Serum and Fecal Amino Acid Profiles in Cats with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Selection of Cats
2.2. Serum Amino Acid Analysis
2.3. Fecal Amino Acid Analysis
2.4. Statistical Analysis
3. Results
3.1. Cats
3.2. Serum Amino Acids
3.3. Fecal Amino Acids
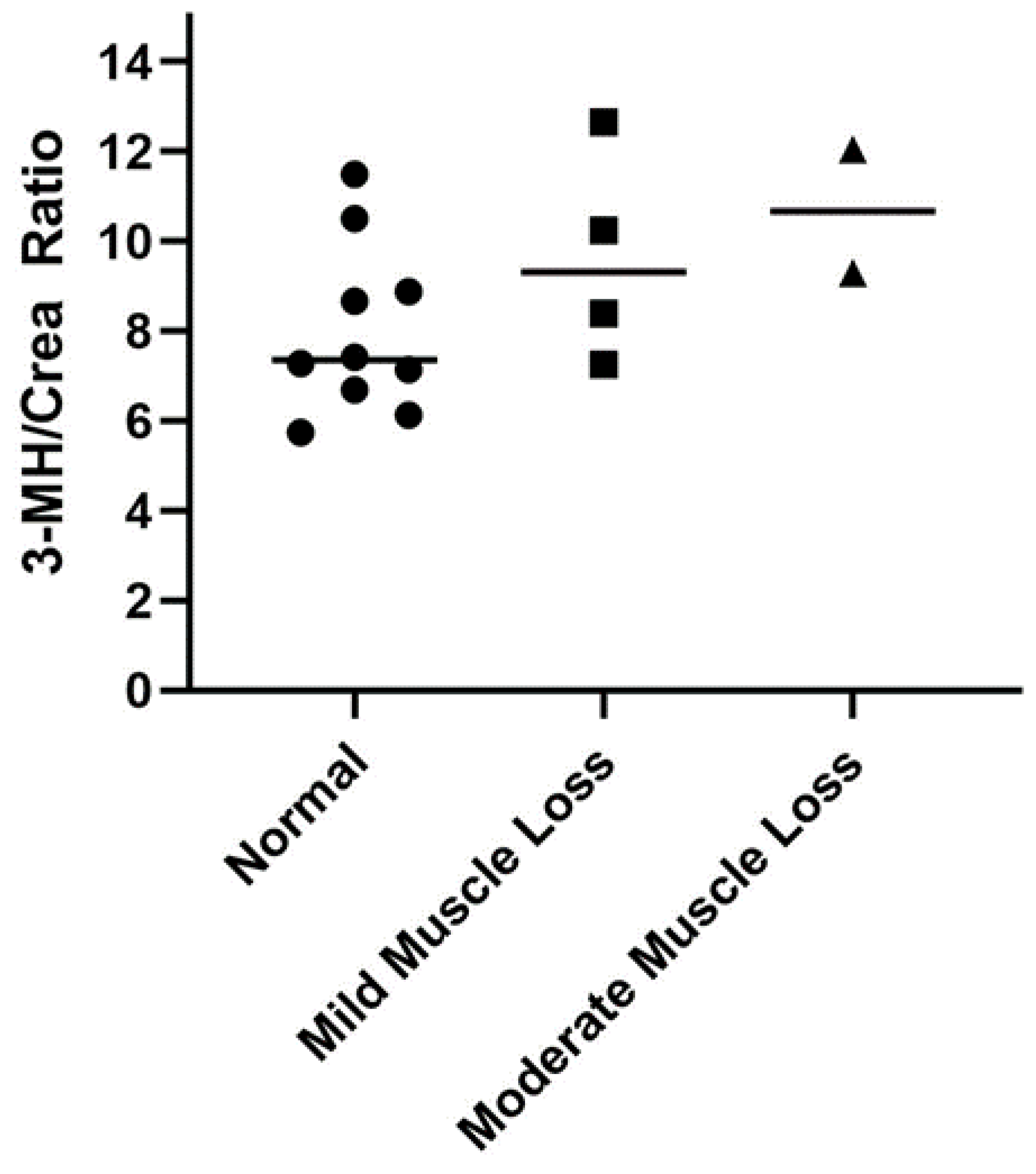
3.4. Serum 3-Methylhistidine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Summers, S.; Quimby, J.M.; Phillips, R.K.; Stockman, J.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J. Preliminary evaluation of fecal fatty acid concentrations in cats with chronic kidney disease and correlation with indoxyl sulfate and p-cresol sulfate. J. Vet. Intern. Med. 2020, 34, 206–215. [Google Scholar] [CrossRef]
- Freeman, L.M.; Lachaud, M.P.; Matthews, S.; Rhodes, L.; Zollers, B. Evaluation of weight loss over time in cats with chronic kidney disease. J. Vet. Intern. Med. 2016, 30, 1661–1666. [Google Scholar] [CrossRef] [Green Version]
- Freeman, L.M. Cachexia and sarcopenia: Emerging syndromes of importance in dogs and cats. J. Vet. Intern. Med. 2012, 26, 3–17. [Google Scholar] [CrossRef]
- Zha, Y.; Qian, Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef]
- Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int. 2003, 64, 2196–2203. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Yu, J.; Wang, Y.; Lu, J.; Shang, E.X.; Zhu, Z.; Guo, J.; Duan, J. Disorder of gut amino acids metabolism during CKD progression is related with gut microbiota dysbiosis and metagenome change. J. Pharm. Biomed. Anal. 2018, 149, 425–435. [Google Scholar] [CrossRef]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef] [Green Version]
- Graboski, A.L.; Redinbo, M.R. Gut-derived protein-bound uremic toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef]
- Goldstein, R.E.; Marks, S.L.; Cowgill, L.D.; Kass, P.H.; Rogers, Q.R. Plasma amino acid profiles in cats with naturally acquired chronic renal failure. Am. J. Vet. Res. 1999, 60, 109–113. [Google Scholar]
- Parker, V.J.; Fascetti, A.J.; Klamer, B.G. Amino acid status in dogs with protein-losing nephropathy. J. Vet. Intern. Med. 2019, 33, 680–685. [Google Scholar] [CrossRef]
- Hansen, B.; DiBartola, S.P.; Chew, D.J.; Browner, C.; Berrie, H.K. Amino acid profiles in dogs with chronic renal failure fed two diets. Am. J. Vet. Res. 1992, 53, 335–341. [Google Scholar]
- Kumar, M.A.; Bitla, A.R.; Raju, K.V.; Monohar, S.M.; Kumar, V.S.; Narasimha, S. Branched chain amino acid profile in early chronic kidney disease. Saudi J. Kidney Dis. Transpl. 2012, 23, 1202–1207. [Google Scholar]
- Zeng, L.; Yu, Y.; Cai, X.; Xie, S.; Chen, J.; Zhong, L.; Zhang, Y. Differences in serum amino acid phenotypes among patients with diabetic nephropathy, hypertensive nephropathy, and chronic nephritis. Med. Sci. Monit. 2019, 25, 7235–7242. [Google Scholar] [CrossRef]
- Laidlaw, S.A.; Berg, R.L.; Kopple, J.D.; Naito, H.; Walker, W.G.; Walser, M. Patterns of fasting plasma amino acid levels in chronic renal insufficiency: Results from the feasibility phase of the Modification of Diet in Renal Disease Study. Am. J. Kidney Dis. 1994, 23, 504–513. [Google Scholar] [CrossRef]
- Liu, S.; Liang, S.; Liu, H.; Chen, L.; Sun, L.; Wei, M.; Jiang, H.; Wang, J. Metabolite profiling of feces and serum in hemodialysis patients and the effect of medicinal charcoal tablets. Kidney Blood Press. Res. 2018, 43, 755–767. [Google Scholar] [CrossRef]
- Kochlik, B.; Gerbracht, C.; Grune, T.; Weber, D. The influence of dietary habits and meat consumption on plasma 3-methylhistidine—A potential marker for muscle protein turnover. Mol. Nutr. Food Res. 2018, 62, e1701062. [Google Scholar] [CrossRef]
- Hall, J.A.; Jackson, M.I.; Farace, G.; Yerramilli, M.; Jewell, D.E. Influence of dietary ingredients on lean body percent, uremic toxin concentrations, and kidney function in senior-adult cats. Metabolites 2019, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Jewell, D.E. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L-carnitine, and medium-chain triglycerides. Vet. J. 2014, 202, 588–596. [Google Scholar] [CrossRef]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachexia Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef]
- Kochlik, B.; Stuetz, W.; Pérès, K.; Feart, C.; Tegner, J.; Rodriguez-Manas, L.; Grune, T.; Weber, D. Associations of plasma 3-methylhistidine with frailty status in French cohorts of the FRAILOMIC Initiative. J. Clin. Med. 2019, 8, 1010. [Google Scholar] [CrossRef] [Green Version]
- Peterson, M.E.; Castellano, C.A.; Rishniw, M. Evaluation of body weight, body condition, and muscle condition in cats with hyperthyroidism. J. Vet. Intern. Med. 2016, 30, 1780–1789. [Google Scholar] [CrossRef]
- Muscle Condition Score—Cat. Available online: https://wsava.org/global-guidelines/global-nutrition-guidelines/ (accessed on 18 January 2022).
- IRIS Staging of CKD. Available online: http://www.iris-kidney.com/guidelines/staging.html (accessed on 18 January 2022).
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- van de Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; Dejong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Suliman, M.E.; Qureshi, A.R.; Stenvinkel, P.; Pecoits-Filho, R.; Barany, P.; Heimburger, O.; Anderstam, B.; Ayala, E.R.; Filho, J.C.D.; Alvestrand, A.; et al. Inflammation contributes to low plasma amino acid concentrations in patients with chronic kidney disease. Am. J. Clin. Nutr. 2005, 82, 342–349. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Moller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef]
- Ceballos, I.; Chauveau, P.; Guerin, V.; Bardet, J.; Parvy, P.; Kamoun, P.; Jungers, P. Early alterations of plasma free amino acids in chronic renal failure. Clin. Chim. Acta 1990, 188, 101–108. [Google Scholar] [CrossRef]
- Bouby, N.; Hassler, C.; Parvy, P.; Bankir, L. Renal synthesis of arginine in chronic renal failure: In vivo and in vitro studies in rats with 5/6 nephrectomy. Kidney Int. 1993, 44, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Suliman, M.E.; Bárány, P.; Filho, J.C.; Lindholm, B.; Bergstrom, J. Accumulation of taurine in patients with renal failure. Nephrol. Dial. Transpl. 2002, 17, 528–529. [Google Scholar] [CrossRef] [Green Version]
- Bergström, J.; Alvestrand, A.; Fürst, P. Plasma and muscle free amino acids in maintenance hemodialysis patients without protein malnutrition. Kidney Int. 1990, 38, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Summers, S.C.; Quimby, J.M.; Isaiah, A.; Suchodolski, J.S.; Lunghofer, P.J.; Gustafson, D.L. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Intern. Med. 2018, 33, 662–669. [Google Scholar] [CrossRef]
- Cheng, F.P.; Hsieh, M.J.; Chou, C.C.; Hsu, W.L.; Lee, Y.J. Detection of indoxyl sulfate levels in dogs and cats suffering from naturally occurring kidney diseases. Vet. J. 2015, 205, 399–403. [Google Scholar] [CrossRef]
- Lin, C.N.; Wu, I.W.; Huang, Y.F.; Peng, S.Y.; Huang, Y.C.; Ning, H.C. Measuring serum total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease using UPLC-MS/MS. J. Food Drug Anal. 2018, 27, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Freeman, L.M.; Michel, K.E.; Zanghi, B.M.; Boler, B.M.V.; Fages, J. Usefulness of muscle condition score and ultrasonographic measurements for assessment of muscle mass in cats with cachexia and sarcopenia. Am. J. Vet. Res. 2020, 81, 254–259. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Zheng, Z.; Grams, M.E.; Appel, L.J.; Sarnak, M.J.; Inker, L.A.; Levey, A.S.; Coresh, J. Serum metabolites associated with dietary protein intake: Results from the Modification of Diet in Renal Disease (MDRD) randomized clinical trial. Am. J. Clin. Nutr. 2019, 109, 517–525. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Caney, S.; Chalhoub, S.; Elliott, J.; Finch, N.; Gajanayake, I.; Langston, C.; Lefebvre, H.P.; White, J.; Quimby, J. ISFM consensus guidelines on the diagnosis and management of feline chronic kidney disease. J. Feline Med. Surg. 2016, 18, 219–239. [Google Scholar] [CrossRef]
- Diets for Cats with Chronic Kidney Disease. Available online: http://www.iris-kidney.com/education/protein_restriction_feline_ckd.html#:~:text=Based%20on%20evidence%20from%20clinical,pharmaceuticals%20to%20manage%20medical%20conditions (accessed on 18 January 2022).
- Hall, J.A.; Fritsch, D.A.; Jewell, D.E.; Burris, P.A.; Gross, K.L. Cats with IRIS stage 1 and 2 chronic kidney disease maintain body weight and lean muscle mass when fed food having increased caloric density, and enhanced concentrations of carnitine and essential amino acids. Vet. Rec. 2019, 184, 190. [Google Scholar] [CrossRef] [Green Version]
- Małgorzewicz, S.; Gałęzowska, G.; Cieszyńska-Semenowicz, M.; Ratajczyk, J.; Wolska, L.; Rutkowski, P.; Jankowska, M.; Rutkowski, B.; Debska-Slizien, A. Amino acid profile after oral nutritional supplementation in hemodialysis patients with protein-energy wasting. Nutrition 2019, 57, 231–236. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Volpi, E. Amino acid metabolism and regulatory effects in aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, G.; Botting, H.G.; Collins, M. A comparison of fasting serum amino acid profiles of young and elderly subjects. J. Am. Coll. Nutr. 1991, 10, 668–674. [Google Scholar] [CrossRef]
- Phipps, W.S.; Jones, P.M.; Patel, K. Amino and organic acid analysis: Essential tools in the diagnosis of inborn errors of metabolism. Adv. Clin. Chem. 2019, 92, 59–103. [Google Scholar]
- Camara, A.; Verbrugghe, A.; Cargo-Froom, C.; Hogan, K.; DeVries, T.J.; Sanchez, A.; Robinson, L.E.; Shoveller, A.K. The daytime feeding frequency affects appetite-regulating hormones, amino acids, physical activity, and respiratory quotient, but not energy expenditure, in adult cats fed regimens for 21 days. PLoS ONE 2020, 15, e0238522. [Google Scholar] [CrossRef]
- Pion, P.D.; Lewis, J.; Greene, K.; Rogers, Q.R.; Morris, J.G.; Kittleson, M.D. Effect of meal-feeding and food deprivation on plasma and whole blood taurine concentrations in cats. J. Nutr. 1991, 121, S177–S178. [Google Scholar] [CrossRef]
- Heinze, C.R.; Larsen, J.A.; Kass, P.H.; Fascetti, A.J. Plasma amino acid and whole blood taurine concentrations in cats eating commercially prepared diets. Am. J. Vet. Res. 2009, 70, 1374–1382. [Google Scholar] [CrossRef] [Green Version]
| Variable (Reference Interval) * | Healthy Controls (n = 16) | CKD Stages 1 and 2 (n = 19) | CKD Stages 3 and 4 (n = 16) |
|---|---|---|---|
| Creatinine (0.8–2.4 mg/dL) | 1.4 (1.1–1.8) a | 2.0 (1.3–2.7) b | 3.7 (2.8–13.1) c |
| BUN (18–35 mg/dL) | 21 (18–29) a | 39 (24–49) b | 58 (36–117) c |
| Phosphorus (3.0–6.0 mg/dL) | 3.6 (2.9–4.6) a | 3.9 (2.7–5.4) a,b | 4.1 (3.1–7.2) b |
| Potassium (3.7–5.4 mEq/L) | 4.4 (3.6–5.2) | 4.7 (3.7–5.5) | 4.5 (3.5–5.1) |
| Albumin (3.1–4.4 g/dL) | 3.8 (3.2–4.4) | 3.6 (3.2–4.5) | 3.6 (3.0–3.9) |
| USG (>1.035) | 1.049 (1.038–1.056) a | 1.021 (1.010–1.035) b | 1.015 (1.006–1.023) c |
| Amino Acid (µM) * | Healthy Cats (n = 16) | All CKD Cats (n = 26) | Adjusted p-Value (Welch t-Test) | Mean Difference ± SEM | CKD Stage 1 and 2 (n = 12) | CKD Stage 3 and 4 (n = 14) |
|---|---|---|---|---|---|---|
| Essential Amino Acids | ||||||
| Arginine | 115.3 ± 25.5 | 114.1 ± 30.0 | 0.63 | 1.2 ± 8.7 | 109.8 ± 25.9 | 117.7 ± 33.7 |
| Histidine | 107.6 ± 12.7 | 112.5 ± 16.5 | 0.35 | 4.9 ± 4.5 | 109.9 ± 15.1 | 114.8 ± 18.0 |
| Isoleucine | 72.5 ± 21.1 | 63.1 ± 24.1 | 0.43 | 9.4 ± 7.1 | 72.8 ± 31.1 | 54.7 ± 11.1 |
| Leucine | 143.1 ± 30.0 a | 120.4 ± 40.3 | 0.14 | 22.7 ± 10.9 | 135.3 ± 52.2 a,b | 107.5 ± 20.6 b |
| Lysine | 99.4 ± 40.7 | 91.2 ± 28.8 | 0.47 | 8.2 ± 11.6 | 100.2 ± 34.8 | 83.5 ± 20.8 |
| Methionine | 46.2 ± 15.6 | 37.9 ± 20.7 | 0.33 | 8.3 ± 5.6 | 39.4 ± 24.1 | 36.7 ± 18.1 |
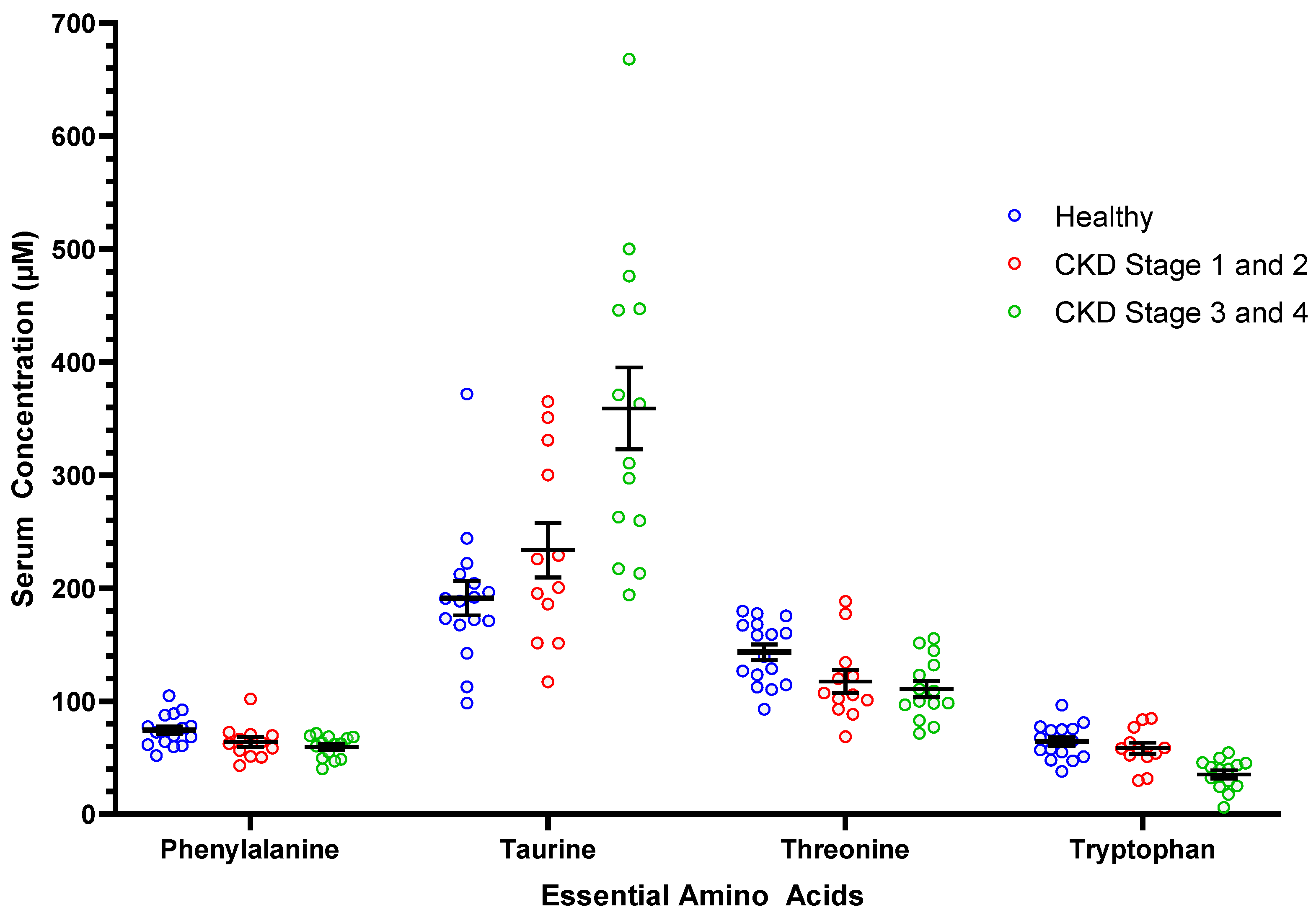
| Phenylalanine | 74.3 ± 13.9 a | 61.6 ± 12.4 | 0.03 | 12.7 ± 4.3 | 64.0 ± 15.0 a,b | 59.6 ± 9.8 b |
| Taurine | 191.3 ± 61.1 a | 301.2 ± 129.2 | 0.01 | 109.9 ± 29.6 | 233.7 ± 83.6 a | 359.1 ± 135.5 b |
| Threonine | 143.3 ± 28.0 a | 113.9 ± 30.5 | 0.03 | 29.6 ± 9.2 | 117.4 ± 35.0 b | 110.8 ± 27.1 b |
| Tryptophan | 64.4 ± 15.2 a | 46.1 ± 19.2 | 0.005 | 18.4 ± 5.4 | 58.5 ± 17.5 a | 35.4 ± 13.5 b |
| Valine | 179.5 ± 41.2 a | 151.9 ± 52.3 | 0.19 | 27.7 ± 14.5 | 172.5 ± 66.6 a,b | 134.2 ± 27.8 b |
| Nonessential Amino Acids | ||||||
| Alanine | 580.2 ± 116.2 | 545.8 ± 108.2 | 0.38 | 34.3 ± 36.0 | 531.6 ± 110.9 | 558.0 ± 108.4 |
| Asparagine | 100.7 ± 25.1 | 85.0 ± 23.4 | 0.19 | 15.7 ± 7.8 | 85.4 ± 25.0 | 84.8 ± 22.9 |
| Aspartic acid | 18.7 ± 5.5 a | 23.4 ± 7.6 | 0.005 | 4.7 ± 2.0 | 18.3 ± 4.9 a | 27.7 ± 6.8 b |
| β-alanine | 3.2 ± 3.5 a | 6.6 ± 3.8 | 0.005 | 3.4 +/1.2 | 4.1 ± 2.4 a | 8.8 ± 3.5 b |
| Citrulline | 12.2 ± 4.0 a | 17.9 ± 6.2 | 0.01 | 5.7 ± 1.6 | 15.0 ± 3.9 a | 20.4 ± 6.8 b |
| Glutamic acid | 38.0 ± 17.8 | 34.1 ± 12.1 | 0.30 | 3.9 ± 5.0 | 37.2 ± 14.0 | 31.5 ± 9.9 |
| Glutamine | 678.8 ± 98.0 | 701.7 ± 176.4 | 0.34 | 22.9 ± 42.4 | 661.9 ± 159.4 | 735.9 ± 188.8 |
| Glycine | 308.6 ± 61.6 | 308.2 ± 82.5 | 0.89 | 0.4 ± 22.3 | 300.5 ± 71.1 | 314.8 ± 93.3 |
| Hydroxyproline | 28.8 ± 15.0 | 34.1 ± 18.6 | 0.52 | 5.0 ± 5.1 | 29.9 ± 15.7 | 37.1 ± 20.6 |
| Ornithine | 14.6 ± 4.9 | 13.0 ± 4.3 | 0.57 | 1.5 ± 1.5 | 12.8 ± 4.8 | 13.2 ± 4.0 |
| Proline | 194.9 ± 70.8 | 145.6 ± 41.2 | 0.10 | 49.3 ± 19.5 | 143.6 ± 50.6 | 147.3 ± 33.0 |
| Serine | 146.4 ± 35.5 a | 116.6 ± 45.4 | 0.03 | 29.8 ± 12.6 | 132.6 ± 48.2 a,b | 103.0 ± 39.5 b |
| Tyrosine | 55.1 ± 13.4 a | 43.5 ± 9.0 | 0.01 | 11.6 ± 3.8 | 41.5 ± 10.0 b | 45.3 ± 8.0 b |
| 3-Methylhistidine | 11.6 ± 2.8 a | 42.7 ± 42.5 | 0.005 | 31.1 ± 8.4 | 27.3 ± 13.5 a | 55.8 ± 53.9 b |
| Serum Amino Acid | Spearman’s Rank Correlation Coefficient (95% Confidence Interval (Upper Limit, Lower Limit) | Adjusted p-Value |
|---|---|---|
| Essential Amino Acids | ||
| Isoleucine | −0.36 (−0.60, −0.05) | 0.02 |
| Leucine | −0.45 (−0.67, −0.16) | 0.005 |
| Methionine | −0.39 (−0.63, −0.10) | 0.01 |
| Phenylalanine | −0.44 (−0.66, −0.15) | 0.005 |
| Threonine | −0.39 (−0.63, −0.10) | 0.01 |
| Tryptophan | −0.58 (−0.76, −0.33) | <0.001 |
| Valine | −0.45 (−0.67, −0.16) | 0.005 |
| Nonessential Amino Acids | ||
| Aspartic acid | 0.68 (0.47, 0.82) | <0.001 |
| β-alanine | 0.58 (0.32, 0.75) | <0.001 |
| Citrulline | 0.49 (0.20, 0.69) | 0.002 |
| Serine | −0.43 (−0.66, −0.14) | 0.005 |
| Taurine | 0.67 (0.46, 0.81) | <0.001 |
| Tyrosine | −0.33 (−0.58, −0.02) | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summers, S.C.; Quimby, J.; Blake, A.; Keys, D.; Steiner, J.M.; Suchodolski, J. Serum and Fecal Amino Acid Profiles in Cats with Chronic Kidney Disease. Vet. Sci. 2022, 9, 84. https://doi.org/10.3390/vetsci9020084
Summers SC, Quimby J, Blake A, Keys D, Steiner JM, Suchodolski J. Serum and Fecal Amino Acid Profiles in Cats with Chronic Kidney Disease. Veterinary Sciences. 2022; 9(2):84. https://doi.org/10.3390/vetsci9020084
Chicago/Turabian StyleSummers, Stacie C., Jessica Quimby, Amanda Blake, Deborah Keys, Joerg M. Steiner, and Jan Suchodolski. 2022. "Serum and Fecal Amino Acid Profiles in Cats with Chronic Kidney Disease" Veterinary Sciences 9, no. 2: 84. https://doi.org/10.3390/vetsci9020084
APA StyleSummers, S. C., Quimby, J., Blake, A., Keys, D., Steiner, J. M., & Suchodolski, J. (2022). Serum and Fecal Amino Acid Profiles in Cats with Chronic Kidney Disease. Veterinary Sciences, 9(2), 84. https://doi.org/10.3390/vetsci9020084








