Analysis of Uterine Blood Flow in Breeding Sows through the Estrus and Early Diestrus, and after Artificial Insemination
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics
2.2. Animals and Study Design
2.3. Semen Collection
2.4. Seminal Dose Preparation and Conservation
2.5. Sperm Analysis
2.5.1. Motility Analysis by CASA
2.5.2. Viability and Acrosome Status Assay
2.5.3. Mitochondrial Activity
2.6. Doppler Ultrasound
2.7. Estrus Detection and Artificial Insemination (AI)
2.8. Return to Estrus and Pregnancy Diagnosis
2.9. Farrowing and Litter Performance
2.10. Statistical Analysis
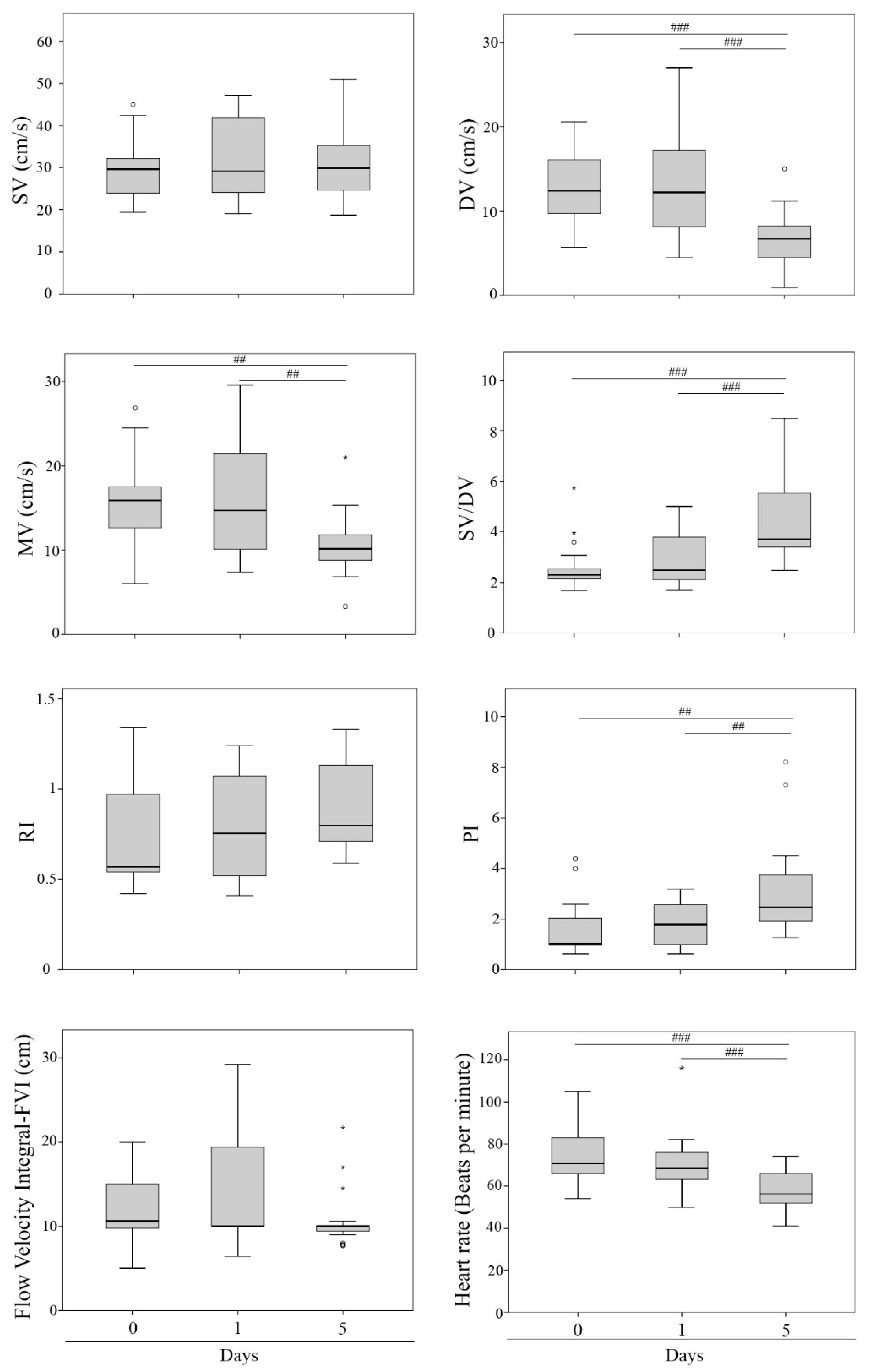
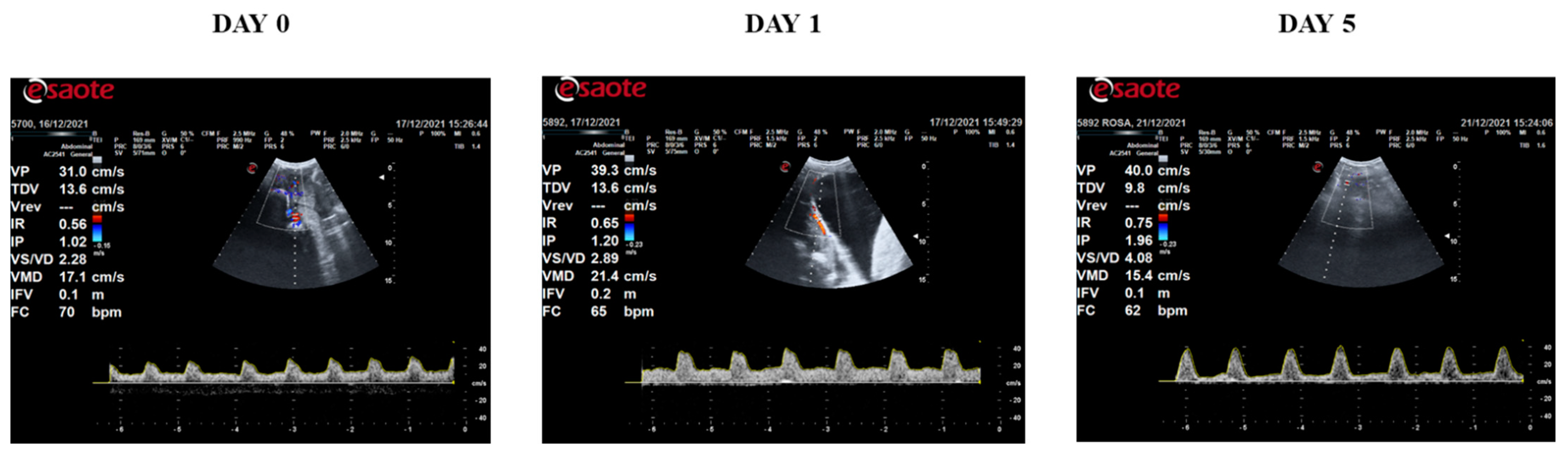
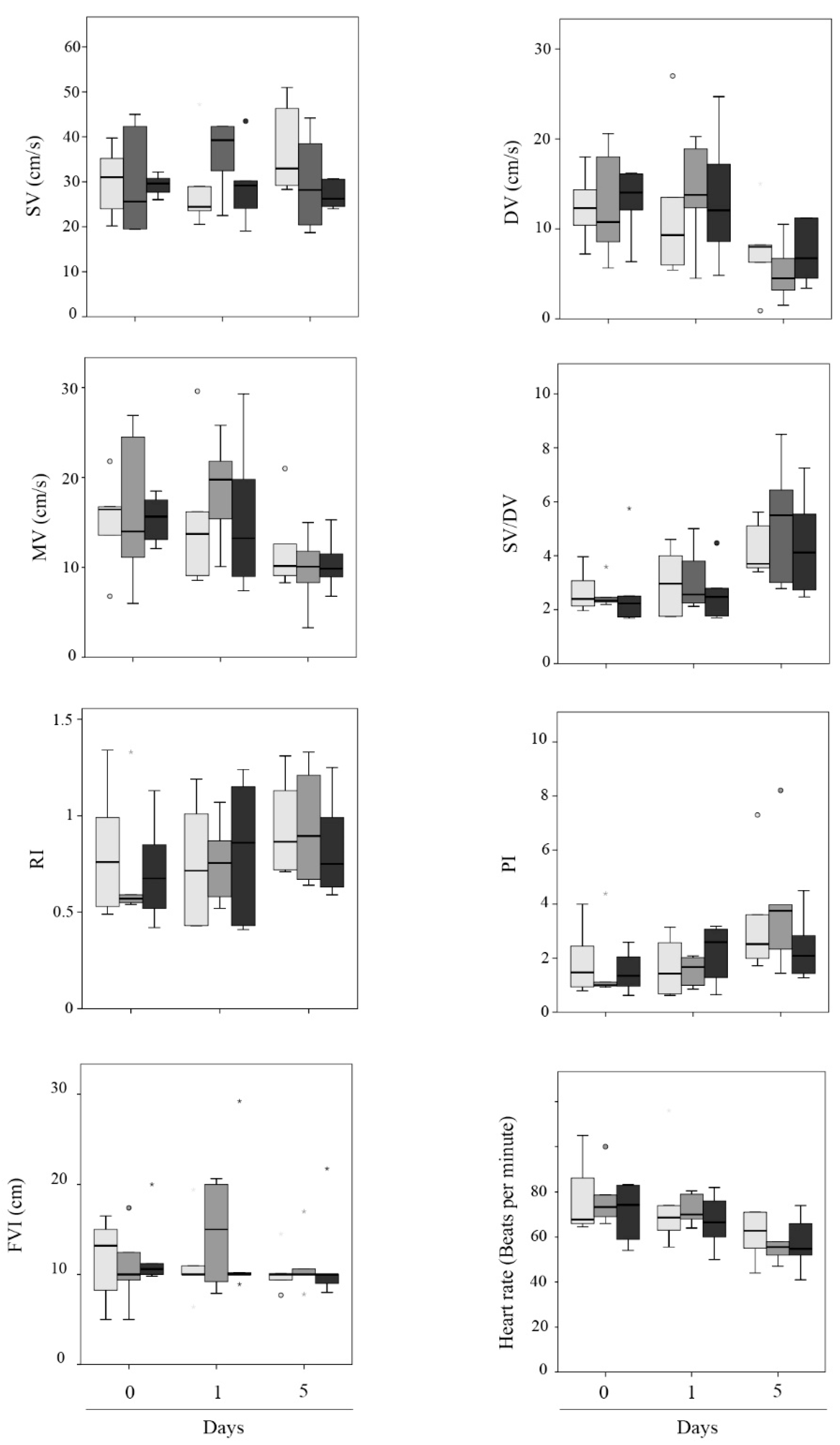
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinat-Botté, F.; Royer, E.; Venturi, E.; Boisseau, C.; Guillouet, P.; Furstoss, V.; Terqui, M. Determination by echography of uterine changes around puberty in gilts and evaluation of a diagnosis of puberty. Reprod. Nut. Dev. 2003, 43, 225–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kauffold, J.; Rautenberg, T.; Richter, A.; Wähner, M.; Sobiraj, A. Ultrasonographic characterization of the ovaries and the uterus in prepubertal and pubertal gilts. Theriogenology 2004, 61, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.I.; de la Sota, R.L. Follicular dynamics and ovulation time in gilts and post-weaning sows. Can. Vet. J. 2017, 58, 65–69. [Google Scholar]
- Kauffold, J.; Peltoniemi, O.; Wehrend, A.; Althouse, G.C. Principles and Clinical Uses of Real-Time Ultrasonography in Female Swine Reproduction. Animals 2019, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, H.; Heppelmann, M.; Lüttgenau, J. Ultrasonographic Doppler Use for Female Reproduction Management. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.H.; Urashiro, E.K.; Siqueira, L.G.; Ghetti, A.; Areas, V.; Guimarães, C.R.; Palhão, M.P.; Camargo, L.S.A.; Fernandes, C.A. Doppler ultrasonography as a tool for ovarian management. Anim. Reprod. 2013, 10, 215–222. [Google Scholar]
- Alvarez-Clau, A.; Liste, F. Ultrasonographic characterization of the uterine artery in the nonestrus bitch. Ultrasound Med. Biol. 2005, 31, 1583–1587. [Google Scholar] [CrossRef]
- Bollwein, H.; Meyer, H.H.D.; Maierl, J.; Weber, F.; Baumgartner, U.; Stolla, R. Transrectal Doppler sonography of uterine blood flow in cows during the estrous cycle. Theriogenology 2000, 53, 1541–1552. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Ignácio, F.S.; Rocha, N.S.; Thompson, D.L., Jr.; Pinto, C.R.; Meira, C. Real-time characterization of the uterine blood flow in mares before and after artificial insemination. Anim. Reprod. Sci. 2015, 160, 90–96. [Google Scholar] [CrossRef]
- Rathmacher, R.P.; Anderson, L.L. Blood flow and progesterone levels in the ovary of cycling and pregnant pigs. Am. J. Physiol. 1968, 214, 1014–1018. [Google Scholar] [CrossRef]
- Ford, S.P.; Christenson, R.K. Blood flow to uteri of sows during the estrous cycle and early pregnancy: Local effect of the conceptus on the uterine blood supply. Biol. Reprod. 1979, 21, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.P.; Reynolds, L.P.; Magness, R.R. Blood flow to the uterine and ovarian vascular beds of gilts during the estrous cycle or early pregnancy. Biol. Reprod. 1982, 27, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Hard, D.L.; Anderson, L.L. Interaction of maternal blood volume and uterine blood flow with porcine fetal development. Biol. Reprod. 1982, 27, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Ford, S.P.; Ferrell, C.L. Blood flow and steroid and nutrient uptake of the gravid uterus and fetus of sows. J. Anim. Sci. 1985, 61, 968–974. [Google Scholar] [CrossRef]
- Guenther, A.E.; Conley, A.J.; Van Orden, D.E.; Farley, D.B.; Ford, S.P. Structural and Mechanical Changes of Uterine Arteries during Pregnancy in the Pig. J. Anim. Sci. 1988, 66, 3144–3152. [Google Scholar] [CrossRef]
- Anderson, L.H.; Christenson, L.K.; Ford, S.P. Estrogen associated changes in uterine blood flow and maternal recognition of pregnancy in Chinese (Meishan) pigs. Anim. Reprod. Sci. 1994, 36, 135–143. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Golba, G.; Kisielewska, J. Effect of human chorionic gonadotropin on uterine blood flow in intact and ovariectomized gilts. Exp. Clin. Endocrinol. Diabetes 1996, 104, 158–163. [Google Scholar] [CrossRef]
- Brüssow, K.P.; Kurth, J.; Vernunft, A.; Becker, F.; Tuchscherer, A.; Kanitz, W. Laparoscopy guided Doppler ultrasound measurement of fetal blood flow indices during early to mid-gestation in pigs. J. Reprod. Dev. 2012, 58, 243–247. [Google Scholar] [CrossRef]
- Père, M.C.; Etienne, M. Uterine blood flow in sows: Effects of pregnancy stage and litter size. Reprod. Nutr. Dev. 2000, 40, 369–382. [Google Scholar] [CrossRef]
- Herlt, C.; Stark, R.; Sigmarsson, H.L.; Kauffold, J. Feasibility of transabdominal Doppler sonography for studying uterine blood flow characteristics in cycling gilts. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere. 2018, 46, 154–163. [Google Scholar] [CrossRef]
- Stenhouse, C.; Tennant, P.; Duncan, W.C.; Ashworth, C.J. Doppler ultrasound can be used to monitor umbilical arterial blood flow in lightly sedated pigs at multiple gestational ages. Reprod. Fertil. Dev. 2018, 30, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Dickson, W.M.; Bosc, M.J.; Locatelli, A. Effect of estrogen and progesterone on uterine blood flow of castrate sows. Am. J. Physiol. 1969, 217, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Krzymowski, T.; Stefańczyk-Krzymowska, S. Uterine blood supply as a main factor involved in the regulation of the estrous cycle—A new theory. Reprod. Biol. 2002, 2, 93–114. [Google Scholar] [PubMed]
- Ford, S.P.; Weber, L.J.; Stormshak, F. Role of estradiol 17- and progesterone in regulating constriction of ovine uterine arteries. Biol. Reprod. 1977, 17, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.P.; Christenson, R.K.; Ford, J.J. Uterine blood flow and uterine arterial, venous and luminal concentrations of oestrogens on days 11, 13 and 15 after oestrus in pregnant and non-pregnant sows. J. Reprod. Fertil. 1982, 64, 185–190. [Google Scholar] [CrossRef]
- Ford, S.P.; Reynolds, L.P.; Farley, D.B.; Bhatnagar, R.K.; Van Orden, D.E. Interaction of ovarían steroids and periarterial α1-adrenergic receptors in altering uterine blood flow during the estrous cycle of gilts. Am. J. Obstet. Gynecol. 1984, 150, 480–484. [Google Scholar] [CrossRef]
- Stefanczyk-Krzymowska, S.; Szafranska, B.; Kaminski, T.; Krzymowski, T. Total content of prostaglandin F2α in the endometrium and myometrium from various sections of the porcine uterine horn during the estrous cycle. Prostaglandins 1994, 48, 99–108. [Google Scholar]
- O’Leary, S.; Jasper, M.J.; Warnes, G.M.; Armstrong, D.T.; Robertson, S.A. Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction 2004, 128, 237–247. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, M.; Martinez, C.A.; Wright, D.; Rodriguez-Martinez, H. The role of semen and seminal plasma in inducing large-scale genomic changes in the female porcine peri-ovulatory tract. Sci. Rep. 2020, 10, 5061. [Google Scholar] [CrossRef]
- De Souza, M.B.; Barbosa, C.D.C.; Pereira, B.S.; Monteiro, C.L.B.; Pinto, J.N.; Linhares, J.C.S.; Da Silva, L.D.M. Doppler velocimetric parameters of the testicular artery in healthy dogs. Res. Vet. Sci. 2014, 96, 533–536. [Google Scholar] [CrossRef]
- Pinggera, G.-M.; Mitterberger, M.; Bartsch, G.; Strasser, H.; Gradl, J.; Aigner, F.; Pallwein, L.; Frauscher, F. Assessment of the intratesticular resistive index by colour Doppler ultrasonography measurements as a predictor of spermatogenesis. BJU Int. 2008, 101, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Caravaca, I.; Izquierdo-Rico, M.J.; Matás, C.; Carvajal, J.A.; Vieira, L.; Abril, D.; Soriano-Úbeda, C.; García-Vázquez, F.A. Reproductive performance and backflow study in cervical and post-cervical artificial insemination in sows. Anim. Reprod. Sci. 2012, 136, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Dickson, W.M.; Bosc, M.J.; Du Mesnil du Buisson, F.; Locatelli, A. The use of an indicator diffusion procedure to measure the uterine blood flow in sows during the estrous cycle and pregnancy. Biol. Reprod. 1969, 1, 295–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ford, S.P. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J. Anim. Sci. 1982, 55, 32–42. [Google Scholar] [PubMed]
- Kaeoket, K.; Persson, E.; Dalin, A.M. The sow endometrium at different stages of the oestrous cycle: Studies on morphological hanges and infiltration by cells of the immune system. Anim. Reprod. Sci. 2001, 65, 95–114. [Google Scholar] [CrossRef]
- Magness, R.R.; Christenson, R.K.; Ford, S.P. Ovarian blood flow throughout the estrous cycle and early pregnancy in sows. Biol. Reprod. 1983, 28, 1090–1096. [Google Scholar] [CrossRef]
- Soede, N.M.; Langendijk, P.; Kemp, B. Reproductive cycles in pigs. Anim. Reprod. Sci. 2011, 124, 251–258. [Google Scholar] [CrossRef]
- Mendelsohn, M.E. Genomic and nongenomic effects of estrogen in the vasculature. Am. J. Cardiol. 2002, 90, 3F–6F. [Google Scholar] [CrossRef]
- Moonmanee, T.; Navanukraw, C.; Yama, P.; Jitjumnong, J. Uterine artery flow velocity waveform, arterial flow indices, follicular dynamics, and sex hormones during preovulatory period in synchronized ovulatory cycle of Bos indicus beef cows. Reprod. Biol. 2018, 18, 99–108. [Google Scholar] [CrossRef]
- Huckabee, W.E.; Crenshaw, C.; Curet, L.B.; Mann, L.; Barron, D.H. The effect of exogenous oestrogen on the blood flow and oxygen consumption of the uterus of the non-pregnant ewe. Q. J. Exp. Physiol. Cogn. Med. Sci. 1970, 55, 16–24. [Google Scholar] [CrossRef]
- Magness, R.R.; Rosenfeld, C.R. Local and systemic estradiol-17 beta: Effects on uterine and systemic vasodilation. Am. J. Physiol. 1989, 256, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Van Buren, G.A.; Yang, D.S.; Clark, K.E. Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, and inhibitor of nitric oxide synthesis. Am. J. Obstet. Gynecol. 1992, 167, 828–833. [Google Scholar] [CrossRef]
- Roman-Ponce, H.; Thatcher, W.W.; Caton, D.; Barron, D.H.; Wilcox, C.J. Thermal stress effects on uterine blood flow in dairy cows. J. Anim. Sci. 1978, 46, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Ruiz, S. New approaches to assess fertility in domestic animals: Relationship between arterial blood flow to the testicles and seminal quality. Animals 2021, 11, 12. [Google Scholar] [CrossRef]
- Soede, N.M.; Roelofs, J.B.; Verheijen, R.J.; Schouten, W.P.; Hazeleger, W.; Kemp, B. Effect of repeated stress treatments during the follicular phase and early pregnancy on reproductive performance of gilts. Reprod. Domest. Anim. 2007, 42, 135–142. [Google Scholar] [CrossRef]
- Rozeboom, K.J.; Troedsson, M.H.; Crabo, B.G. Characterization of uterine leukocyte infiltration in gilts after artificial insemination. J. Reprod. Fertil. 1998, 114, 195–199. [Google Scholar] [CrossRef]
- Rozeboom, K.J.; Troedsson, M.H.; Molitor, T.W.; Crabo, B.G. The effect of spermatozoa and seminal plasma on leukocyte migration into the uterus of gilts. J. Anim. Sci. 1999, 77, 2201–2206. [Google Scholar] [CrossRef]
- Still, J.G.; Greiss, F. The effect of prostaglandins and other vasoactive substances on uterine blood flow and myometrial activity. Am. J. Obstet. Gynecol. 1978, 130, 1–8. [Google Scholar] [CrossRef]
- Luongo, C.; Llamas-López, P.J.; Hernández-Caravaca, I.; Matás, C.; García-Vázquez, F.A. Should all fractions of the boar ejaculate be prepared for insemination rather than using the sperm rich only? Biology 2022, 11, 210. [Google Scholar] [CrossRef]
- Bollwein, H.; Sowade, C.; Stolla, R. The effect of semen extender, seminal plasma and raw semen on uterine and ovarian blood flow in mares. Theriogenology 2003, 60, 607–616. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, M.; Abrol, A.; Soni, T. Doppler sonography of uterine blood flow at mid-oestrus during different degree of clinical endometritis in dairy cows. Reprod. Domest. Anim. 2019, 54, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
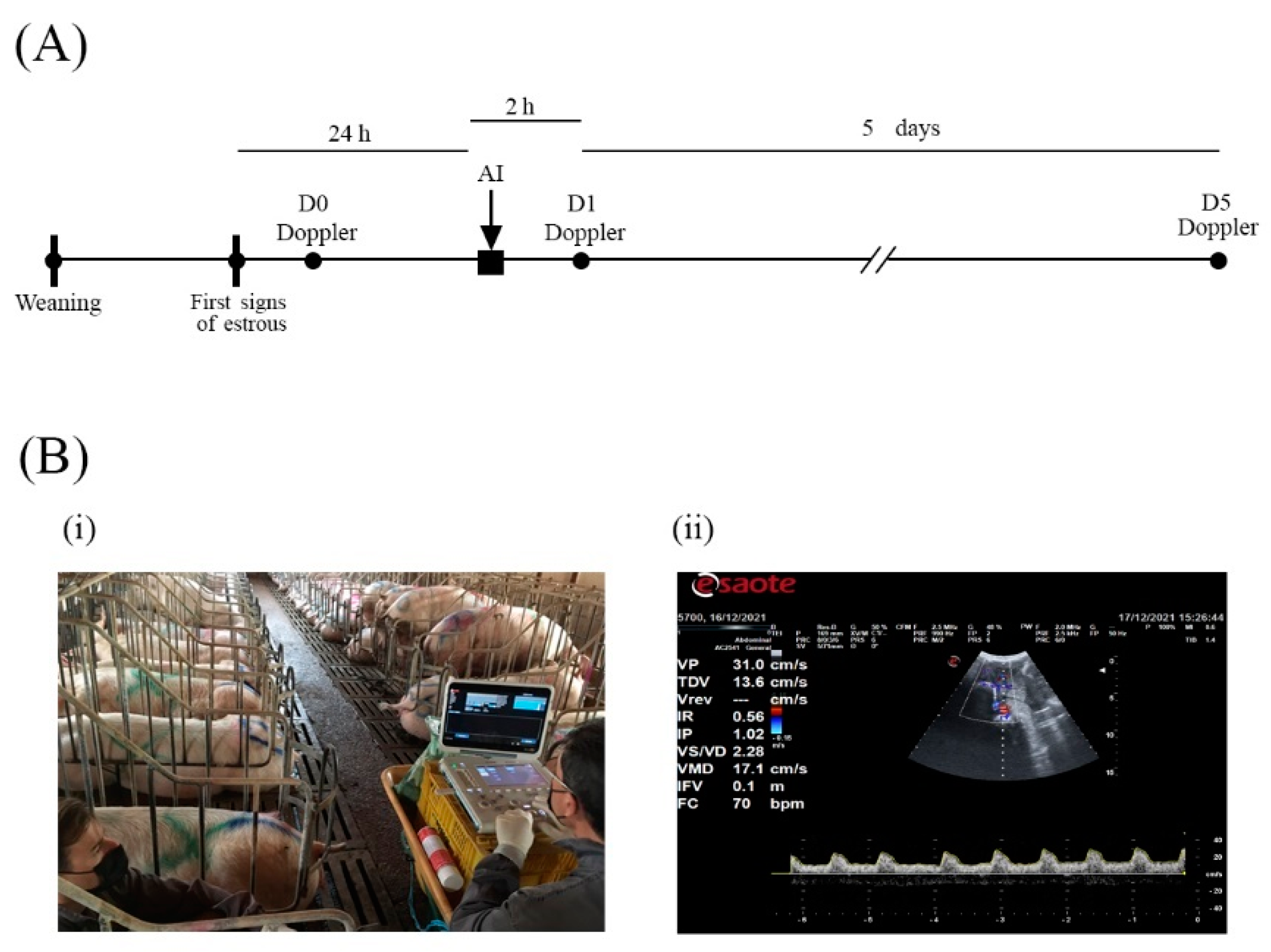
| Experimental Group | Sows (n) | Parity | Weaning-To-Estrus Interval (Days) |
|---|---|---|---|
| Global | 18 | 4.22 ± 0.87 | 3.77 ± 0.42 |
| F1 | 6 | 4.33 ± 1.03 | 3.83 ± 0.40 |
| F2 | 6 | 4.16 ± 0.75 | 3.83 ± 0.40 |
| F3 | 6 | 3.92 ± 0.98 | 3.66 ± 0.51 |
| Doppler Ultrasound Parameters | Abbreviation | Units | Description |
|---|---|---|---|
| Systolic velocity | SV | cm/s | Peak systolic velocity |
| Diastolic velocity | DV | cm/s | End diastolic velocity |
| Mean velocity | MV | cm/s | Mean velocity |
| Systolic velocity/diastolic velocity | SV/DV | - | SV/DV relationship |
| Resistance Index | RI | - | (SV − DV)/SV |
| Pulsatility Index | PI | - | (SV − DV)/MV |
| Flow velocity integral | FVI | cm | Area within the spectral curve |
| Heart rate | HR | bpm | Beats per minute |
| Total Motility (%) | Progressive Motility (%) | Viability (%) | Acrosome Integrity (%) | Mitochondrial Activity (%) | |
|---|---|---|---|---|---|
| Global | 90.5 ± 2.7 | 51.6 ± 6.0 | 94.0 ± 0.6 | 97.0 ± 0.2 | 91.6 ± 1.0 |
| F1 | 93.0 ± 1.0 | 49.5 ± 14.5 | 93.5 ± 1.5 | 97.0 ± 0.0 | 92.5 ± 1.5 |
| F2 | 87.5 ± 8.5 | 53.5 ± 6.5 | 94.0 ± 1.0 | 97.0 ± 0.0 | 91.5 ± 1.5 |
| F3 | 91.5 ± 3.1 | 52.0 ± 17.0 | 94.5 ± 1.5 | 97.0 ± 1.0 | 91.0 ± 3.0 |
| F1 | F2 | F3 | p-Value | |
|---|---|---|---|---|
| SV (cm/s) | 31.73 ± 2.23 | 31.88 ± 2.40 | 28.52 ± 1.21 | 0.54 |
| DV (cm/s) | 10.64 ± 1.41 | 10.49 ± 1.49 | 11.22 ± 1.32 | 0.93 |
| MV (cm/s) | 14.12 ± 1.41 | 14.88 ± 1.68 | 13.71 ± 1.32 | 0.89 |
| SV/DV | 3.28 ± 0.28 | 3.51 ± 0.44 | 3.22 ± 0.40 | 0.69 |
| RI | 0.83 ± 0.07 | 0.80 ± 0.07 | 0.79 ± 0.07 | 0.95 |
| PI | 2.26 ± 0.39 | 2.26 ± 0.46 | 1.99 ± 0.25 | 0.81 |
| FVI (cm) | 11.09 ± 0.89 | 12.08 ± 1.15 | 12.18 ± 1.45 | 0.84 |
| HR (bpm) | 70.52 ± 4.17 | 67.66 ± 3.07 | 65.07 ± 3.09 | 0.67 |
| Experimental Group | Sows (n) | Pregnancy Rate (%) | Farrowing Rate (%) | Total Born Piglets (n) | Live-Born Piglets (n) | Fecundity Index * (n) |
|---|---|---|---|---|---|---|
| Global | 18 | 100 | 94.4 | 20.17 ± 4.6 | 15.58 ± 4.7 | 1471.5 ± 450.3 |
| F1 | 6 | 100 | 100 | 22.66 ± 4.4 | 14.50 ± 5.6 | 1450.0 ± 568.3 |
| F2 | 6 | 100 | 83.3 | 20.60 ± 1.6 | 18.20 ± 2.8 | 1516.0 ± 238.5 |
| F3 | 6 | 100 | 100 | 17.33 ± 5.5 | 14.50 ± 4.9 | 1450.0 ± 92.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, S.; Gardón, J.C.; Hernández-Caravaca, I.; Luongo, C.; García-Vázquez, F.A. Analysis of Uterine Blood Flow in Breeding Sows through the Estrus and Early Diestrus, and after Artificial Insemination. Vet. Sci. 2022, 9, 260. https://doi.org/10.3390/vetsci9060260
Ruiz S, Gardón JC, Hernández-Caravaca I, Luongo C, García-Vázquez FA. Analysis of Uterine Blood Flow in Breeding Sows through the Estrus and Early Diestrus, and after Artificial Insemination. Veterinary Sciences. 2022; 9(6):260. https://doi.org/10.3390/vetsci9060260
Chicago/Turabian StyleRuiz, Salvador, Juan Carlos Gardón, Iván Hernández-Caravaca, Chiara Luongo, and Francisco Alberto García-Vázquez. 2022. "Analysis of Uterine Blood Flow in Breeding Sows through the Estrus and Early Diestrus, and after Artificial Insemination" Veterinary Sciences 9, no. 6: 260. https://doi.org/10.3390/vetsci9060260
APA StyleRuiz, S., Gardón, J. C., Hernández-Caravaca, I., Luongo, C., & García-Vázquez, F. A. (2022). Analysis of Uterine Blood Flow in Breeding Sows through the Estrus and Early Diestrus, and after Artificial Insemination. Veterinary Sciences, 9(6), 260. https://doi.org/10.3390/vetsci9060260






