Evaluation of Temporary Urethral Stents in the Management of Malignant and Nonmalignant Urethral Diseases in Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
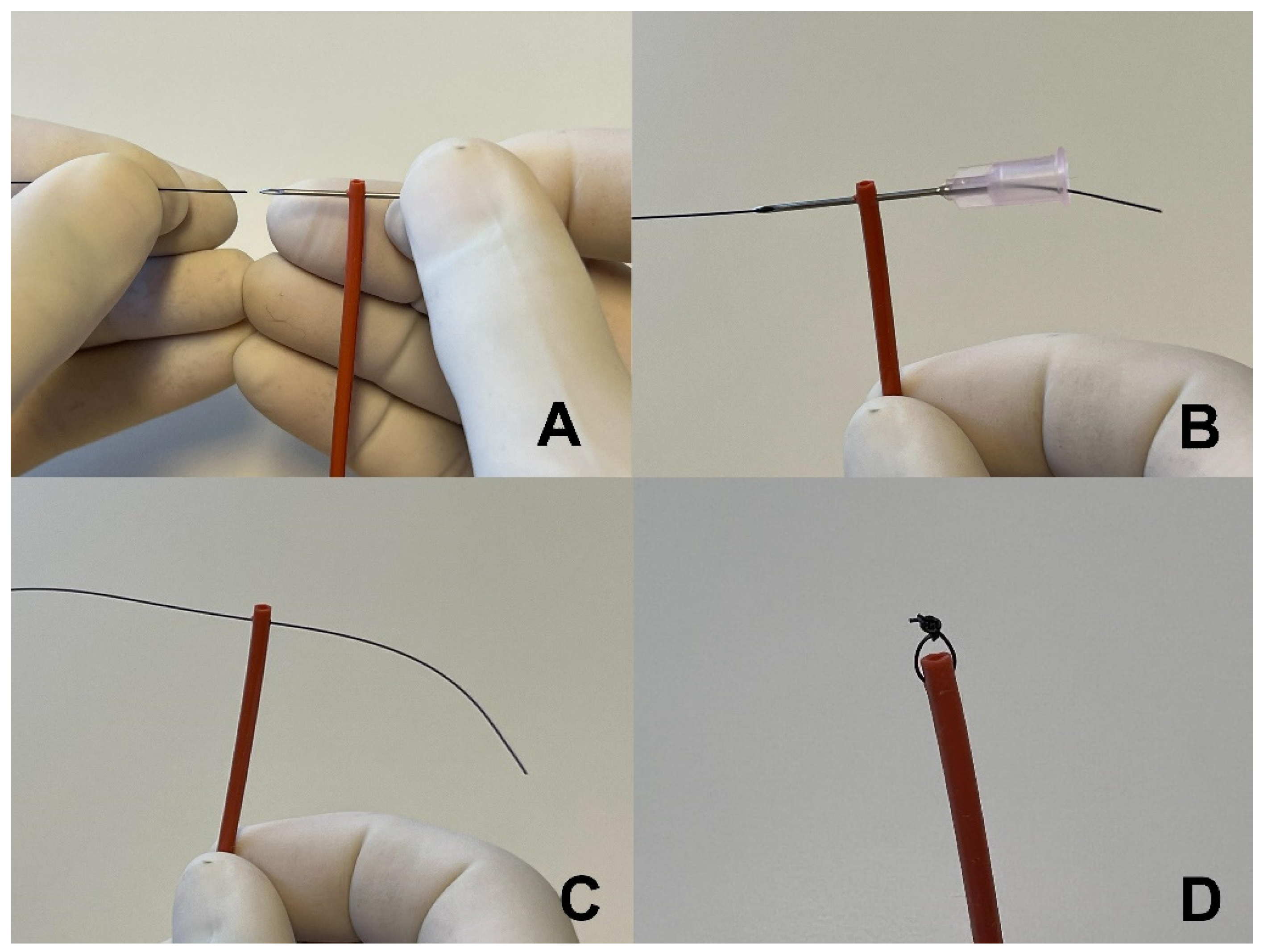
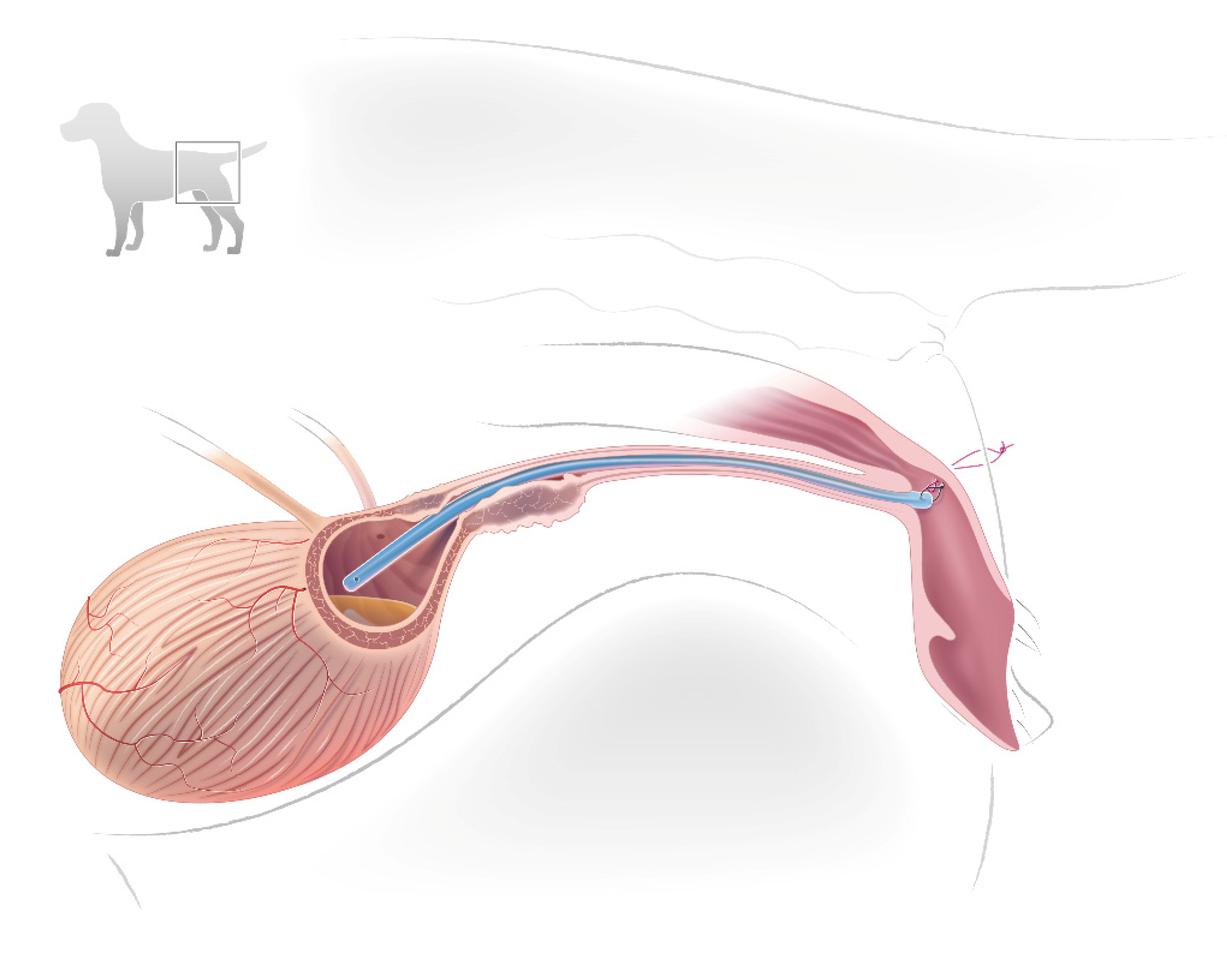
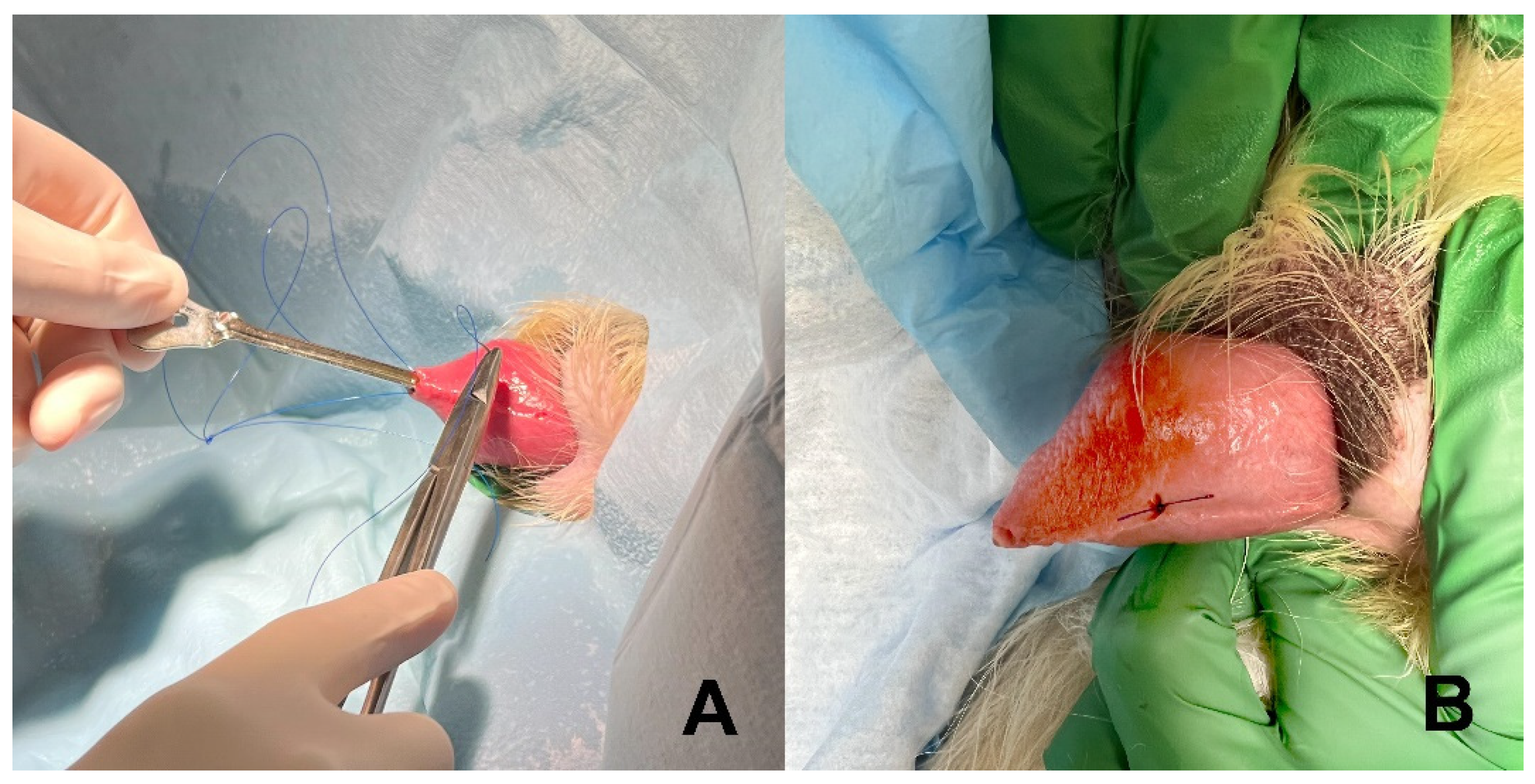
2.2. Stent Construction and Placement
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lippert, A.C.; Fulton, R.B.; Parr, A.M. Nosocomial infection surveillance in a small animal intensive care unit. J. Am. Anim. Hosp. Assoc. 1988, 24, 627–636. [Google Scholar]
- Smarick, S.D.; Haskins, S.C.; Alsrich, J.; Foley, J.E.; Kass, P.H.; Fudge, M.; Ling, G.V. Incidence of catheter-associated urinary tract infection among dogs in a small animal intensive care unit. J. Am. Vet. Med. Assoc. 2004, 224, 1936–1940. [Google Scholar] [CrossRef] [PubMed]
- Cocca, G.; Piva, S.; Magno, S.D.; Scarpellini, R.; Giacometti, F.; Serraino, A.; Giunti, M. Prevalence and Patterns of Antimicrobial Resistance among Escherichia coli and Staphylococcus spp. in a Veterinary University Hospital. Vet. Sci. 2021, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.A.; Campbell, V.L.; Onuma, S.C. Evaluation of open versus closed urine collection systems and development of nosocomial bacteriuria in dogs. J. Am. Vet. Med. Assoc. 2010, 237, 187–190. [Google Scholar] [CrossRef]
- Ogeer-Gyles, J.; Mathews, K.; Weese, J.S.; Prescott, J.F.; Boerlin, P. Evaluation of catheter-associated urinary tract infections and multi-drug-resistent Escherichia coli isolated from the urinary of dogs with indwelling urinary catetheters. J. Am. Vet. Med. Assoc. 2006, 229, 1584–1590. [Google Scholar] [CrossRef]
- Weisse, C.; Berent, A.; Todd, K.; Clifford, C.; Solomon, J. Evaluation of palliative stenting for management of malignant urethral obstructions in dogs. J. Am. Vet. Med. Assoc. 2006, 229, 226–234. [Google Scholar] [CrossRef]
- McMillan, S.K.; Knapp, D.W.; Ramos-Vara, J.A.; Bonney, P.L.; Adams, L.G. Outcome of urethral stent placement for management of urethral obstruction secondary to transitional cell carcinoma in dogs: 19 cases (2007–2010). J. Am. Vet. Med. Assoc. 2012, 241, 1627–1632. [Google Scholar] [CrossRef]
- Blackburn, A.L.; Berent, A.C.; Weiss, C.W.; Brown, D.C. Evaluation of outcome following urethral stent placement for the treatment of obstructive carcinoma of the urethra in dogs: 42 cases (2004–2008). J. Am. Vet. Med. Assoc. 2013, 242, 59–68. [Google Scholar] [CrossRef]
- Hill, T.L.; Berent, A.C.; Weisse, C.W. Evaluation of urethral stent placement for benign urethral obstruction in dogs. J. Vet. Intern. Med. 2014, 28, 1384–1390. [Google Scholar] [CrossRef] [Green Version]
- Fulkerson, C.M.; Knapp, D.W. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet. J. 2015, 205, 217–225. [Google Scholar] [CrossRef]
- Beal, M.W. Interventional management of urethral obstructions. Vet. Clin. Small Anim. 2018, 48, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A. Urethral Stenting for Obstructive Uropathy Utilizing Digital Radiography for Guidance: Feasibility and Clinical Outcome in 26 Dogs. J. Vet. Intern. Med. 2017, 31, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsch, J.A.; Hauptman, J.G.; Walshaw, R. A Urethropexy Technique for Surgical Treatment of Urethral Prolapse in the Male Dog. J. Am. Anim. Hosp. Assoc. 2002, 38, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, T.M.; Jenseb, A.B.; Damborg, P.; Bjornvad, C.R.; Guaedabassi, L.; Jennsen, L.R. Evaluation of different sampling methods and criteria for diagnosing canine urinary tract infection by quantitative bacterial culture. Vet. J. 2016, 216, 168–173. [Google Scholar] [CrossRef]
- Arnold, S.; Weber, U. Urethral sphincter mechanism incompetence in male dogs. Curr. Vet. Ther. XIII 2000, 11, 896–899, Saunders: Philadelphia, PA, USA. [Google Scholar]
- Abdul-Muhsin, H.M.; Jakob, N.J.; McLemore, R.M.; McAdams, S.B.; Humphreys, M.R. Infectious complications associated with the use of temporary prostatic stents in patients with benign prostatic hyperplasia. Can. J. Urol. 2016, 23, 8465–8470. [Google Scholar]
- Tambyah, P.A. Catheter-associated urinary tract infections: Diagnosis and prophylaxis. Int. J. Antimicrob. Agents 2004, 24, 44–48. [Google Scholar] [CrossRef]
- Bubenik, L.; Hosgood, G. Urinary tract infection in dogs with thoracolumbar intervertebral disc herniation. Vet. Surg. 2008, 37, 791–800. [Google Scholar] [CrossRef]
- Stiffler, K.S.; Stevenson, M.A.; Sanchez, S.; Barsanti, J.A.; Hofmeister, E.; Budsberg, S.C. Prevalence and characterization of urinary tract infections in dogs with surgically treated type 1 thoracolumbar intervertebral disc extrusion. Vet. Surg. 2006, 35, 330–336. [Google Scholar] [CrossRef]
- Sabharwal, S.; Sabharwal, S. Using temporary prostatic stents to eliminate bacterial colonization in men with chronic indwelling catheters: A pilot study. Cureus 2018, 10, e3152. [Google Scholar] [CrossRef] [Green Version]
- Boothe, H. Managing Traumatic Urethral Injuries. Clin. Tech. Small Anim. Pract. 2000, 15, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Mann, F.A.; Barrett, R.T.; Henderson, R.A. Use of a Retained Urethral Catheter in 3 dogs with Prostatic Neoplasia. Vet. Surg. 1992, 21, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Skinner, O.T.; Boston, S.E.; Maxwell, P.L. Interventions and experience after complicated total cystectomy in a dog with transitional cell carcinoma. Vet. Surg. 2020, 49, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, M.; Berent, A.C.; Weisse, C.; Donovan, T.; Lamb, K.E. Retospective study of prliferative urethritis in dog: Clinical presentation and outcome using various treatment modalities in 11 dogs. J. Vet. Intern. Med. 2021, 35, 312–320. [Google Scholar] [CrossRef]
- Maggiore, A.D.; Steffey, M.A.; Westropp, J.L. Treatment of traumatic penile urethral stricture in a dog with a self-expanding covered nitinol stent. J. Am. Vet. Med. Assoc. 2013, 242, 1117–1121. [Google Scholar] [CrossRef]
- Saulnier-Troff, F.; Busoni, V.; Hamaide, A. A technique for resection of invasive tumors involving the trigone area of the bladder in dogs: Preliminary results in two dogs. Vet. Surg. 2008, 37, 427–437. [Google Scholar] [CrossRef]
- Holmes, E.S.; Weisse, C.; Berent, A.C. Use of fluoroscopically guided percutaneous antegrade urethral catheterization for the treatement of urethral obstruction in male cats: 9 cases (2000–2009). J. Am. Vet. Med. Assoc. 2012, 241, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Culler, C.A.; Fick, M.; Vigani, V. Ultrasound-guided placement of pigtail cystotomy tubes in dogs with urethral obstruction. J. Vet. Emerg. Crit. Care 2019, 29, 331–336. [Google Scholar] [CrossRef]
- Stiffler, K.S.; Stevenson, M.A.M.; Cornell, K.K.; Glerum, L.E.; Smith, J.D.; Miller, N.A.; Rawlings, C.A. Clinical use of low-profile cystostomy tubes in four dogs and a cat. J. Am. Vet. Med. Assoc. 2003, 223, 325–329. [Google Scholar] [CrossRef]
- Smith, J.D.; Stone, E.A.; Gilson, S.D. Placement of a permanent cystostomy catheter to relieve urine outflow obstruction in dogs with transitional cell carcinoma. J. Am. Vet. Med. Assoc. 1995, 206, 496–499. [Google Scholar]
| Dog | Stent | Patient Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Breed | Urethral Diagnosis | Indication | Duration (days) | Adverse Consequences | |||
| Incontinence | Migration | Urine Infection | |||||||
| Urethral Cancer | |||||||||
| 11 | FS | Labrador | TCC | Obstruction | NFU | Yes | NFU | NFU | NFU |
| 7 | FS | Lhasa | TCC | Obstruction | NFU | Yes | NFU | NFU | NFU |
| 1 | MN | Golden | Lymphoma | Obstruction | 1 | Yes | No | NFU * | Urethrostomy |
| 11 | FS | Corgi | TCC | Obstruction | 6 | Yes | No | NFU | Permanent stent |
| 12 | FS | Beagle | TCC | Obstruction | 7 | Yes | No | NFU * | Euthanized |
| 6 | FS | Labrador | TCC | Obstruction | 24 | Yes | No | NFU * | Euthanized |
| 10 | FS | Labrador | Leiomyosarcoma | Obstruction | 57 | Yes | Yes | Enteroc * (day 13) | Euthanized |
| 10 | FS | Mixed | TCC | Obstruction | 62 | Yes | Yes | NFU * | Permanent stent |
| 12 | FS | Lhasa | TCC | Obstruction | 105 | Yes | No | Staph * (day 56) | Euthanized |
| Extraurethral Cancer | |||||||||
| 13 | FS | Staffordshire terrier | Abdominal sarcoma | Obstruction | 1 | Yes | No | NFU | Euthanized |
| 10 | FS | Beagle | Intrapelvic Hemangiosarcoma | Obstruction | 10 | Yes | No | NG (day 7) | Euthanized |
| 13 | FS | Bichon | Anal gland adenocarcinoma | Obstruction | 337 | Yes | ? | NFU | Euthanized |
| Urethral Inflammation | |||||||||
| 8 | MN | Bichon | Urethral swelling after stone removal | Obstruction | 5 | Yes | No | NFU | Normal voiding |
| 8 | MN | Labrador | Urethral swelling after urethrostomy | Obstruction | 13 | No | No | NFU | Normal voiding |
| Functional Urethral Obstruction | |||||||||
| 5 | MN | St. Bernard | Functional | Obstruction | 11 | Yes | No | NFU | Urethral obstruction, euthanized |
| 12 | FS | Labrador | Functional | Obstruction | 13 | Yes | Yes | Pseudom * Enterococ * E. coli (day 16) | Urethral obstruction, euthanized |
| Urethral Perforation | |||||||||
| 8 | FS | Boxer | Urethral tear | Urine diversion | 9 | Yes | Yes | NFU | Healed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lulich, J.P. Evaluation of Temporary Urethral Stents in the Management of Malignant and Nonmalignant Urethral Diseases in Dogs. Vet. Sci. 2022, 9, 63. https://doi.org/10.3390/vetsci9020063
Lulich JP. Evaluation of Temporary Urethral Stents in the Management of Malignant and Nonmalignant Urethral Diseases in Dogs. Veterinary Sciences. 2022; 9(2):63. https://doi.org/10.3390/vetsci9020063
Chicago/Turabian StyleLulich, Jody P. 2022. "Evaluation of Temporary Urethral Stents in the Management of Malignant and Nonmalignant Urethral Diseases in Dogs" Veterinary Sciences 9, no. 2: 63. https://doi.org/10.3390/vetsci9020063
APA StyleLulich, J. P. (2022). Evaluation of Temporary Urethral Stents in the Management of Malignant and Nonmalignant Urethral Diseases in Dogs. Veterinary Sciences, 9(2), 63. https://doi.org/10.3390/vetsci9020063






