Altered Intestinal Production of Volatile Fatty Acids in Dogs Triggered by Lactulose and Psyllium Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Treatments and Samplings
2.2. Laboratory Analyses
2.3. Statistics
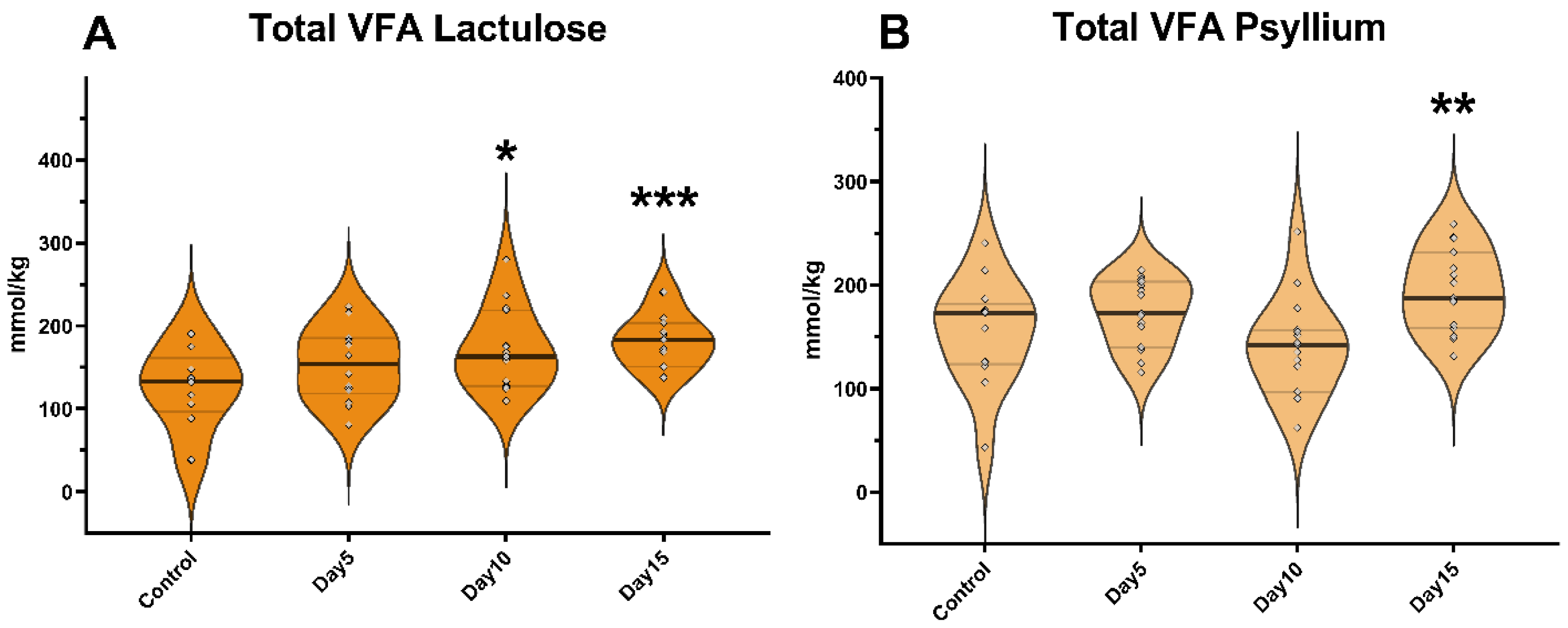
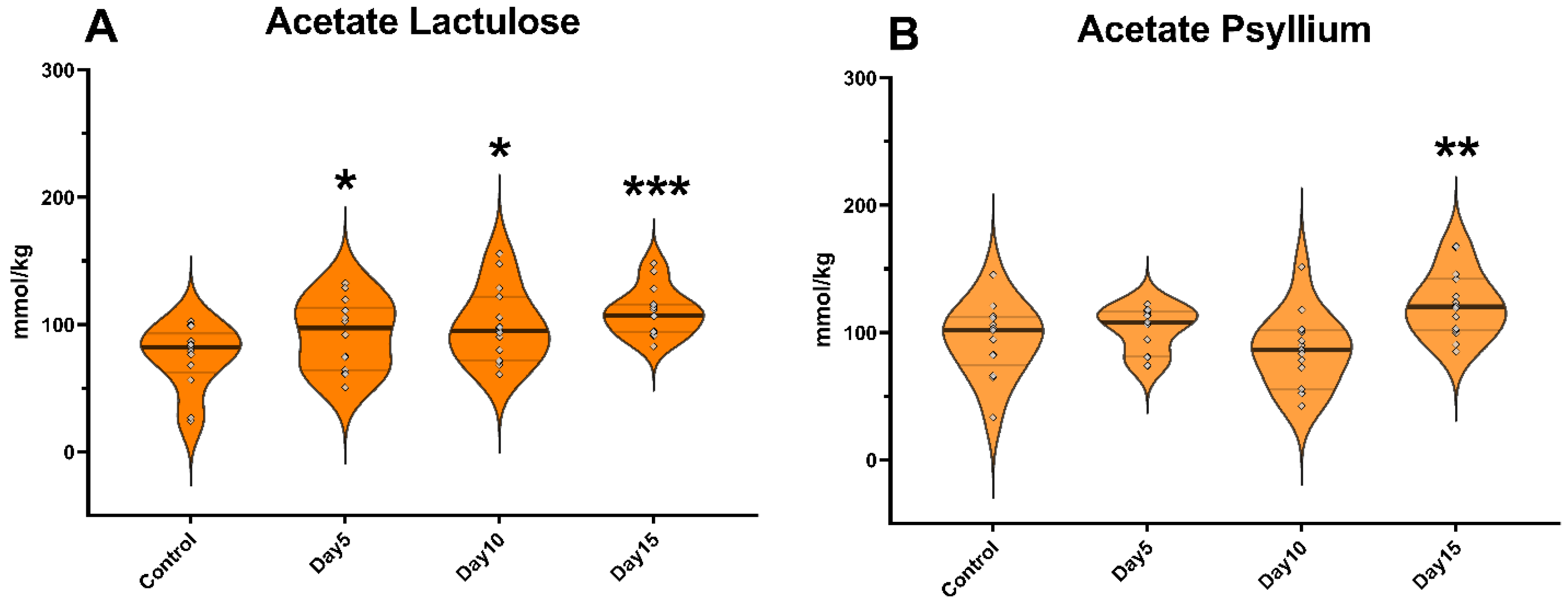
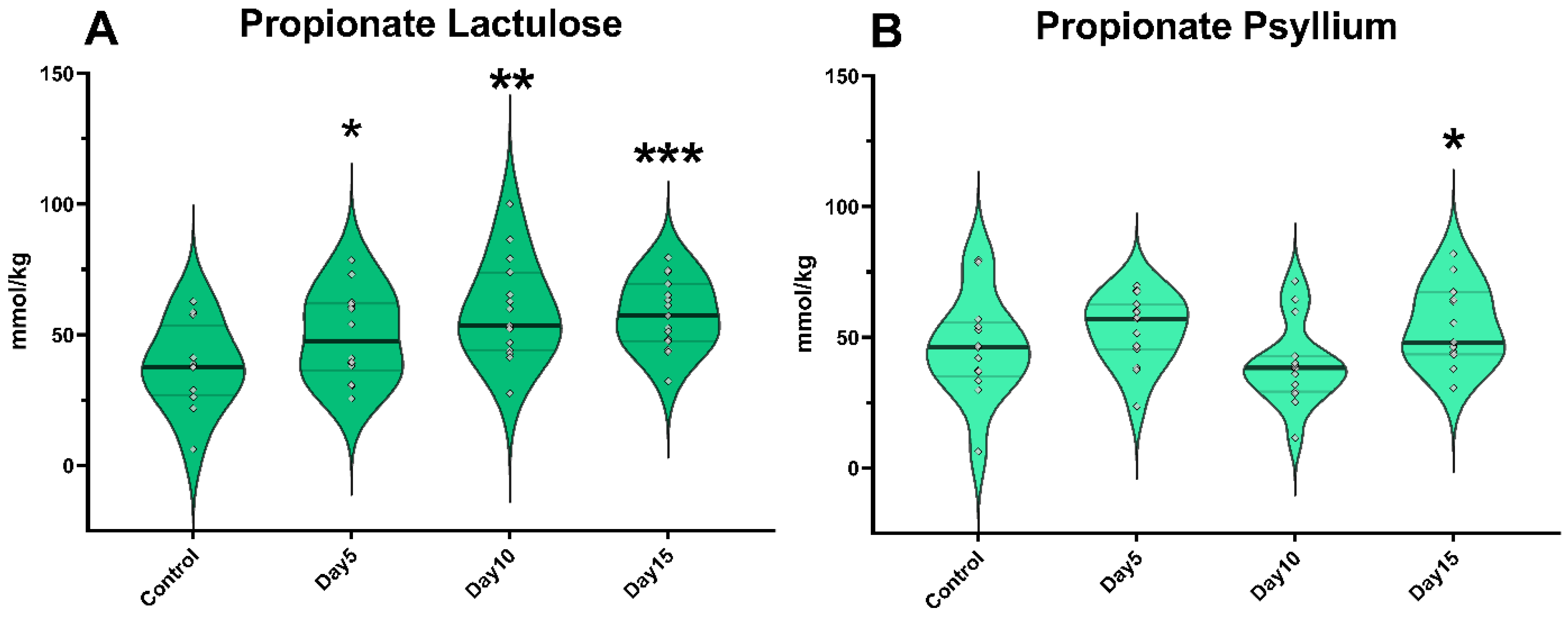
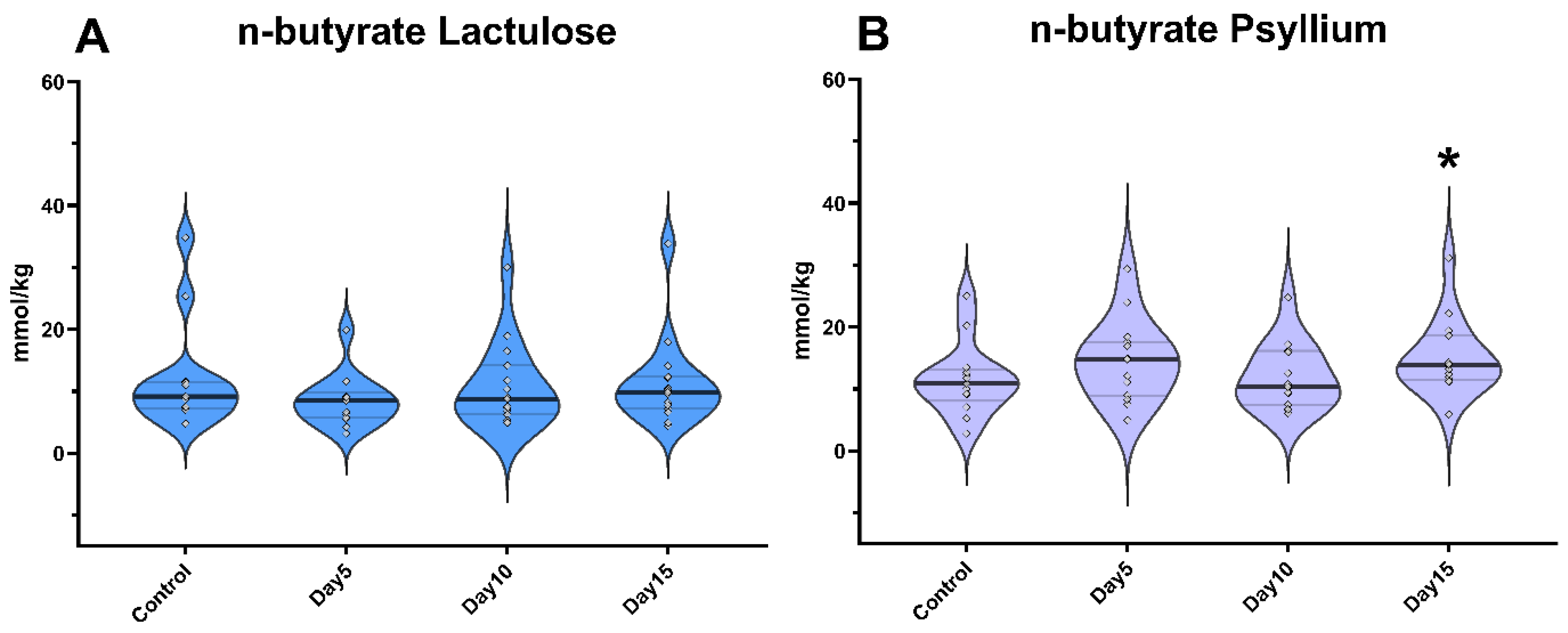
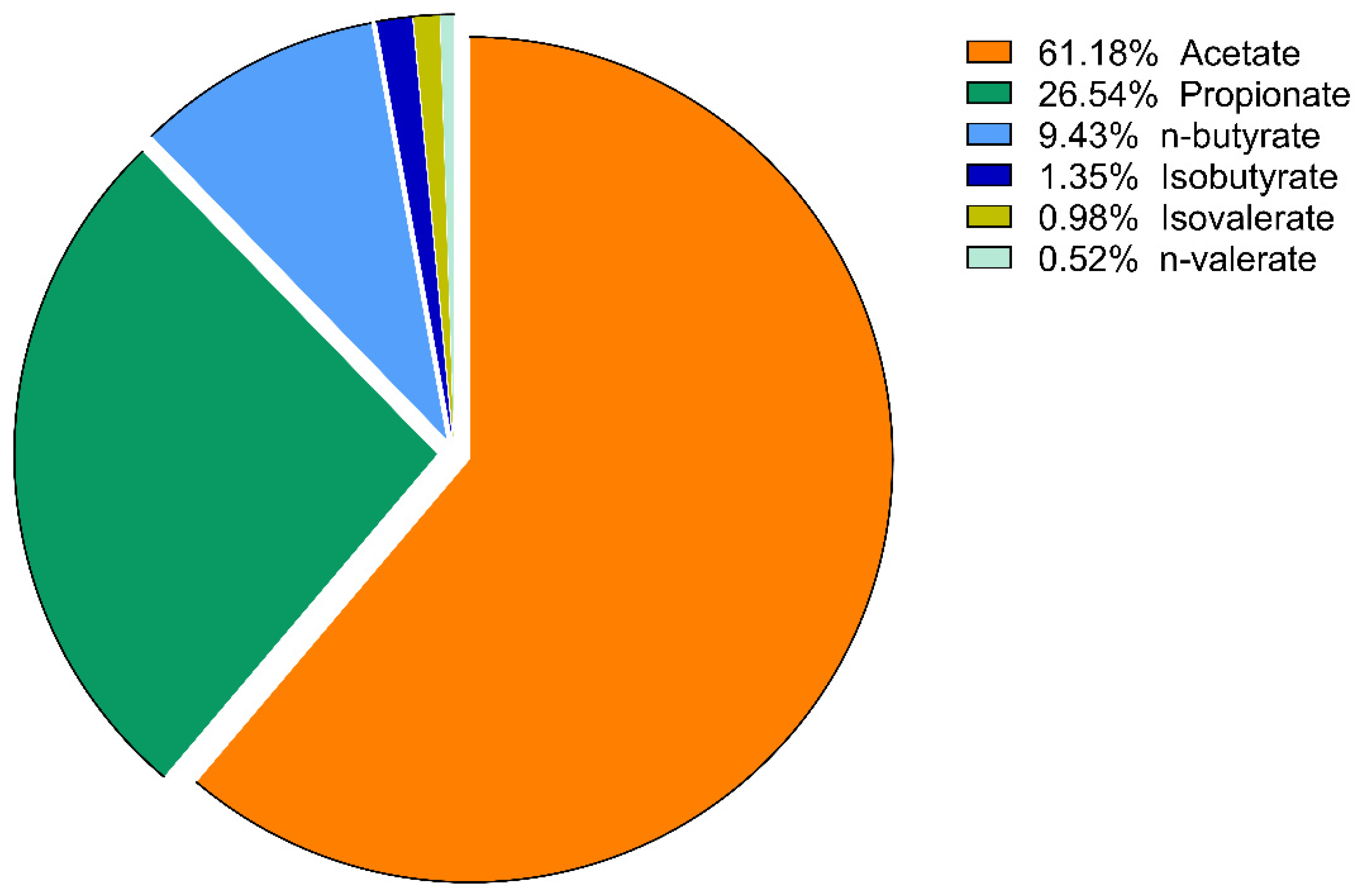
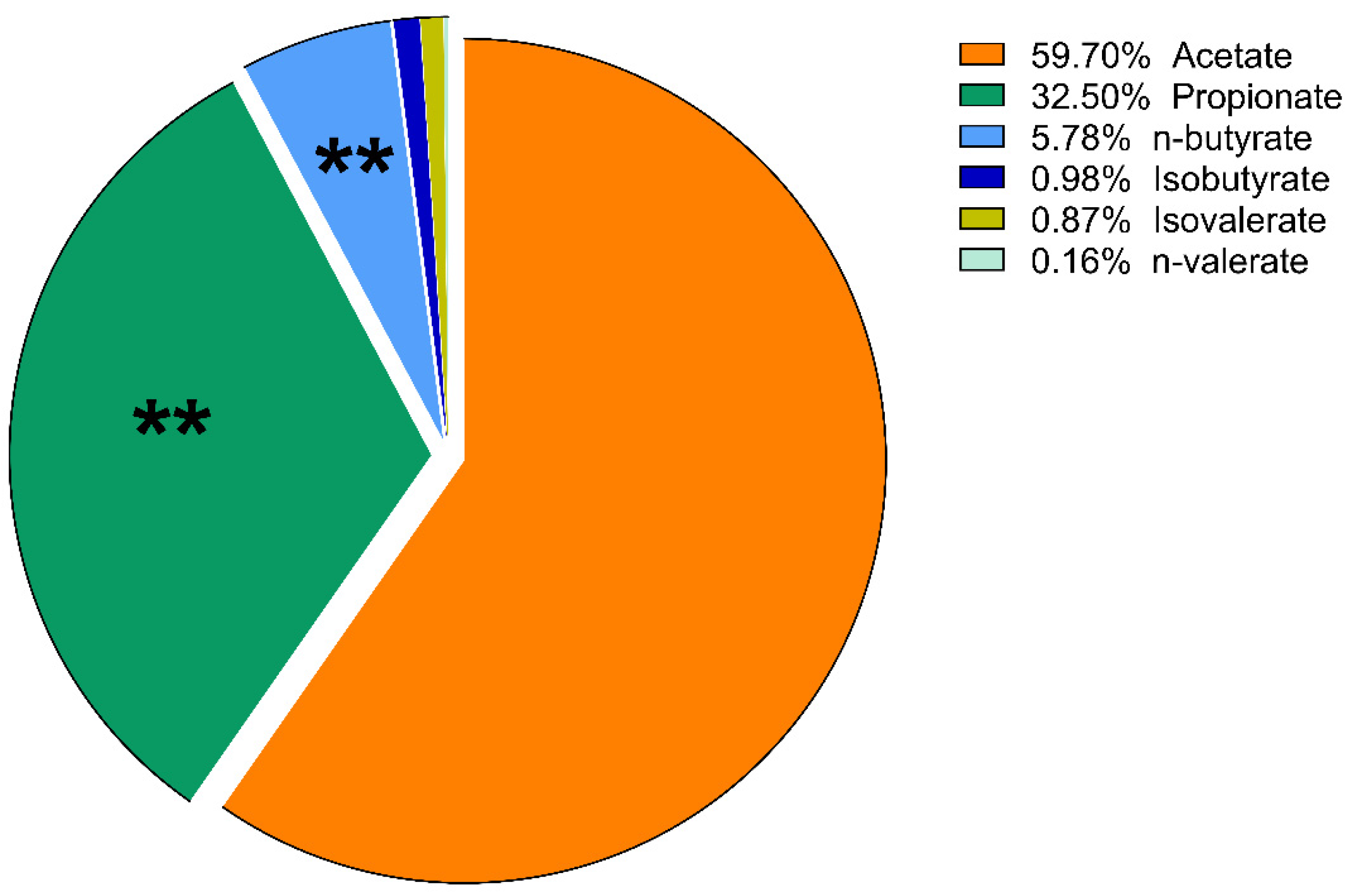
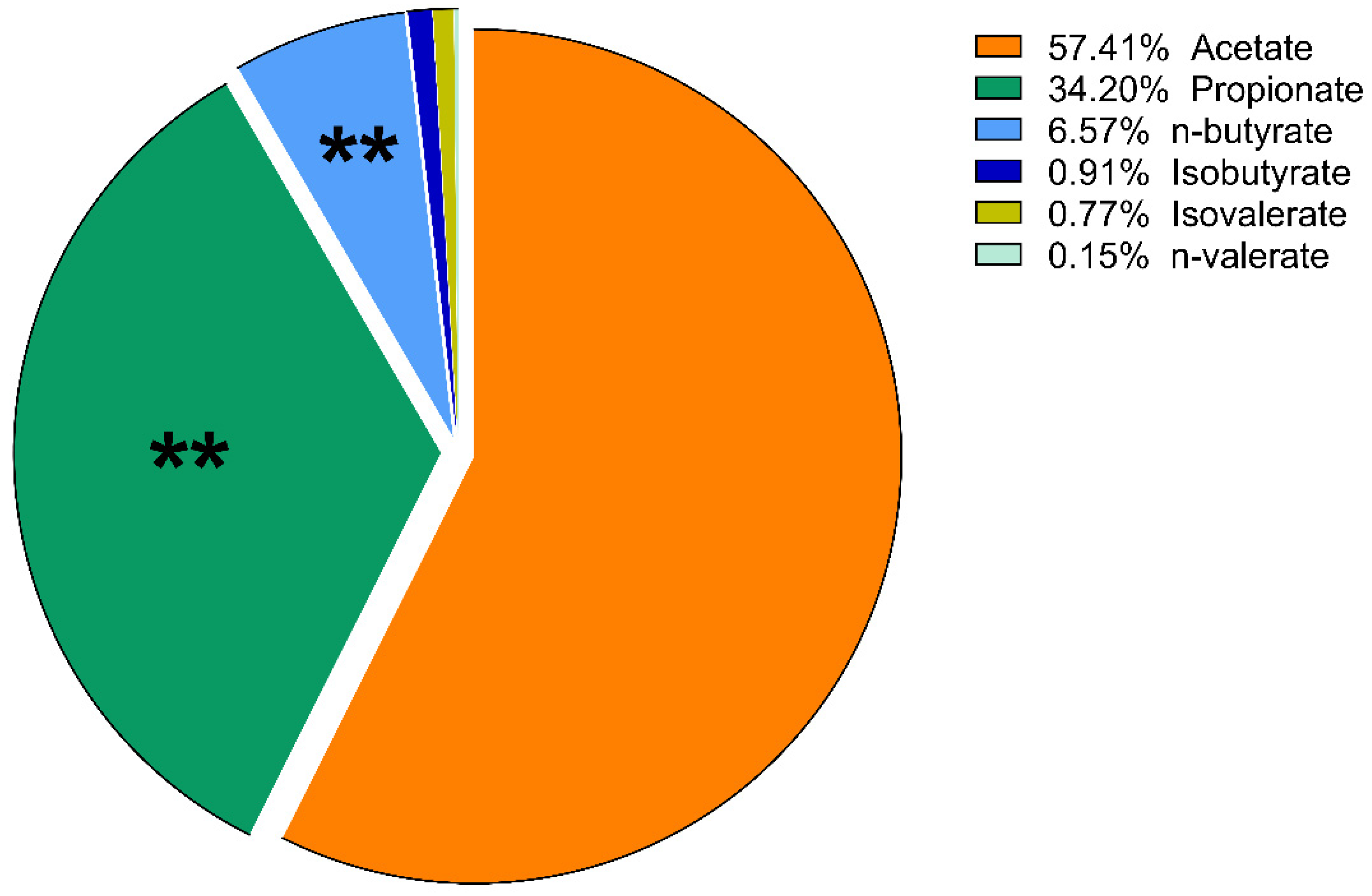
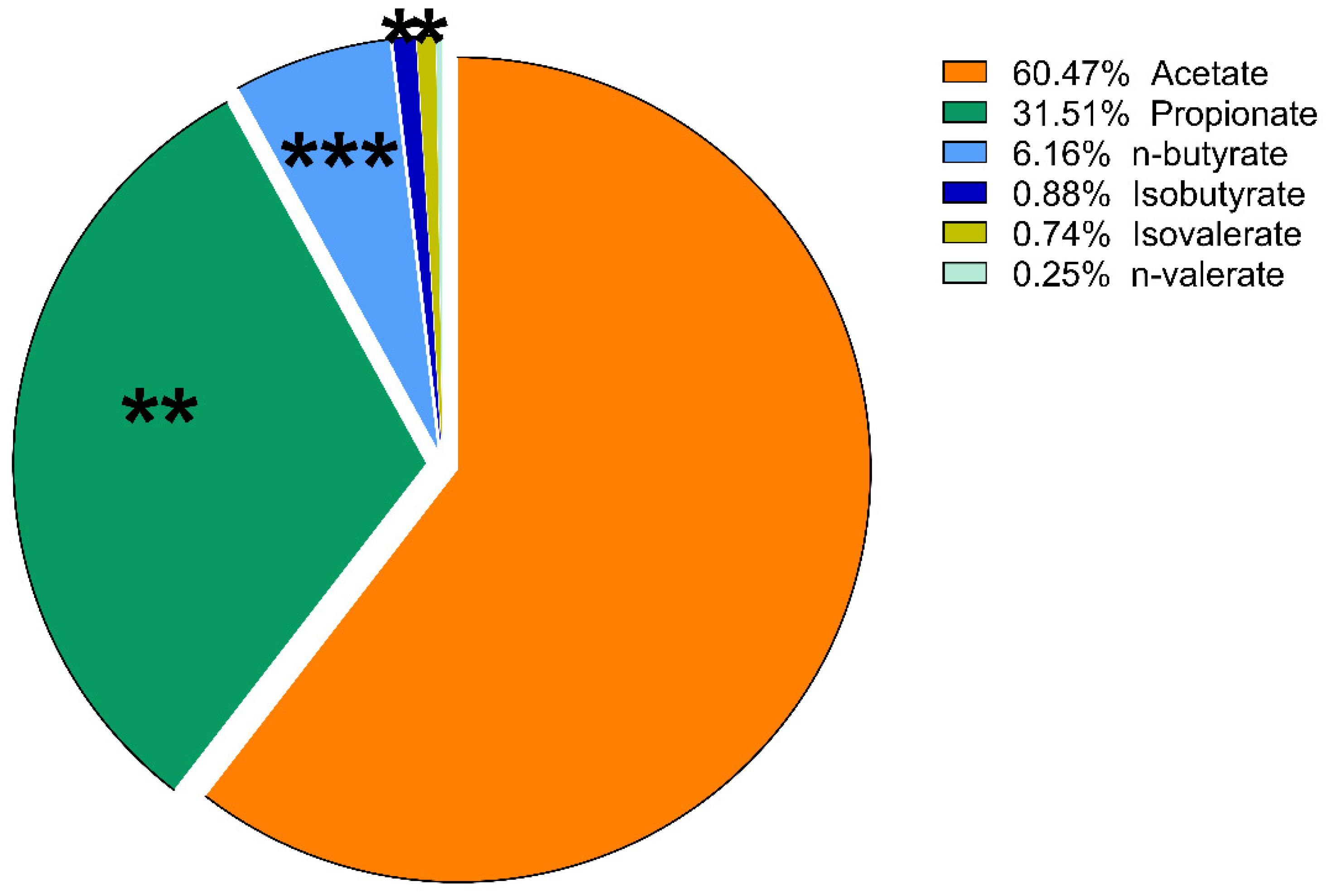
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr. 2006, 46, 459–471. [Google Scholar] [CrossRef]
- Jiang, F.; Du, C.; Jiang, W.; Wang, L.; Du, S.K. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2020, 150, 1155–1161. [Google Scholar] [CrossRef]
- Asp, N.G. Dietary carbohydrates: Classification by chemistry and physiology. Food Chem. 1996, 57, 9–14. [Google Scholar] [CrossRef]
- Cummings, J.H.; Englyst, H.N. Fermentation in the human large intestine and the available substrates. Am. J. Clin. Nutr. 1987, 45, 1243–1255. [Google Scholar] [CrossRef]
- Calabrò, S.; Carciofi, A.C.; Musco, N.; Tudisco, R.; Gomes, M.O.; Cutrignelli, M.I. Fermentation characteristics of several carbohydrate sources for dog diets using the in vitro gas production technique. Ital. J. Anim. Sci. 2013, 12, e4. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Borda-Molina, D.; Mátis, G.; Mackei, M.; Neogrády, Z.; Huber, K.; Seifert, J.; Camarinha-Silva, A. Caeca microbial variation in broiler chickens as a result of dietary combinations using two cereal types, supplementation of crude protein and sodium butyrate. Front. Microbiol. 2021, 11, 3453. [Google Scholar] [CrossRef]
- Mátis, G.; Kulcsár, A.; Mackei, M.; Petrilla, J.; Neogrády, Z. Comparative study on the modulation of incretin and insulin homeostasis by butyrate in chicken and rabbit. PLoS ONE 2018, 13, e0205512. [Google Scholar] [CrossRef]
- Elkington, S.G.; Floch, M.H.; Conn, H.O. Lactulose in the treatment of chronic portal-systemic encephalopathy: A double-blind clinical trial. N. Engl. J. Med. 1969, 281, 408–412. [Google Scholar] [CrossRef]
- Beynen, A.C.; Kappert, H.J.; Yu, S. Dietary lactulose decreases apparent nitrogen absorption and increases apparent calcium and magnesium absorption in healthy dogs. J. Anim. Physiol. Anim. Nutr. 2001, 85, 67–72. [Google Scholar] [CrossRef]
- Lee-Robichaud, H.; Thomas, K.; Morgan, J.; Nelson, R.L. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst. Rev. 2010, 7. [Google Scholar] [CrossRef]
- Aldridge, D.R.; Tranah, E.J.; Shawcross, D.L. Pathogenesis of Hepatic Encephalopathy: Role of Ammonia and Systemic Inflammation. J. Clin. Exp. Hepatol. 2015, 5, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Yakubov, G.E.; Linter, B.R.; MacNaughtan, W.; Foster, T.J. Temperature fractionation, physicochemical and rheological analysis of psyllium seed husk heteroxylan. Food Hydrocoll. 2020, 104, 105737. [Google Scholar] [CrossRef]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef] [Green Version]
- Belorio, M.; Gómez, M. Psyllium: A useful functional ingredient in food systems. Crit. Rev. Food. Sci. Nutr. 2020, 62, 527–538. [Google Scholar] [CrossRef]
- Leib, M.S. Treatment of chronic idiopathic large-bowel diarrhea in dogs with a highly digestible diet and soluble fiber: A retrospective review of 37 cases. J. Vet. Intern. Med. 2000, 14, 27–32. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Pitães, A. The use of soluble fibre for the management of chronic idiopathic large-bowel diarrhoea in police working dogs. BMC Vet. Res. 2021, 17, 100. [Google Scholar] [CrossRef]
- Singh, B. Psyllium as therapeutic and drug delivery agent. Int. J. Pharm. 2007, 334, 1–14. [Google Scholar] [CrossRef]
- Iwasa, M.; Nakao, M.; Kato, Y.; Kobayashi, Y.; Takagi, K.; Kaito, M.; Adaci, Y. Dietary fiber decreases ammonia levels in patients with cirrhosis. Hepatology 2005, 41, 217–218. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef]
- Sa’ad, H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar]
- Zentek, J.; Marquart, B.; Pietrzak, T. Intestinal effects of mannanoligosaccharides, transgalactooligosaccharides, lactose and lactulose in dogs. J. Nutr. 2002, 132, 1682S–1684S. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.D.F.; Salavati Schmitz, S.; Schoenebeck, J.J.; Clements, D.N.; Campbell, S.M.; Gaylor, D.E.; Mellanby, R.J.; Gow, A.G.; Salavati, M. Lactulose drives a reversible reduction and qualitative modulation of the faecal microbiota diversity in healthy dogs. Sci. Rep. 2019, 9, 13350. [Google Scholar] [CrossRef] [Green Version]
- Mendel, M.; Chłopecka, M.; Dziekan, N.; Karlik, W. Phytogenic feed additives as potential gut contractility modifiers—A review. Anim. Feed. Sci. Technol. 2017, 230, 30–46. [Google Scholar] [CrossRef]
- Pinna, C.; Biagi, G. The utilisation of prebiotics and synbiotics in dogs. Ital. J. Anim. Sci. 2014, 13, 3107. [Google Scholar] [CrossRef]
- Foreman, M.; Cherubini, G.B. Managing canine status epilepticus in practice. Companion Anim. 2020, 25, 228–232. [Google Scholar] [CrossRef]
- Gómez-Gallego, C.; Junnila, J.; Männikkö, S.; Hämeenoja, P.; Valtonen, E.; Salminen, S.; Beasley, S. A canine-specific probiotic product in treating acute or intermittent diarrhea in dogs: A double-blind placebo-controlled efficacy study. Vet. Microbiol. 2016, 197, 122–128. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Cheng, W.; Li, Y.; Ma, J.; Zhong, F. Quantitative optimization and assessments of supplemented tea polyphenols in dry dog food considering palatability, levels of serum oxidative stress biomarkers and fecal pathogenic bacteria. RSC Adv. 2016, 6, 16802–16807. [Google Scholar] [CrossRef]
- Dhakal, J.; Aldrich, C.G. Use of medium chain fatty acids to mitigate Salmonella typhimurium (ATCC 14028) on dry pet food kibbles. J. Food Prot. 2020, 83, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- de Brito, C.B.M.; Menezes Souza, C.M.; Bastos, T.S.; Mesa, D.; Oliveira, S.G.; Félix, A.P. Effect of dietary inclusion of dried apple pomace on faecal butyrate concentration and modulation of gut microbiota in dogs. Arch. Anim. Nutr. 2021, 75, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Da, H.; Ra, A.; M, S.; Ce, S. Absorption of volatile fatty acid, Na, and H2O by the colon of the dog. Am. J. Vet. Res. 1981, 42, 1118–1124. [Google Scholar]
- Stevens, C.E.; Hume, I.D. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol. Rev. 1998, 78, 393–427. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Knarreborg, A.; Miquel, N.; Granli, T.; Jensen, B.B. Establishment and application of an in vitro methodology to study the effects of organic acids on coliform and lactic acid bacteria in the proximal part of the gastrointestinal tract of piglets. Anim. Feed Sci. Technol. 2002, 99, 131–140. [Google Scholar] [CrossRef]
- Sun, Y.; O’Riordan, M.X.D. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar]
- McQuaid, T.S. Medical management of a patent ductus venosus in a dog. Can. Vet. J. 2005, 46, 352–356. [Google Scholar]
- Schuster-Wolff-Bühring, R.; Fischer, L.; Hinrichs, J. Production and physiological action of the disaccharide lactulose. Int. Dairy J. 2010, 20, 731–741. [Google Scholar] [CrossRef]
- Bliss, D.Z.; Weimer, P.J.; Jung, H.J.G.; Savik, K. In vitro degradation and fermentation of three dietary fiber sources by human colonic bacteria. J. Agric. Food Chem. 2013, 61, 4614–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Căpriţă, A.; Căpriţă, R.; Simulescu, V.O.G.; Drehe, R.M. Dietary fiber: Chemical and functional properties. J. Agroaliment. Processes Technol. 2010, 16, 406–410. [Google Scholar]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Huang, J.Y.; Ho, L.T.; Verdin, E. Acetate metabolism and aging: An emerging connection. Mech. Ageing Dev. 2010, 131, 511–516. [Google Scholar] [CrossRef]
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate metabolism in physiology, cancer, and beyond. Trends Cell Biol. 2019, 29, 695–703. [Google Scholar] [CrossRef]
- Gebreselassie, E.E.; Jackson, M.I.; Yerramilli, M.; Jewell, D.E. Anti-aging food that improves markers of health in senior dogs by modulating gut microbiota and metabolite profiles. bioRxiv 2018, 324327. [Google Scholar] [CrossRef] [Green Version]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef] [Green Version]
- Green, M.H. Are fatty acids gluconeogenic precursors? J. Nutr. 2020, 150, 2235–2238. [Google Scholar] [CrossRef]
- Tirosh, A.; Calay, E.S.; Tuncman, G.; Claiborn, K.C.; Inouye, K.E.; Eguchi, K.; Alcala, M.; Rathaus, M.; Hollander, K.S.; Ron, I.; et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019, 11, eaav0120. [Google Scholar] [CrossRef]
- González-Fandos, E.; Maya, N.; Pérez-Arnedo, I. Effect of propionic acid on Campylobacter jejuni attached to chicken skin during refrigerated storage. Int. Microbiol. 2015, 171–175. [Google Scholar]
- Ormsby, M.J.; Johnson, S.A.; Carpena, N.; Meikle, L.M.; Goldstone, R.J.; McIntosh, A.; Wessel, H.M.; Hulme, H.E.; McConnachie, C.C.; Connolly, J.P.R.; et al. Propionic Acid Promotes the Virulent Phenotype of Crohn’s Disease-Associated Adherent-Invasive Escherichia coli. Cell Rep. 2020, 30, 2297–2305.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panasevich, M.R.; Morris, E.M.; Chintapalli, S.V.; Wankhade, U.D.; Shankar, K.; Britton, S.L.; Koch, L.G.; Thyfault, J.P.; Rector, R.S. Gut microbiota are linked to increased susceptibility to hepatic steatosis in low-aerobic-capacity rats fed an acute high-fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, 166–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, C.E.; Dodd, S.; Abood, S.K.; Verbrugghe, A. Commercial diet recommendations and follow-up for a large breed puppy with an intrahepatic portosystemic shunt. Can. Vet. J. 2021, 62, 598. [Google Scholar]
- Favier, R.P.; de Graaf, E.; Corbee, R.J.; Kummeling, A. Outcome of non-surgical dietary treatment with or without lactulose in dogs with congenital portosystemic shunts. Vet. Q. 2020, 40, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Petrilla, J.; Mátis, G.; Kulcsár, A.; Talapka, P.; Bíró, E.; Mackei, M.; Fébel, H.; Neogrády, Z. Effect of dietary cereal type, crude protein and butyrate supplementation on metabolic parameters of broilers. Acta Vet. Hung. 2018, 66, 408–452. [Google Scholar] [CrossRef]
- Galfi, P.; Bokori, J. Feeding trial in pigs with a diet containing sodium n-butyrate. Acta Vet. Hung. 1990, 38, 3–17. [Google Scholar]
- Lan, R.X.; Li, S.Q.; Zhao, Z.; An, L.L. Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet. Med. Sci. 2020, 6, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Jirsova, Z.; Heczkova, M.; Dankova, H.; Malinska, H.; Videnska, P.; Vespalcova, H.; Micenkova, L.; Bartonova, L.; Sticova, E.; Lodererova, A.; et al. The effect of butyrate-supplemented parenteral nutrition on intestinal defence mechanisms and the parenteral nutrition-induced shift in the gut microbiota in the rat model. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Bordin, M.; D’Atri, F.; Guillemot, L.; Citi, S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol. Cancer Res. 2004, 12, 692–701. [Google Scholar]
- Fernández-Rubio, C.; Ordóñez, C.; Abad-González, J.; Garcia-Gallego, A.; Honrubia, M.P.; Mallo, J.J.; Balaña-Fouce, R. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 2009, 88, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Immerseel, F.; Methner, U.; Rychlik, I.; Nagy, B.; Velge, P.; Martin, G.; Foster, N.; Ducatelle, R.; Barrow, P. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: Exploitation of innate immunity and microbial activity. Epidemiol. Infect. 2006, 133, 959–978. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mátis, G.; Neogrády, Z.; Csikó, G.; Kulcsár, A.; Kenéz, Á.; Huber, K. Effects of orally applied butyrate bolus on histone acetylation and cytochrome P450 enzyme activity in the liver of chicken–a randomized controlled trial. Nutr. Metab. 2013, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, H.E.; Hamaker, B.; Rajan, K.B.; Mutlu, E.; Green, S.J.; Brown, M.; Kaur, A.; Keshavarzian, A. Starch-entrapped microsphere fibers improve bowel habit but do not exhibit prebiotic capacity in those with unsatisfactory bowel habits: A phase I, randomized, double-blind, controlled human trial. Nutr. Res. 2017, 44, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Jaskiewicz, J.; Zhao, Y.; Hawes, J.W.; Shimomura, Y.; Crabb, D.W.; Harris, R.A. Catabolism of isobutyrate by colonocytes. Arch. Biochem. Biophys. 1996, 327, 265–270. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Q.; Wang, C.; Zhang, Y.; Pei, C.; Wang, Y.; Guo, G.; Huo, W.; Zhang, S.; Shi, C.; et al. Effects of isobutyrate on gene expressions of ruminal and small intestinal mucosa of calves. Chin. J. Anim. Nutr. 2015, 27, 2483–2492. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackei, M.; Talabér, R.; Müller, L.; Sterczer, Á.; Fébel, H.; Neogrády, Z.; Mátis, G. Altered Intestinal Production of Volatile Fatty Acids in Dogs Triggered by Lactulose and Psyllium Treatment. Vet. Sci. 2022, 9, 206. https://doi.org/10.3390/vetsci9050206
Mackei M, Talabér R, Müller L, Sterczer Á, Fébel H, Neogrády Z, Mátis G. Altered Intestinal Production of Volatile Fatty Acids in Dogs Triggered by Lactulose and Psyllium Treatment. Veterinary Sciences. 2022; 9(5):206. https://doi.org/10.3390/vetsci9050206
Chicago/Turabian StyleMackei, Máté, Rebeka Talabér, Linda Müller, Ágnes Sterczer, Hedvig Fébel, Zsuzsanna Neogrády, and Gábor Mátis. 2022. "Altered Intestinal Production of Volatile Fatty Acids in Dogs Triggered by Lactulose and Psyllium Treatment" Veterinary Sciences 9, no. 5: 206. https://doi.org/10.3390/vetsci9050206
APA StyleMackei, M., Talabér, R., Müller, L., Sterczer, Á., Fébel, H., Neogrády, Z., & Mátis, G. (2022). Altered Intestinal Production of Volatile Fatty Acids in Dogs Triggered by Lactulose and Psyllium Treatment. Veterinary Sciences, 9(5), 206. https://doi.org/10.3390/vetsci9050206






