 2013, 8, e71502. [Google Scholar] [CrossRef]
- Fabriek, B.O.; van Bruggen, R.; Deng, D.M.; Ligtenberg, A.J.; Nazmi, K.; Schornagel, K.; Vloet, R.P.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 2009, 113, 887–892. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor CD163. Immunobiology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Schwartz, E.J.; West, R.B.; Warnke, R.A.; Arber, D.A.; Natkunam, Y. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am. J. Surg. Pathol. 2005, 29, 617–624. [Google Scholar] [CrossRef]
- Cheng, M.; Hu, S. Lung-resident gammadelta T cells and their roles in lung diseases. Immunology 2017, 151, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.C.; Lopes, N.; Silva-Santos, B. gammadelta T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 2021, 21, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995, 57, 827–872. [Google Scholar] [CrossRef]
- Gaashan, M.M.; Al-Mubarak, A.I.; Hussen, J. Leukocyte populations and their cell adhesion molecules expression in newborn dromedary camel calves. Vet. World 2020, 13, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Harjunpaa, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Dalmarco, E.M.; Medeiros, Y.S.; Frode, T.S. Cyclosporin A inhibits CD11a/CD18 adhesion molecules due to inhibition of TNFalpha and IL-1 beta levels in the mouse model of pleurisy induced by carrageenan. Cell Adh. Migr. 2008, 2, 231–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Roller, J.; Menger, M.D.; Thorlacius, H. Sepsis-induced leukocyte adhesion in the pulmonary microvasculature in vivo is mediated by CD11a and CD11b. Eur. J. Pharmacol. 2013, 702, 135–141. [Google Scholar] [CrossRef]
- DeLisser, H.M.; Albelda, S.M. The function of cell adhesion molecules in lung inflammation: More questions than answers. Am. J. Respir. Cell Mol. Biol. 1998, 19, 533–536. [Google Scholar] [CrossRef]
- Baharom, F.; Rankin, G.; Blomberg, A.; Smed-Sorensen, A. Human Lung Mononuclear Phagocytes in Health and Disease. Front. Immunol. 2017, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.Y. Phagocytic defense in the lung. Antibiot. Chemother. (1971) 1985, 36, 74–87. [Google Scholar]
- Quie, P.G. Lung defense against infection. J. Pediatr. 1986, 108, 813–816. [Google Scholar] [CrossRef]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.; Hosseini, E.; Shahbaz Ghasabeh, A.; Mousavi Hosseini, K. Reactive Oxygen Species Generated by CD45-Cells Distinct from Leukocyte Population in Platelet Concentrates Is Correlated with the Expression and Release of Platelet Activation Markers during Storage. Transfus. Med. Hemother. 2018, 45, 33–41. [Google Scholar] [CrossRef]
- Balaiya, S.; Chalam, K.V. An In vitro Assay to Quantify Nitrosative Component of Oxidative Stress. J. Mol. Genet. Med. 2014, 8, 120. [Google Scholar] [CrossRef]
- Roesslein, M.; Hirsch, C.; Kaiser, J.P.; Krug, H.F.; Wick, P. Comparability of in vitro tests for bioactive nanoparticles: A common assay to detect reactive oxygen species as an example. Int. J. Mol. Sci. 2013, 14, 24320–24337. [Google Scholar] [CrossRef]
- Osman, R.; Malmuthuge, N.; Gonzalez-Cano, P.; Griebel, P. Development and Function of the Mucosal Immune System in the Upper Respiratory Tract of Neonatal Calves. Annu. Rev. Anim. Biosci. 2018, 6, 141–155. [Google Scholar] [CrossRef]
- Schneberger, D.; Aharonson-Raz, K.; Singh, B. Monocyte and macrophage heterogeneity and Toll-like receptors in the lung. Cell Tissue Res. 2011, 343, 97–106. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Hostetter, S.J.; Clark, S.K.; Gilbertie, J.M.; Wiechert, S.A.; Jones, D.E.; Sponseller, B.A. Age-related variation in the cellular composition of equine bronchoalveolar lavage fluid. Vet. Clin. Pathol. 2017, 46, 344–353. [Google Scholar] [CrossRef]
- Elghetany, M.T.; Lacombe, F. Physiologic variations in granulocytic surface antigen expression: Impact of age, gender, pregnancy, race, and stress. J. Leukoc. Biol. 2004, 75, 157–162. [Google Scholar] [CrossRef]
- Drosten, C.; Meyer, B.; Muller, M.A.; Corman, V.M.; Al-Masri, M.; Hossain, R.; Madani, H.; Sieberg, A.; Bosch, B.J.; Lattwein, E.; et al. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014, 371, 828–835. [Google Scholar] [CrossRef]
- Mok, C.K.P.; Zhu, A.; Zhao, J.; Lau, E.H.Y.; Wang, J.; Chen, Z.; Zhuang, Z.; Wang, Y.; Alshukairi, A.N.; Baharoon, S.A.; et al. T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: An observational cohort study. Lancet Infect. Dis. 2020, 21, 385–395. [Google Scholar] [CrossRef]
- Adney, D.R.; van Doremalen, N.; Brown, V.R.; Bushmaker, T.; Scott, D.; de Wit, E.; Bowen, R.A.; Munster, V.J. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg. Infect. Dis. 2014, 20, 1999–2005. [Google Scholar] [CrossRef]
- Haagmans, B.L.; van den Brand, J.M.; Raj, V.S.; Volz, A.; Wohlsein, P.; Smits, S.L.; Schipper, D.; Bestebroer, T.M.; Okba, N.; Fux, R.; et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016, 351, 77–81. [Google Scholar] [CrossRef]
- Reusken, C.B.; Haagmans, B.L.; Muller, M.A.; Gutierrez, C.; Godeke, G.J.; Meyer, B.; Muth, D.; Raj, V.S.; Smits-De Vries, L.; Corman, V.M.; et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect. Dis. 2013, 13, 859–866. [Google Scholar] [CrossRef]
- Harcourt, J.L.; Rudoler, N.; Tamin, A.; Leshem, E.; Rasis, M.; Giladi, M.; Haynes, L.M. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Public Health 2018, 65, 749–754. [Google Scholar] [CrossRef]
- Gossner, C.; Danielson, N.; Gervelmeyer, A.; Berthe, F.; Faye, B.; Kaasik Aaslav, K.; Adlhoch, C.; Zeller, H.; Penttinen, P.; Coulombier, D. Human-Dromedary Camel Interactions and the Risk of Acquiring Zoonotic Middle East Respiratory Syndrome Coronavirus Infection. Zoonoses Public Health 2016, 63, 1–9. [Google Scholar] [CrossRef]
- Reusken, C.; Haagmans, B.L.; Koopmans, M.P. Dromedary camels and Middle East respiratory syndrome: MERS coronavirus in the ‘ship of the desert’. Ned. Tijdschr. Geneeskd. 2014, 158, A7806. [Google Scholar]
- Reusken, C.B.; Farag, E.A.; Jonges, M.; Godeke, G.J.; El-Sayed, A.M.; Pas, S.D.; Raj, V.S.; Mohran, K.A.; Moussa, H.A.; Ghobashy, H.; et al. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Eurosurveillance 2014, 19, 20829. [Google Scholar] [CrossRef]
- Al Sulayyim, H.J.; Khorshid, S.M.; Al Moummar, S.H. Demographic, clinical, and outcomes of confirmed cases of Middle East Respiratory Syndrome coronavirus (MERS-CoV) in Najran, Kingdom of Saudi Arabia (KSA); A retrospective record based study. J. Infect. Public Health 2020, 13, 1342–1346. [Google Scholar] [CrossRef]



| Antigen | Antibody Clone | Labeling | Source | Isotype |
|---|---|---|---|---|
| CD14 | Tuk4 | PerCP | Thermofisher | Mouse IgG1 |
| MHCII | TH81A5 | - | Kingfisher | Mouse IgG2a |
| CD172a | DH59b | WSU | Mouse IgG1 | |
| CD163 | LND68A | - | Kingfisher | Mouse IgG1 |
| CD4 | GC50A1 | - | Xceltis | Mouse IgM |
| WC1 | BAQ128A | - | Xceltis | Mouse IgG1 |
| CD11a | HUH73A | - | WSU | Mouse IgG1 |
| Mouse IgM | poly | APC | Thermofisher | Goat IgG |
| Mouse IgG1 | poly | FITC | Thermofisher | Goat IgG |
| Mouse IgG2a | poly | PE | Thermofisher | Goat IgG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussen, J.; Shawaf, T.; Al Humam, N.A.; Alhojaily, S.M.; Al-Sukruwah, M.A.; Almathen, F.; Grandoni, F. Flow Cytometric Analysis of Leukocyte Populations in the Lung Tissue of Dromedary Camels. Vet. Sci. 2022, 9, 287. https://doi.org/10.3390/vetsci9060287
Hussen J, Shawaf T, Al Humam NA, Alhojaily SM, Al-Sukruwah MA, Almathen F, Grandoni F. Flow Cytometric Analysis of Leukocyte Populations in the Lung Tissue of Dromedary Camels. Veterinary Sciences. 2022; 9(6):287. https://doi.org/10.3390/vetsci9060287
Chicago/Turabian StyleHussen, Jamal, Turke Shawaf, Naser Abdallah Al Humam, Sameer M. Alhojaily, Mohammed Ali Al-Sukruwah, Faisal Almathen, and Francesco Grandoni. 2022. "Flow Cytometric Analysis of Leukocyte Populations in the Lung Tissue of Dromedary Camels" Veterinary Sciences 9, no. 6: 287. https://doi.org/10.3390/vetsci9060287
APA StyleHussen, J., Shawaf, T., Al Humam, N. A., Alhojaily, S. M., Al-Sukruwah, M. A., Almathen, F., & Grandoni, F. (2022). Flow Cytometric Analysis of Leukocyte Populations in the Lung Tissue of Dromedary Camels. Veterinary Sciences, 9(6), 287. https://doi.org/10.3390/vetsci9060287






